Abstract
Hard tissue regeneration and regrowth have continued to be a challenge in the field of conventional medicine in this 21st century. Over the years, the regrowth of broken bones and diseased hard tissue has remained a major concern in medical research. Since the discovery of hydroxyapatite (HA), a bioceramic compound that possesses the ability to activate bone regrowth and bond directly with regenerated bone, it has subsequently become an indispensable biomaterial. Currently, it is being used across the medical fields due to its exceptional biocompatibility. This became plausible because the main mineral phase of mammalian bones is HA. It has found application in various medical fields like medical instruments, drug delivery, bone and tooth fillers, prosthetics, orthotics, and in-vitro implants. As the importance of HA geometrically increases, it is necessary to critically evaluate and propose the most economic process of synthesizing and manufacturing this important bioceramic material. This review, therefore, highlights the different sources of HA and the synthesis/production methods for each source with a strong emphasis on the environment. Thus, the appraisal was carried out based on the properties of the derived HA. Such properties include but are not limited to geometry, particle size, morphology, thermal stability, and stoichiometry to suggest the most economic and environmentally sustainable sources and processing routes.
Keywords: Materials science, Biomedical engineering, Materials chemistry, Biomaterials, Bioceramics, Hydroxyapatite, Osteoinductive, Hard tissue regeneration, Prosthesis, In-vitro implants, Pollution control, Environmental impact assessment, Biocomposites
Materials Science; Biomedical Engineering; Materials Chemistry; Biomaterials; Bioceramics; Hydroxyapatite; Osteoinductive; Hard tissue regeneration; Prosthesis; In-vitro implants; Pollution control; Environmental impact assessment; Biocomposites
1. Introduction
Human beings are susceptible to numerous kind of diseases and accidental mishaps some of which result in bone, joint and teeth injury. From time immemorial, various forms of treatment and coping measures have been used to improve the life of the patients or victims of such mishaps and make them more comfortable. Various equipment including wooden stents, clutches, scaffolds and various types of wheelchairs have been utilized to better the lives of people with bone injuries. With the advancement of technology, efforts have been intensified and these have been yielding better results over time. The development of artificial body parts and prosthetic limbs are examples of the result of human effort in collaboration with technological advancement in the medical field to yield much better aid to the injured.
With the advancement of research into proffering solutions to these issues, various materials are being developed for a variety of applications, majority of which fall into the calcium phosphate (CaP) spectrum of biocompatible compounds shown in Table 1, one of the most important of these being a calcium apatite-calcium, phosphorous, and oxygen-ceramic material known as Hydroxyapatite. This calcium phosphate compound occurs naturally and it is usually found within the mineral phase of teeth, shells, and bones of many living organisms. Its crystal system is hexagonal connoting that it grows in hexagonal (stop sign shaped) crystals. Hydroxyapatite makes up most of the human bone structure, builds tooth enamel, and collects in tiny amounts within some parts of the brain.
Table 1.
Common calcium phosphate compounds in the CaP spectrum and their applications (Akram et al., 2014).
| No. | Compound Name | Application | Chemical Formula | Crystal Structure | Ca/P Ratio |
|---|---|---|---|---|---|
| 1. | Monocalcium phosphate monohydrate | Enhances the uptake of root fluoride in the body (Takagi et al., 1987) | Ca(H2PO4)2.H2O | Triclinic | 0.5 |
| 2. | α-Tricalcium phosphate | Used in the repair of bones as a biodegradable composite (Carrodeguas, 2011) | α-Ca3(PO4)2 | Monoclinic | 1.5 |
| 3. | β-Tricalcium phosphate | Used in orthopaedic surgery | β-Ca3(PO4)2 | Rhombohedral | 1.5 |
| 4. | Tetracalcium phosphate | Utilized in metallic implants as coatings, binders and cement | Ca4(PO4)2O | Monoclinic (Brown and Epstein, 1965) | 2 |
| 5. | Monocalcium phosphate (anhydrous) | It is used to form artificial bone grafts | Ca(H2PO4)2 | Triclinic | 0.5 |
| 6. | Dicalcium phosphate dihydrate | Used in the controlled release of drugs that are highly soluble in water (Mulye and Turco, 1994) | CaHPO4 2H2O | Monoclinic (Nosrati et al., 2019; Beevers, 1958) | 1 |
| 7. | Dicalcium phosphate anhydrous | It is used as a source of calcium and phosphorus in food supplements and also for polishing teeth | CaHPO4 | Orthorhombic (Ouerfelli and Zid, 2016) | 1 |
| 8. | Hydroxyapatite | Used in the repair and regrowth of hard tissues | Ca10(PO4)6 (OH)2 | Hexagonal (Mathai and Shozo, 2001) | 1.67 |
| 9. | Calcium-deficient hydroxyapatite | Used for bone grafting | Ca10-x (HPO4)x (PO4)6-x (OH)2-x | Hexagonal | 1.5–1.6 |
| 10. | Fluorapatite | Used in the making of pharmaceutical products as a source of flourine | Ca10(PO4)6F2 | Hexagonal (Mathai and Shozo, 2001) | 1.67 |
1.1. Hydroxyapatite
Hydroxyapatite also called hydroxylapatite (HA) is represented chemically as Ca5(PO4)3(OH), usually expressed as Ca10(PO4)6(OH)2 meaning two entities make up the unit cell of the crystal. Hydroxyapatite being the hydroxyl end member of the complex apatite group possesses OH− ions that are replaceable by carbonate, chloride or fluoride creating fluorapatite or chlorapatite. Unadulterated hydroxyapatite powder is typically white. Natural apatites, however, may have darker, green, or yellow hues, comparable to the stains of dental fluorosis (Webmineral, 2019). Enamel and dentin found in the teeth are composed mainly of carbonated calcium-deficient hydroxyapatite. Many structures including the pineal gland additionally contain corpora arenacea or 'brain sand' which are tiny calcified crystals of hydroxyapatite (Anthony et al., 2000). Bone mineral is a non-stoichiometric form of hydroxyapatite which makes up about 70% by weight and over half by volume of human bone (Junqueira et al., 2003). The biomedical advantages and mechanical properties of hydroxyapatite have prompted its examination for potential engineering design and use.
1.1.1. Phases
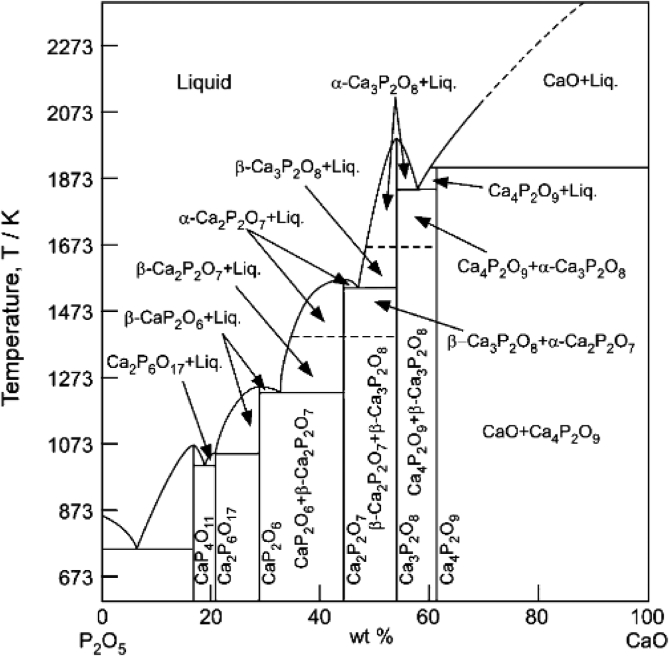
In the discussion of calcium phosphate phases, a ratio of Calcium to Phosphorus known as the Ca/P ratio is used (Angervall et al., 2009). The Ca/P ratio of Stoichiometric apatite Ca10(PO4)6(OH)2 is 10:6 usually conveyed as 1.67, while that of non-stoichiometric hydroxyapatite ranges within 1.5 and 1.9 and that of Calcium deficient hydroxyapatite, Ca10−x (PO4)6−x (HPO4)x (OH)2−x (where x is within 0 and 1) is below 1.67 (Ofudje et al., 2018). Figure 1 shows a phase diagram of the calcium phosphate system spectrum.
Figure 1.
A phase diagram of the calcium phosphate system spectrum.
Phases possessing a hydroxyapatite structure with cation vacancies (Ca2+) and anion (OH–) vacancies are non-stoichiometric. In these phases, phosphate or hydrogen phosphate (HPO42–) anions occupy sites that are usually occupied exclusively by phosphate anions in stochiometric hydroxyapatite (Rey et al., 2011). Calcium deficient phases with the desired Ca/P ratio (for example materials having a Ca/P ratio of 1.6 as shown in the equation 1 can be set up by precipitation out of a blend of calcium nitrate and di-ammonium phosphate (Raynaud et al., 2002).
| 9.6 Ca(NO3)2 + 6 (NH4)2HPO4 → Ca9.6(PO4)5.6(HPO4)0.4(OH)1.6 | (1) |
A solid-phase termed biphasic calcium phosphate, a close blend of tricalcium phosphate and hydroxyapatite, may be formed upon sintering these non-stoichiometric phases according to the reaction equation 2 (Valletregi, 1997).
| Ca10−x(PO4)6−x(HPO4)x(OH)2−x → (1−x) Ca10(PO4)6(OH)2 + 3x Ca3(PO4)2 | (2) |
1.1.2. Sources
HA can be sourced from both natural and synthetic materials, for example, the limbs of an odontodactylus scyllarus (peacock mantis shrimp) which it uses for clubbing are composed of an amazingly thick type of HA possessing higher toughness and strength than other engineered composite material (Weaver et al., 2012). It also possesses other interesting properties that can be adapted for use in engineering applications which include: excellent impact resistance and significant hardness due to the impact region in their dactyl appendages being mostly composed of crystalline hydroxyapatite. This crystalline hydroxyapatite has an intermittent layer beneath the impact layer made up of hydroxyapatite having much lower calcium and phosphorus content, bringing about a lower modulus. The enormous contrast in modulus causes the reflection of some of the incident energy which reduces the energy transferred across both layers and compels new cracks to change direction, therefore, hindering crack growth (Tanner, 2012).
It has been established that bone and teeth contain large amounts of hydroxyapatite. Bone contains 65–70% hydroxyapatite (HA) crystals interspersed in a collagen matrix while dentin and enamel in teeth contain 70–80% with amelogenins and enamelins forming the enamel matrix for HA in teeth instead of collagen (Habibah and Salisbury, 2018).
The local in-vivo disintegration rate of hydroxyapatite is about 10 wt% every year, which is essentially less than the development speed of recently framed bone tissue. Methods are being developed to improve its solvency rate and advance better bioactivity when utilized as a bone substitute material. Although HA can be used as supplements, it has some detrimental biological effects including calcific tendinitis which is the deposition of HA in tendons around joints (Carcia and Scibek, 2013). However, it is highly useful for biological and medical applications, for example, bone grafting, dental repair, implants, and prosthesis are made or coated with HA (Habibah and Salisbury, 2018).
1.1.3. Production
HA can be produced via synthesis from various sources, several methods of HA synthesis have been developed, for example, wet chemical deposition, biomimetic deposition, electro-deposition or sol-gel method (wet-chemical precipitation) (Ferraz et al., 2004). It was proposed that using the following reaction in equation 3 for wet chemical precipitation, hydroxyapatite nanocrystal suspensions may well be prepared (Bouyer et al., 2000).
| 10 Ca(OH)2 + 6 H3PO4 → Ca10(PO4)6(OH)2 + 18 H2O | (3) |
Numerous research has proven that high-power ultrasound can improve hydroxyapatite synthesis through the wet-chemical synthesis method. The high-quality production of nanostructured hydroxyapatite through Sono-synthesis or ultrasonically assisted synthesis has been very successful. The ultrasonic route enables the production of core-shell nanospheres and composites via the production of nano-crystalline and altered hydroxyapatite particles (www.hielscher.com, Sono-Synthesis of Nano-Hydroxyapatite, 2019).
1.1.4. Uses
Although there are numerous sources of HA from both synthetic and non-synthetic origins, the HA within the bones of living organisms are very thin plate-like carbonate structures which have an average of 50 nm length, 2–3 nm thickness and a width of 25 nm, with other very unique properties (www.hielscher.com, Sono-Synthesis of Nano-Hydroxyapatite, 2019). The use of HA for drug delivery and cell carriers also demands a high level of biocompatibility and a very unique set of physical and chemical properties (Oladele et al., 2018a, Oladele et al., 2018b). Another age of biomaterials based on agro-waste that brings the science of aggravation and wound recuperating to the spotlight is developing and serious research is being conducted on them for use as both cell bearers and drug medication conveyers. This makes it very tasking to develop hydroxyapatite possessing mineral components that entirely fit the mineral components possessed by mammalian hard tissue (Nayak, 2010). On the other hand, there is the challenge of improving the osteoinductive properties of HA by incorporating trace elements into the structure of the developed HA (Akram et al., 2014).
This review aims to bring to fore the different sources of HA and the synthesis/production methods for each source. Consequently appraising thoroughly the characteristic properties of the HA derived, such as the geometry, particle size, morphology, thermal stability, and stoichiometry to suggest the most environmentally sustainable source and processing route.
2. Synthetic sources of hydroxyapatite
The stoichiometric Ca/P ratio of synthetic hydroxyapatite is 1.67. The strict control of the process of synthesis using different methods and materials is required to produce stoichiometric hydroxyapatite which is of the required high quality. Generally, hydroxyapatite exists in various forms including cylindrical forms, spherical forms, etc. (Oladele et al., 2018a, Oladele et al., 2018b). A variety of processes and techniques for synthesizing HA powders using different chemicals and reactants exist (Nayak, 2010) (see Table 2). Some of which include:
Table 2.
Synthetic Processes and Techniques for Producing HA Powders using Different Chemicals and Reactants (Nayak, 2010).
| S/N | Processing Technique | Reference/Source |
|---|---|---|
| 1. | Spray pyrolysis | Walsh et al. (2008) |
| 2. | Sol gel technique | (Sanosh et al., 2009) |
| 3. | Solution combustion | (Pandharipande and Sondawale, 2016a) |
| 4. | Hydrolysis | (Shih et al., 2005) |
| 5. | Microwave | (Pandharipande and Sondawale, 2016b) |
| 6. | Solid state reaction | (Guo et al., 2013) |
| 7. | Hydrothermal | (Neira et al., 2008) |
| 8. | Emulsion method | (Chen et al., 2009) |
| 9. | Mechanochemical | (Yeong et al., 2001) |
| 10. | Precipitation methods including: a. Calcium carbonate (CaCO3) based wet chemical precipitation method, b. Calcium hydroxide (Ca(OH)2) based wet chemical precipitation method, c. Di-ammonium hydrogen Ortho-phosphate based wet chemical precipitation method, d. and Ortho-phosphoric acid (H3PO4) based wet chemical precipitation method. |
(Mobasherpour et al., 2007) |
More advanced methods are used to modify the size of the crystals and synthesize HA nanoparticles. Some methods even go further to engineer the structure, shape and surface properties of the nanosized hydroxyapatite crystals. Summarized below are a few of the most recent methods.
A technique to synthesize hydroxyapatite nanoparticles was successfully developed by Ji and Meisha using a dispersed template of block copolymers (Ji and Meisha, 2013). In this technique, a scheme for synthesizing nanoparticles was used that controls the surface chemistry and shapes of the nanoparticles and at the same time produces particles that are spherical and needle-shaped, retaining the block copolymers as surface coatings (Ji and Meisha, 2013). The strategy performed well when a dispersed template of double-hydrophilic block copolymers (DHBCs) was used (Tjandra et al., 2005; Marentette et al., 1997; Öner et al., 1998; Antonietti et al., 1998). However, this strategy was advanced to synthesizing organophilic nanoparticles in organic solvents rather than hydrophilic nanoparticles using alternative block copolymer chemistry having a dispersed template of poly (methyl methacrylate)-b-poly (methacrylic acid) (PMMA-b-PMAA) and an organic solvent -tetrahydrofuran. A systematic variation of the concentration of precursors and the number of block copolymers was carried out to explore a range of shape control possible (Ji and Meisha, 2013). There was a similarity between the results obtained during the synthesis and the results obtained with DHBC template synthesis (Tjandra et al., 2005). From the synthesis results, it was observed that there exists a relationship between the Ca2+/COO− mole ratio and the shape of the nanoparticles produced. This synergy in the quality of the mechanisms for shape control in both synthesis schemes proposes that the connection between the synthesis schemes might be general and as such serve as a mechanism for adjusting the nanoparticle shape of a large variety of chemistries for various other such block copolymers (Ji and Meisha, 2013). Further work is however needed to explore this relationship and confirm this proposition.
Another successful attempt was made to synthesize nanostructured HA. The HA was artificially synthesized using readily available materials via a method based on wet chemical precipitation. The yield improved surface morphology and enhanced porosity when compared to other methods (Hall, 2005). Orthophosphoric acid (H3PO4) was combined with Calcium hydroxide (Ca(OH)2) to conduct an experiment under a controlled environment, the required pH, concentration of solutions and temperature were carefully maintained. Variation in any of these parameters would result in a change in the resultant HA obtained (Monmaturapoj, 2008). After synthesis and characterization of the HA powder, X-ray diffraction, Scanning electron microscopy, and Fourier-transformed infrared spectroscopy were performed to obtain its surface, size and dimensions analysis (Ganachari et al., 2016). The analysis revealed that the HA was between 35 nm to 90 nm which was a desirable output, and pores were observed on the surface which would support the growth and interaction of osteoblasts which implies that the HA was osteoconductive. The X-Ray Diffraction pattern with reference to JCPDS number 09–432 established the existence of calcium in the material and FTIR analysis revealed the presence of -O-H-, NH⁺⁴ and PO₄³-. Due to these characterizations, it was then concluded that nano-structured hydroxyapatite was successfully synthesized (Ganachari et al., 2016). It is, however, necessary to exercise extreme caution whenever this process is used, to minimize the emission of dangerous chemicals and reduce the detrimental effects on the environment.
In a bid to reduce or eliminate harmful emissions which are usually byproducts of most synthetic methods of hydroxyapatite formation, a method for synthesizing HA nanoparticles was developed using Ca and P sources from a reaction of 99.0% Ca(OH)2 and 85.0% H3PO4 (obtained from High Purity Chemical, Japan and Junsei Chemical Co., Ltd. respectively). There was no need for additional washing since these chemicals do not produce residual harmful anions (Kim et al., 2010). To carry out the reaction, a direct mixture of 500 mL of 0.6M H3PO4 solution with 500 mL of 1.0 M Ca(OH)2 suspension was prepared using a magnetic stirrer at 25 °C. Several processes were carried out on the mixture including press-filtering, quenching with liquid nitrogen, and freeze-drying after sampling at different time intervals. After drying the powders were preserved inside a vacuum desiccator to avoid air contact. HA nanoparticles were the only intermediate phase formed from dicalcium phosphate dihydrate (DCPD). The reaction was monitored by Fourier Transformed Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), 1H and 31P Magic-Angle Spinning (MAS) NMR via in-situ observation (Kim et al., 2010). Hetero-nucleation of HA preceded its phase evolution to the DCPD surface. Elliott (1994) revealed via Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-ES) and Scanning Electron Microscopy (SEM) analysis that the HA crystallization process was retarded by the slow diffusion process of Calcium ions into the interface between HA and DCPD and the residual Ca(OH)2, it was concluded from the results that pure HA nanoparticles could be synthesized in large amounts because of the rapidity of the initial synthesis of HA nanoparticles via heterogeneous nucleation on bulky DCPD particles. The slow diffusion progression of Calcium ions as the reaction proceeded into the interface between DCPD and HA and the existence of residual Ca(OH)2 however retarded HA synthesis. This shows that pure HA nanoparticles could be mass-produced in an environmentally friendly manner with highly reduced emissions by controlling the diffusion progression of Calcium ions and the residual Ca(OH)2 (Elliott, 1994). This reaction is however expensive and time-consuming due to the high cost of reagents and equipment required and the slow rate of diffusion.
Minh et al. (2012) investigated calcium hydroxyapatite (Ca-HA) synthesis using starting materials derived from different sources at ambient conditions. Some of these sources are calcium carbonates and phosphorus-based sources, including orthophosphoric acid, potassium orthophosphates, sodium orthophosphates, and ammonium dihydrogen orthophosphates. Orthophosphoric acid remained the lone reactant that leads to the total depletion via precipitation of orthophosphate species used as starting materials within 48 h and was the most capable acid for dissolving calcium carbonate. The reaction began with the dissolution of calcium carbonate in acid after which swift precipitation of calcium cations with orthophosphate species occurred forming particles with sizes ranging from 0.4–1μm based on calcium phosphate. Using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) analysis, an elemental inquiry of the soluble phosphorus and calcium contents within the liquid phase was performed. As the chemical reaction proceeded, these calcium phosphate particles then agglomerated growing into larger aggregates with a diameter of about 350 μm which led to a porous structure in the final products that were quite unstable and had relatively similar size range and a specific surface area (SBET) difference in the solid final products. Thermogravimetric analysis and x-ray diffraction analysis (TG and XRD) characterizations were used on dissimilar intermediates to identify as well as better understand their evolution during the reaction (Elliott, 1994). It was discovered that orthophosphate species were completely precipitated using orthophosphoric acid, and calcium carbonate were also highly dissolved. The maximum pore volume and specific surface area were displayed by potassium dihydrogen orthophosphate. The formation of low crystallinity solid products based on calcium phosphate was also observed under ambient conditions. This led to the identification of different intermediates and the proposal of a reaction pathway (Minh et al., 2012). It is hoped that more research focusing on the use of orthophosphoric acid to dissolve calcium carbonate at higher reaction temperatures will be conducted, thereby deriving a simple one-step production route for HA that is of good properties and highly economical. A special focus should be directed toward handling the environmental effects and the resulting emissions from the high-temperature reactions of phosphoric acid.
Researchers in various previously conducted research, including Masaki et al. (1993) studied the in vitro HA/b-TCP ceramics stability. Jarcho (1981), Driessens (1983), LeGeros (1988) and de Groot (1980) all studied calcium phosphate ceramics for different biomedical applications and proposed that HA ceramics can be considered bone -replacement materials which are both bioactive and nonbiodegradable (Kivrak and Tas, 1998). However, upon utilization for alveolar ridge augmentation the nonbiodegradability, becomes a disadvantage to the tissue that hosts the hydroxyapatite. Giving rise to the requirement of a material which possessed the required biodegradability such that bone can replace it as it degraded. This lead to the development of b-TCP ceramics as a biodegradable bone replacement (Rejda et al., 1977). This, however, did not meet the need because its rate of degradation was too fast, therefore leading to the need for better options to reduce the rate of degradation. Composite ceramics that are made up of mixtures of phases of b-TCP and HA for example biphasic calcium phosphate (BCP) ceramics (i.e.) were developed, and the rate of degradation was studied (Daculsi et al., 1990; Ellinger et al., 1986; LeGeros et al., 1988; Moore et al., 1987; Nery et al., 1975; Renooij et al., 1985; Takeishi et al., 1989).
Kivrak and Tas (1998) made an exhaustive study of the methods of synthesis and sintering behavior of bioceramic Calcium Hydroxyapatite–Tricalcium Phosphate (HA–TCP) composite powders and developed a chemical precipitation technique for composite powder manufacture. This study performed by Kivrak and Tas was perhaps the first investigation into biphasic composite, calcium phosphate ceramics, apart from those accidentally encountered by the previous researchers when studying the synthesis of clean HA or clean whitlockite (b-TCP) phases (Kivrak and Tas, 1998).
The study presented a methodical stride in the area of biomaterials technology. It precisely described the chemical synthesis techniques of Hydroxyapatite–Tricalcium phosphate bioceramic composite powders. These bioceramic composites were bioactive due to the type of TCP in the homogeneous HA–TCP composite powders and precursors and completely resorbable when they interacted with bodily fluids. This TCP phase was alleged to have served as the bioimplant initial-matrix material that later induced different levels of porosity and underwent in situ generations of new areas of bone formation. It was, therefore, hoped that a high level of control over the phase composition and biodegradability of a bioceramic calcium phosphate-centered implant would be gained (Kivrak and Tas, 1998). In the study, biphasic composites of tricalcium phosphate (Ca3(PO4)2) and calcium hydroxyapatite (Ca10(PO4)6(OH)2) — the two most significant inorganic phases of synthetic bone, — were primed via a one-step novel chemical precipitation technique, as chemically homogeneous highly pure ceramic powders of submicron-sizes. Specific amounts of the starting materials, viz diammonium hydrogen phosphate salts and Calcium nitrate tetrahydrate salts were dissolved during powder precipitation runs in corresponding appropriate amounts of distilled water. The proper concentration of diammonium hydrogen phosphate solution was introduced into the solution of calcium nitrate in amounts of 2 mL/min while stirring continuously. Separate solutions of the biphasic bioceramic composite powders within the range of 20–90% were each prepared in 10% increments and mixed with corresponding 10% increments of the diammonium hydrogen phosphate (DAHP) salt solution. The solutions formed were used in preparing pellets which were almost completely sintered to a full density of about 99% in an ~1200 °C atmosphere of dry air via rapid densification for 5 h. Thereafter, phase development in the composite powders was observed and studied within a 1000–1300 °C temperature range using XRD, the behavior upon sintering was observed by SEM, while inductively coupled plasma–atomic emission spectroscopy (ICP-ES) was used for chemical analysis. The study was highly successful and it showed, for the first time, that the manufacture of two of the calcium phosphate system's most essential inorganic compounds which are bioinert Hydroxyapatite and bioresorbable tricalcium phosphate, in the form of an intimate composite powder mixture which is a two-phase and chemically homogeneous substance, was indeed possible using aqueous solutions.
There exist older and more popular synthesis methods for HA production which are a form of the following; hydrothermal techniques, sol-gel techniques, calcium phosphate hydrolysis, wet chemical precipitation methods. The last two of these methods are the most frequently used procedures.
The hydrothermal technique is a very popular method of producing HA powders. It comprises reacting a mixture of di-ammonium hydrogen phosphate with calcium carbonate (CaCO3) at high pressures of 12000 psi and elevated temperatures reaching 275 °C leading to the formation of a well crystallized and chemically homogeneous carbonate substituted hydroxyapatite.
Secondly, wet chemical precipitation methods are of different varieties, the following is a summary of the procedures for two of the most famous varieties.
The first is a wet chemical precipitation method that utilizes a solution of calcium hydroxide and orthophosphoric acid. It involves adding orthophosphoric acid solution dropwise to a dilute calcium hydroxide solution, at a pH greater than 9. The setup is stirred continuously as the orthophosphoric acid is slowly added at a precise rate. The precipitation reaction is slow but as the temperature increases, higher crystallinity products are produced. The range of reaction temperatures is usually from 25 °C to 90 °C (Oladele et al., 2019).
The second is a well-known wet chemical precipitation method that uses ammonium hydroxide, calcium nitrate, and diammonium hydrogen phosphate. During the reaction, the pH is regulated and kept at a constant 9 using ammonium hydroxide. After which distilled water is used to wash the precipitates to remove ammonium hydroxide and additional nitrates upon completion of the reaction. The production rate of this method is faster although both methods are similar. In both methods, calcium-deficient hydroxyapatite may be formed upon processing at pH's lower than 9 and a maturation or aging process involving continuous stirring is usually carried out after a combination of the reactants. This is to enable the slow incorporation of calcium into the apatitic structure as it undergoes morphological changes into more “blocky” crystals from the erstwhile needle-like crystals as the HA is formed in stoichiometric Ca/P ratios (see Table 3).
Table 3.
A summary of the different procedures for the synthesization of HA.
| Methods | General remarks | Advantage | Disadvantage |
|---|---|---|---|
| Wet methods Precipitation (Mobasherpour et al., 2007) |
|
|
|
|
|
|
|
|
|
|
|
| Hydrolysis (Shih et al., 2005) |
|
|
|
|
|
|
|
|
|
||
|
|||
| Emulsion (Chen et al., 2009) |
|
|
|
|
|
||
|
|
||
|
|
||
| Hydrothermal (Neira et al., 2008) |
|
|
|
|
|
||
| Sol-gel (Sanosh et al., 2009) |
|
|
|
|
|
||
|
|||
| Dry methods Solid state (Guo et al., 2013) |
|
|
|
|
|
|
|
|
|
||
| Mechanochemical (Yeong et al., 2001) |
|
|
|
|
|
|
|
|
|
3. Non-synthetic sources of hydroxyapatite
Most non-synthetic methods of hydroxyapatite production are usually variations of the synthetic methods mentioned above. The major difference is that some of the starting materials are sourced from natural sources such as eggshells, snail shells, plants, seashells, cow bones, fish bones, etc. These natural sources may be grouped into four major categories namely; Plant sources; Animal sources; Biogenic sources and Aquatic sources:
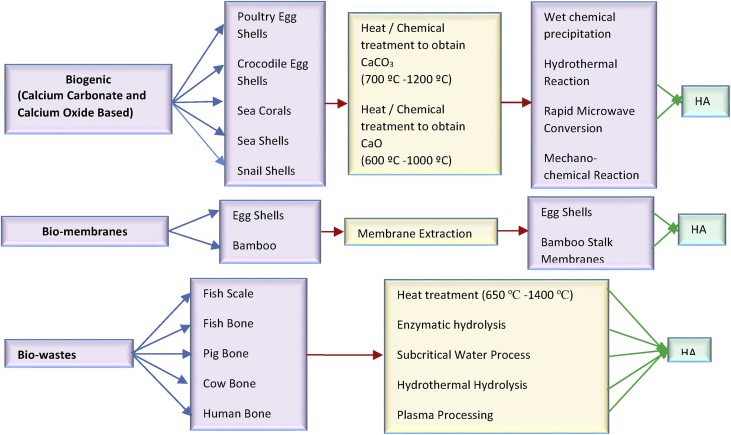
These different sources yield varying amounts of hydroxyapatite, depending on the number of calcium compounds contained in them and the processing methods applied (Oladele et al., 2018a, Oladele et al., 2018b). Therefore, it is essential to critically analyze each, and ascertain the most efficient synthesis procedure for each natural source of HA (see Figure 2).
Figure 2.
Different sources of HA with their preparation and synthesis routes.
3.1. Plant sources
Plants rich in calcite (CaCO3) are the most common sources of hydroxyapatite, examples include red algae (Phymatolithon Calcareum), leaves and stems of green tea, basil, mint, trifolium, khat, and wood.
Leaves of the basil plant contain hydroxyapatite and calcium hydroxide. XRD tests show HA as the major phase of basil leaves and the presence of other ions and elements in trace amounts (Akram et al., 2014). Shaltout et al. (2011) investigated the HA derived from Khat (C. edulis) and Basil plants using XRD and confirmed that HA was the main phase present, while Ca(OH2) was present in minor amounts along with trace amounts of other ions. Mint, green tea, trifolium, Khat stalks, and basil leaves, are five different plant samples that contain among their constituent compounds calcium hydroxyapatite, calcium hydroxide and trace amounts of other ions in varying quantities. The amount of hydroxyapatite extracted from the leaves and stalks of the five different plant samples after washing them in deionized water, drying, grinding, milling and heating to three different temperatures, which are 600 °C, 700 °C, and 800 °C in a muffle furnace, was compared in terms of HA concentration and it was discovered that the leaves contain higher hydroxyapatite content than the stalks. The lowest concentration of HA was noticed in trifolium and the highest concentration was noticed in Khat (C. edulis) leaves. Generally, the concentration was found to increase with an increase in temperature, with the highest relative concentration observed in C. edulis leaves at the highest temperature of 800 °C (Shaltout et al., 2011; Akram et al., 2014).
Red algae contain sufficiently porous interconnected calcite (CaCO3) with high similarity to human bones which are usable for hydroxyapatite synthesis via pyrolysis and hydrothermal synthesis. After using a technique involving the purification of calcite using diammonium hydrogen phosphate and magnesium nitrate while calcining, a type of marine algae containing CaCO3 known as Rodophycophyta division was used in preparing HA in non-stoichiometric and F-substituted forms. This was carried out by first extracting it and performing hydrothermal synthesis involving the reaction of diammonium hydrogen phosphate (DAHP) with CaCO3 and ammonium fluoride at 200 °C for 24–48 h. FTIR, XRD, SEM, and EDX confirmed the phase purity, the presence of A- and B- type carbonate substitution and other chemical and morphological characteristics (Kusmanto et al., 2008). Walsh et al. (2008) observed and confirmed cell adhesion in the HA derived from porous algae using SEM study and biocompatibility testing. Red algae (C. officinals) was used to make HA at low temperature and pressure. The high content of calcium carbonate it contains was extracted via pyrolysis at temperatures ranging between 650 °C–700 °C for 12 h. Granular shaped HA was produced by reacting to the CaCO3 obtained with ammonium dihydrogen phosphate [NH3PO4(OH)2] at 100 °C for 12 h. The calcite produced from calcination of the red algae (pharmatolithon calcareum) was converted into HA that retains its naturally porous nature using a mixture of diammonium hydrogen phosphate (DAHP) and magnesium nitrate (MgNO3).
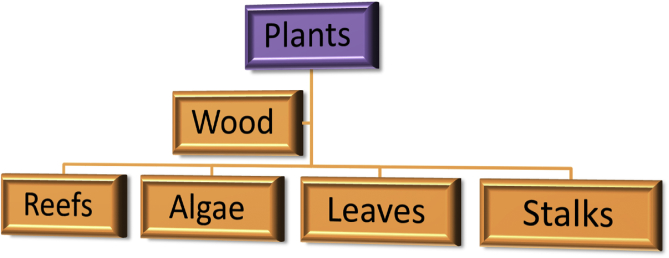
Wood is composed basically of carbon compounds. A method of deriving hydroxyapatite from wood involves processing the carbon compounds in the wood into carbon templates. These carbon templates are then converted into calcium carbide and also used to form calcium oxide which is used as a precursor during the development of calcium carbonate. A process of hydrothermal phosphatization is then performed to produce hydroxyapatite from the calcium carbonate (Akram et al., 2014; Wu et al., 2017). Tampieri et al. (2009) derived HA via this route and characterized it using XRD which confirmed the phase purity of the HA crystals and FTIR to evaluate and confirm the carbonate substitution into the structure of the HA formed. Various plant sources of Hydroxyapatite are illustrated in Figure 3.
Figure 3.
Plant sources of Hydroxyapatite.
3.2. Animal Sources
Animals are a major source of HA, the bones of most animals are primarily composed of calcium compounds, including calcium hydroxyapatite and tricalcium phosphate. The mineral matter in natural bone is made up of about 57%–67% hydroxyapatite and the remaining 33%–43% consisting of organic matter and trace amounts of other ions and elements (Jojor et al., 2015), consequently the hydroxyapatite derived from it is non-stoichiometric, very bioactive and good for bone grafting (Hiller et al., 2003). HA is usually gotten from the ashes of the bones after calcination, this negatively affects its mechanical strength but effectively eliminates the organic phases present in the bones leaving only the ceramic inorganic phases (mainly calcium oxide (CaO) and other trace elements). Generally, the bones are procured from various sources, including abattoirs, they are then carefully selected, boiled and washed in distilled water, to tenderize and remove the meat, blood, tissues and other forms of impurities from the bones. Other methods of dissolution may then be carried out to further dissolve the fats, oils, proteins and other soft tissues that may remain within the bones. One such method is alkaline treatment and fat removal which may be used with or without calcination for the process of hydroxyapatite formation. The bones are then thoroughly dried, cut into smaller sizes and crushed using a crusher. Further reduction in size is accomplished using a ball mill. After milling to the required particle size, the bovine bones are used in the production of hydroxyapatite by various synthesis modes. The choice of synthesis method, bone condition, and calcination temperature usually affects the properties including the particle size distribution and purity of hydroxyapatite produced using natural bones.
Hydroxyapatite exists in different microscopic forms including needles, rod, and spheres. These shapes allow for varying levels of porosity which influences cell proliferation, cell adhesion, and bone or tissue interaction. It is important to enhance the formation of spherical crystallites and as such enhance the crystallinity of hydroxyapatite without causing thermal degradation during calcination. Calcined bones especially bovine bones exhibit the highest degree of crystallinity (Akram et al., 2014).
Michael Mucalo presented a lecture at Waikato in 2017 where he expounded that cow bones can be processed into mineral shells of hydroxyapatite. He explained that the cow bones were procured and washed, after which the meat was shaved off the bones. The bones were then cut to get the spongy middle part known as the condyle. This middle part consisted of bone, fat, blood and gut therefore it was boiled thoroughly at 100 °C for six hours to remove the blood, fat, and guts from the bones. The bones were then put in a muffle furnace at 1000 °C to effectively burn off the remaining organic matter in the bone and leave the mineral phase consisting mainly of the calcium phosphate phase known as hydroxyapatite.
Jojor et al. (2015) extracted HA from bovine bone, the bovine bones were converted to HA via treatment procedures at different high temperatures. To remove organic materials and impurities including fats and collagen, the samples were first soaked in acetone for one hour and then distilled water was used to wash the samples before drying and calcination at 200–1000 °C in ambient conditions for five hours in an open crucible placed inside an electric furnace. After the samples were allowed to cool and subjected to grinding into 200 μm particle size they were characterized using DSC (Differential Scanning Calorimetry) and TGA (Thermo Gravimetric Analysis), to detect and analyze the sample's stretching frequencies of vibrational origin in the range of 450–4000 cm−1, FTIR and spectrum GX spectrometer were used. The crystalline nature of the samples was examined via an X-ray diffractometer and the obtained patterns were matched with reference JCPDS 09–0342/1996 to detect the phases present. The morphological properties and chemical elements of the HA crystals were observed and analyzed using a field emission SEM combined with EDX (In Situ Energy Dispersive X-ray Spectrometer) and results revealed that calcination of clean bovine bone at high temperatures ranging from 700 – 1000 °C gives rise to the formation of hydroxyapatite that exhibits a crystalline nature and purity directly related to the calcining temperature. In other words, as the temperature got higher the particle size grew bigger (Jojor et al., 2015). XRD results confirmed that the purity of the HA synthesized by thermal calcination was satisfactory upon comparison with the standard data. Microcrystals were observed to form as soon as the temperatures rose above 700 °C. The optimum temperature was perceived as being between 700–900 °C for the derivation and synthesis of high purity HA from bovine bone. The derived HA possessed good crystallinity, excellent stability, and almost no organic portion, which makes it suitable for use in biomedical applications. This process of HA extraction is relatively easy and economical, it also doesn't involve the use of dangerous chemicals. But the calcination of the bovine bones, however, releases greenhouse gases that must be collected and possibly reused (see Figure 4.).
Figure 4.
Bovine bones (animal source of hydroxyapatite).
3.3. Biogenic Sources
These include but are not limited to eggshells, snail shells, and seashells. Biogenic sources are excellent choices to synthesize biocompatible and bioactive biomaterials usable within the human body due to their characteristic properties.
Eggshells are waste materials that are cheap and easily accessible (Idris et al., 2014). An annual waste of more than a million tons of eggshells is generated from hatcheries, houses, restaurants, bakeries, etc. all over the world, collecting this waste is easy, because they are readily available and cheap (Oladele et al., 2018a, Oladele et al., 2018b). In the same vein, calcium-rich materials including seashells and snail shells are existing in large quantities all over the world (Akram et al., 2014). All these are good sources for large quantity HA synthesis (Akram et al., 2014; Rejda et al., 1977). Eggshells account for about 11% of the egg and are excellent suppliers of calcium. They are osteophilic and easily incorporated into bone tissues. They are chemically constituted of about (94%) Calcium carbonate, (1%) calcium phosphate, (1%) Magnesium carbonate and the remaining (4%) consisting of trace amount of other organic substrates and insoluble proteins (Li-Chan and Kim, 2008 and Rivera et al., 1999). Structurally, eggshells are constituted of three layers, they are the cuticle, the lamellar and the spongeous layers (Daculsi et al., 1990). The ceramic looking outer surface (cuticle) is made up of proteins and contains tiny amounts of needle-like hydroxyapatite while the lamellar and spongeous layers consist of a matrix of calcite calcium carbonate with protein fiber reinforcements in a ratio of 1:50 (Jojor et al., 2015). Snail shells especially the garden snail variety also serve as very good sources of high purity Hydroxyapatite.
To convert the eggshells, seashells and snail shells generated from food wastes to HA they are first collected and cleaned then boiled and eroded with salty water to eradicate all traces of the eggs or snails left over within the shells (Núñez et al., 2018). Snail shells (Helix Aspersa) contain calcium carbonate skeleton and so do eggshells (Jojor et al., 2015).
Generally, the process of furnishing HA from these shells is the same. However minor variations may be required, due to specific properties in the shells and depending on the type of shells. The process generally requires the use of a ball mill in pulverizing the shells to the appropriate grain size after which the shell samples are calcined at a temperature of about 850 °C in a furnace to decompose it into calcium oxide. After calcination, the shells are then subjected to chemical treatment using the appropriate chemicals and the required processes are carried out to transform the resulting powder into bioactive, stoichiometric, extremely pure and extremely fine hydroxyapatite (HA) crystals in form of a filter cake having a Ca/P molar ratio of 1.67 with a 15 m2/g specific surface area.
Seashells, for example, the shell of the mollusk (Pomacea Lineata) possess high mechanical strength compared to other shells and contain approximately 97% CaCO3. They are collected boiled in water and thoroughly washed to remove impurities, after which the samples are pulverized into powders of required grain size using a ball mill, put in a crucible and charged into a furnace for calcination at high temperatures to yield CaO. Specific chemical processes are then performed on this resultant CaO to prepare hydroxyapatite. FTIR and XRD analysis are used in the confirmation of the development of hydroxyapatite.
In previous work by Rivera et al. (1999) eggshells were gathered and subjected to mechanical and thermal treatment to clean off the unwanted materials and burn off the organic content respectively. The thermal treatment was performed in a couple of stages. First, the eggshells were heated at a rate of 5 °C/min up to a temperature of 450 °C and held for 2 h to allow all the proteins to escape and destroy all the organic residue. At the second stage the eggshells were calcined at a heating rate of 0.5 °C/min then held at 900 °C for 2 h to burn out and liberate the carbon dioxide CO2 and transform the eggshells into calcium oxide. CaO derived from the eggshells was converted into HA using a solution of phosphates, via the reaction stoichiometry: 5.54 g CaO corresponds to 1 g Ca3(PO4)2 for the determination of the reactants and the Ca/P ratio of HA which is 1.67 and for the determination of the products. The reaction was performed in a vessel engineered to reproduce an atmosphere that remained moist during the reaction time. A phosphate solution was prepared in the vessel and the CaO derived from the calcined eggshells was added to the phosphate solution in the vessel which was then completely sealed and the temperature was raised to 1050 °C at the rate of 10 C/min then held for 3 h. After this, filtering was performed on the resulting solution and the filtrate was dried at 80 °C within an oven overnight. SEM, XRD, and FTIR tests were performed on the samples, and it was inferred through the results, that the Ca3(PO4)2 XRD analysis exhibited hydroxyapatite traces and CaHPO4 impurities in the starting reactant which, however, had a slight if any effect on the concluding material. Hydroxyapatite was discovered to be the main unique, crystalline apatite phase identified in the product of the procedure. FTIR analysis of the final sample revealed the existence of bands at 605 cm−1and 1050 cm−1. These bands match with the (PO)4 functional groups in HA and TCP. A hydration process occurred when calcium carbonate mixed with water and produced calcium hydroxide:
| CaO + H2O Ca(OH)2 |
Which was the initial reactant phase present, while the total reaction process could be described using the formulae:
| 3Ca3(PO4)2 + Ca(OH)2 Ca10 (PO4)6(OH)2 |
This explained the presence of CaHPO4 as an impurity:
| 6CaHPO4 + 4CaO Ca10 (PO4)6(OH)2 + 2H2O |
| CaHPO4 + 4Ca(OH)2 Ca10(PO4)6(OH)2 + 6H2O |
The SEM micrographs of the different samples obtained show that at varying periods, morphologies of the materials subjected to heating were visible and shown to have a morphology similar to that of the inner side of an eggshell in pore size and fiber structure. These fiber-like structures were maintained throughout the thermal treatment and didn't seem to affect this morphology significantly.
Hydroxyapatite derived from eggshells are non-cytotoxic and support osteoblast cell adhesion perhaps because CaCO3 improves the properties of HA. The large quantity of calcite in eggshells causes an increase in the mechanical strength and better performance in terms of density, cell culture, and hardness after sintering of the derived hydroxyapatite. Krishna et al., 2007). Eggshell derived HA also possesses good crystallinity, morphology, and smaller particle size; an advantage resulting in better cellular response (Kim et al., 2006). Furthermore, the characteristics of the hydroxyapatite derived from eggshells are independent of the nature, source, calcination temperature and method of extraction of the HA from eggshells (Idris et al., 2014). Eggshell derived HA are osteophilic and easily incorporated into bone tissues. A prominent method of inducing new and functional tissue using eggshell derived HA is the development of a three dimensional (3D) extracellular matrix scaffold to which cells attach themselves and grow thereby regenerating the affected tissue. These scaffolds may be created by 3D printing nano-hydroxyapatite in an ordered interconnected structure. This is proposed as a very good replacement for bone and very suitable for tissue both in-vivo or in-vitro conditions.
Other methods of synthesizing hydroxyapatite from eggshells include; Hydrothermal method; High energy mechanochemical activation; Ethylene, Diamine, Tetra Acetic Acid (EDTA) and Sol-gel precipitation.
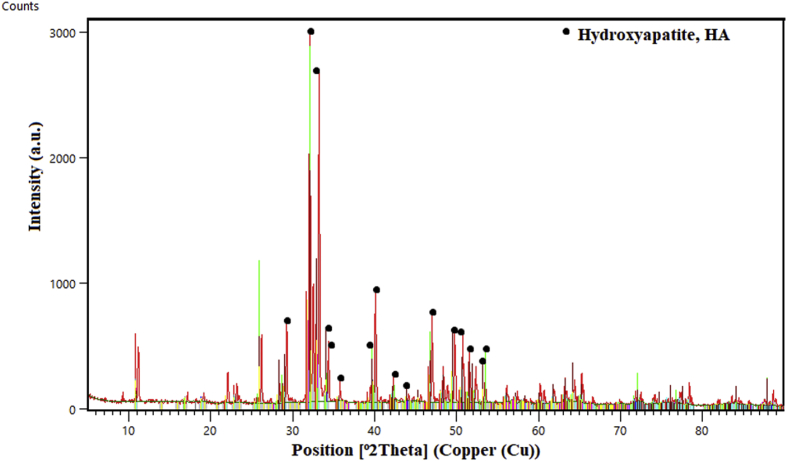
One of the most popular and most convenient methods of HA synthesis is the hydrothermal method, which utilizes a mixture of eggshells in a phosphate solution at a high temperature to generate hydroxyapatite single crystals (Zhang et al., 2009). It utilizes Ca(OH)2 and CaHP4·2H2O as starting materials in a reaction performed at high temperature and pressure, which yields HA as the main phase of the reaction product (Elizondo-Villarreal et al., 2012). This method involves a lot of time and labor but has the advantage of yielding all the characteristic bands and properties of HA as shown by FTIR, SEM, TEM, and XRD characterization (Rivera et al., 1999). An example of such XRD characterization is shown in Figure 5.
Figure 5.
XRD pattern of Synthesized HA.
Oladele et al., 2019 worked on giant African snails with a weight ranging from 250 - 450 g, and an average age of about 3–10 yrs. Distilled water was used in washing the shells thoroughly to eliminate mud from the shells and they were dried at 300 °C for 5 h within an oven. After drying the shells were treated hydrothermally and utilized as fillers as indicated by methodology explained by Ağaoğullari et al. (2012). A ball mill of laboratory-scale running continuously for 48 h at 300 rpm was used to pulverize the treated shells into powder. The samples in powdery form were heated in a furnace to 1000 °C and 1200 °C at a rate of 5 °C/min. A combination of sodium hydroxide and deionized water in solution was used to remove the black coloration caused by trapped carbon dioxide emitted during the calcination process and purify the heated powdered samples now composed mostly of calcite (CaO). After calcination, the purified powders were allowed to cool to room temperature and then treated with concentrated phosphoric acid (H3PO4) which converted the calcite to HA. The reaction was constantly monitored and the pH kept at 10 by adding a hydroxide solution. The results exhibited that the optimum temperature for the synthesis of bioactive HA of optimum porosity and biocompatibility from snail shells was 1200 °C (see Figure 6).
Figure 6.
Snail shells (Biogenic Source of Hydroxyapatite).
Singh and Purohit (2010) worked on snail shells of the garden variety which they obtained, gathered together and worked on carefully to remove their protective covering. After which they were meticulously washed using distilled water and sun-dried for 48 h. This was done to completely remove debris, sand and other impurities before calcination. Calcination treatment was performed at 1000 °C on the dried and cleaned samples for two hours to eliminate the organic matter, burn out fats, oils and proteins leaving the ceramic phase. The samples were then cooled to room temperature and pulverized into powders which were further subjected to chemical treatment using nitric acid and ammoniacal solution. The nitric acid was added first to convert the sample into Ca(NO3)2 then 130 ml of 1.63(N) ammoniacal Ca(NO3)2 solution was added in drops into a mixture of ammoniacal (NH4)2HPO4 solution while stirring constantly using a magnetic stirrer while maintaining the pH of the solution at 10. Hydroxyapatite formation followed the reaction as shown in equation 4:
| 10Ca(NO3)2 + 6(NH4)2HPO4 + 2H2O → Ca10(PO4)6(OH)2 + 12NH4NO3 + 8HNO3 | (4) |
The suspension obtained was subjected to 10 min of boiling after which it was cooled overnight using an ice bath to form white precipitates that were gelatinous. The samples were filtered and the residue dried at 80 °C using an oven. After drying the HA was crushed to powdery form and subjected to heating in a furnace from ambient temperature to 1200 °C at a rate of 10 °C/min. This was done to observe the reaction of the material to heat and its weight loss when subjected to elevated temperatures. The ground snail shells, calcined and resultant HA samples were each examined using an X-ray Diffractometer with a high resolution by Cu-Kα radiation which was recorded in intervals of 0.010 with a 1s counting time per step. A spectrum of the produced HA powder was generated and recorded via FTIR analysis. The particle size was analyzed and the morphology of the sample surfaces was observed using SEM and surface area analyzer (BET) was used to examine and find out the surface area of the formed powdered HA. The crystallinity and phase purity of the powder was determined using XRD analysis. TG/DTA results exposed that shells of garden snails are composed largely of calcium carbonate (CaCO3). The SEM analysis showed that the shape of the uncalcined HA powders are round and almost regular; while that of the calcined HA powders are agglomerated. The BET surface area analysis agrees with the SEM microstructure and particle size analysis. Simulated Body Fluid (SBF) evaluation of bioactivity was performed by submerging the samples in SBF mediums and allowing them to soak for various periods after which agglomerated tiny bone-like apatite specks were observed on the surface of the powdered hydroxyapatite samples. The synthesized powdered hydroxyapatite exhibited high bioactivity which was comparable to biological apatite. When compared with the usual HA it possessed higher bioactivity. The results of these tests showed that a highly suitable bioactive, pure, stoichiometric and thermally stable hydroxyapatite powder with fine particle size, suitable for biomedical use was synthesized by chemical precipitation from shells of Snail (Helix aspersa). This means that HA from the shell of the garden snail (Helix aspersa) may be more effective and useful in promoting bone formation, bone replacement and the treatment of oral bone defects when compared with synthetic HA.
These research and studies meet the aim of synthesizing hydroxyapatite from waste materials (i.e. snail shell, eggshells, and waste seashells) for biomedical purposes, but the chemicals used in the extraction of HA from these shells contain certain elements (e.g phosphorus) that have proven harmful to the environment and a majority of the processes, therefore, require safety measures (e.g. protective clothing, nose masks, fume cupboards, e.t.c.) to be put in place and used to reduce the detrimental effects on human health and improve environmental safety.
3.4. Aquatic sources
This encompasses all marine life forms and waste that contain high quantities of calcium including, corals, fishes, and crustaceans. Fishes are an important part of the everyday diet of many humans. They contain varieties of essential nutrients including calcium, phosphates, and carbonates which are major building blocks of hydroxyapatite (Akram et al., 2014). To synthesize hydroxyapatite from aquatic sources, the necessary crustacean parts and the bones of the fishes are collected and washed thoroughly before they are boiled. Alkaline solutions were used to treat the boiled sample to eliminate all traces of proteins and impurities and to improve crystallinity as well as particle size distribution. The treatment was followed by calcination at elevated temperatures of up to 1200 °C to synthesize hydroxyapatite. However, above this temperature, it becomes unstable. The hydroxyapatite derived from these sources is usually spherical and is ideal for biomedical applications (Akram et al., 2014). Fish scales are also an excellent source of biocompatible and bioactive HA.
Mwambungu et al. (2014) discovered that crocodile eggshells are made up of mineral constituents which are similar to hen eggshells, as shown in Table 4, this makes it quite viable that HA can be produced from them using processes similar to those used to synthesize HA from eggshells. Some crocodile eggshells were subjected to hydrothermal processing by reaction with diammonium hydrogen phosphate ((NH4)2HPO4), tricalcium phosphate (Ca3(PO4)2) and phosphoric acid (H3PO4). It was observed that upon heat treatment at 250 °C, the crocodile eggshells combined with ((NH4)2HPO4) transformed completely into HA after 25 h and those combined with (Ca3(PO4)2) completely transformed into the HA phase after 8 h. The morphology also revealed that the crystals within the samples were agglomerated into plate-like clusters (Boonyang et al., 2010). The process was however not fully environmentally viable because of the harmful nature of some of the chemicals used for the synthesis.
Table 4.
A comparison of the mineral constituents of Hen and Crocodile Egg Shells (Mwambungu et al., 2014).
| Minerals | Hen Egg Shells (mg/100g) | Crocodile Egg Shells (mg/100g) |
|---|---|---|
| Copper | 0.93 ± 0.00 | 0.97 ± 0.02 |
| Cobalt | 0.93 ± 0.01 | 0.79 ± 0.06 |
| Manganese | 0.93 ± 0.01 | 0.79 ± 0.05 |
| Iron | 7.64 ± 0.02 | 7.23 ± 0.14 |
| Potassium | 82.2 ± 0.7 | 44.0 ± 0.6 |
| Sodium | 168.9 ± 0.3 | 151.2 ± 1.2 |
| Lead | 1.36 ± 0.1 | 2.5 ± 0.2 |
| Zinc | 0.93 ± 0.02 | 0.79 ± 0.05 |
| Chromium | 6.0 ± 0.3 | 6.3 ± 0.3 |
| Magnesium | 247.7 ± 0.3 | 110.0 ± 1.3 |
| Calcium | 2534.4 ± 10.6 | 2271.7 ± 8.8 |
| Phosphorus | 139.8 ± 0.2 | 5.0 ± 0.1 |
Ivankovic et al. (2010) prepared HA which had a diameter of 200–300 nm on average and the physical appearance of tiny nano-rods and nano-plates which were highly porous along with aragonite and brushite via hydrothermal reaction of DAHP with the cleaned inner lamellae spacing within the bone matrix of aragonite cuttlefish bones (Sepia Officinalis Linnaeus). It was observed via XRD that at a hydrothermal temperature approaching 160 °C aragonite was transformed to brushite and then to HA. However, above 180 °C phase-pure HA was formed. Carbonate groups were substituted and confirmed by FTIR analysis. The HA formed also possessed the tissue engineering advantage of retaining the original porous bone network throughout the hydrothermal treatment, as observed by SEM.
Coelho et al. (2006) calcined the bones of pentado (Pseudoplatystoma corruscans), jau' (Paulicea lutkeni), and cachara (Pseudoplatystoma fasciatum) at 900 °C for 4–12 h to obtain hydroxyapatite at a Ca/P ratio of 1.64, then they were crushed using a high powered ball mill for 2–4 h which reduced the particle size to about 300 nm with a spherical particle shape. The stainless steel milling balls and other processing factors were however attributed to being responsible for the presence of Fe2+, Cr3+, Ni2+, Mn2+, Cu2+, Zn2+, K+, and Na+ discovered via elemental analysis in trace amounts within the synthesized HA.
Boutinguiza et al. (2012) used the bones of the northern Atlantic sea Swordfish (Xiphias gladius) and Tuna fish (Thunnus thunnus) to synthesize HA. The bones were first boiled in water to eliminate the organic matter, then calcination at 600 °C and 950 °C was carried out in a furnace for 12 h. The bones were then characterized by TEM, FTIR, Raman, and XRD analysis which confirmed that in the two species, rod-like particles having submicron particle sizes were separated, B-type HA was formed after calcining at 600 °C while b-TCP was formed after calcining at 950 °C. The B-type carbonate substitution was however ascribed to be responsible for the nonconformity of lattice parameters from the usual HA standard.
Tilapia (Oreochromis sp.) scales were used by Huang et al. (2011) in the synthesis of Porous HA which possessed enhanced MG63 cell viability via enzymatic hydrolysis with 1 % protease N and 0.5 % flavourzyme solution. The HA formed had a Ca/P ratio of 1.78 and exhibited a smaller particle size of 719.8 nm which was suspected to be responsible for the improved cell viability when compared against the commercial HA.
Kongsri et al. (2013) synthesized phase-pure nanocrystalline cHA from Fish scales (Tilapia nilotica) obtained as waste products. The scales were deproteinized and heated in 50 % sodium hydroxide alkaline for 1 h at 100 °C to synthesize HA with a 1.67 Ca/P ratio as confirmed by ICP-OES. Some phosphate groups were replaced with carbonate group as per B-type substitution which was confirmed by FTIR analysis.
Piccirillo et al. (2013) calcined the raw bones of Codfish at high temperatures ranging from 900 °C -1200 °C and then characterized the powder obtained using XRD and FTIR. The characterization showed that the powder contained a biphasic mixture of HA and b-TCP with evident characteristic peaks. Elemental analysis revealed that the HA was calcium deficient with a Ca/P ratio of 1.49. It also revealed the presence of trace elements including Na+, F−, and Cl− in an amount that was a bit higher than mammalian bones.
The gases and fumes released during the process of enzymatic hydrolysis and calcination, should, however, be carefully collected and reused to improve the economic viability and environmental safety of these extraction processes. This will ensure that the production of phase pure HA from aquatic sources will be safer, cheaper and more effective.
3.5. Environmental effects
From the acute review above of both synthetic and non-synthetic sources of HA, a few major trends are evident.
In terms of the sustainability of the source, non-synthetic or natural sources of HA have proven to be more economically viable and advantageous when compared to synthetic sources because, in most cases, the natural sources are usually waste products of other human activities which are usually obtained at absolutely no cost or at a small price. Whereas their synthetic counterparts are usually derived from chemicals that are in most cases quite expensive, scarce, dangerous and harmful to both humans and the environment and as such are usually not easily obtainable.
In terms of sustainability of the processing route or ease of processing, the natural sources usually require mostly calcination at a specific temperature and hardly anything else to furnish HA with similar properties to human bone apatite, with the right characteristics at an appropriate, Ca/P ratio, while the synthetic sources require carefully controlled chemical processes and stoichiometric reactions to synthesize HA of the right, characteristics, calcium content and Ca/P ratio.
In terms of the effect on the environment, a major concern about furnishing HA from natural sources is the carbon and greenhouse gas emission, which occurs as a result of calcination. When calcination is performed within a furnace, the effect on the environment can be easily minimized by controlling the atmosphere around the furnace and taking steps to recover the emitted greenhouse gases and reuse them. Meanwhile for the synthetic route there exist the danger of chemical spillage, emission of dangerous fumes e.t.c. as the reaction is performed, and this can be harmful to both the humans in the vicinity and the environment at large. Therefore, steps must be taken to improve the safety of the process and protect the environment by using protective clothing, fume cupboards and the protective gear required by the processing route.
It is also well known that certain chemicals used in the synthetic route are dangerous and corrosive in their raw undiluted state including phosphorus (P) which can cause phossy jaw, serious cough and damage to blood vessels when inhaled. Nitrogen and its oxides which may cause sudden death upon exposure to massive concentrations, and cause nausea, tiredness, coughing, and shortness of breath upon exposure to lighter concentrations but are used in most wet chemical precipitation. Methods along with some compounds such as ammonia which may also lead to death upon severe exposure to high concentrations and may cause blindness, lung damage upon inhalation as well as coughing, immediate burning and irritation of the nose, throat, respiratory tract and lungs upon slight exposure. Cetyltrimethylammonium used in the process of hydrolysis which causes respiratory tract irritations when inhaled, eye burns, and can be harmful when absorbed through the skin. Therefore, it is evident that any accidents during the process can be critical or even fatal for the parties involved in the worst case and very uncomfortable in the best case.
These risks and factors suggest that the furnishing of HA from natural sources without the use of chemicals (sources where HA is already present e.g. bovine, pig, and fish bones and teeth) is safer, simpler and more beneficial to the environment when compared to producing it from synthetic sources (including those with natural starting materials). Although the calcination of bones may lead to the emission of CO2 and other greenhouse gases, the effects can be mitigated and controlled via calcining them in a controlled environment and recovering the gaseous emissions using a chemical hood and/or other better-suited methods.
4. Conclusion
The importance of ceramic material known as hydroxyapatite (a biomaterial) is evident from its recent uses as in-vivo and in-vitro coatings and reinforcements in various materials utilized for the treatment of a plethora of bone and tooth diseases as well as other functions including drug delivery. This is mainly based on its bioactivity and resemblance to the mineral parts of teeth and bones. Consequently, a lot of research into its formation and synthesis via natural and synthetic routes are available. This review has carefully considered and outlined the major synthetic and non-synthetic routes of HA production while considering the effect of each on humans and the environment and attempted to select the most environmentally benign methods of deriving hydroxyapatite suitable for biomedical applications. It was concluded that the calcination of bovine, swine and human bones and teeth stand out as the most environmentally sustainable processes of HA production available from both natural and synthetic sources, as far as the CO2 and other greenhouse gasses emitted during the process can be reduced and/or recovered to limit their effects on the environment. It is hoped that the choice of a safer and greener route to hydroxyapatite synthesis is selected by the researcher from the methods outlined herein concomitant on fit environmental conditions. Further research efforts are still being expected to investigate better and more viable options for hydroxyapatite synthesis to make the material readily available globally.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to appreciate Landmark University Centre for Research, Innovation and Development (LUCRID), Omu-Aran, Nigeria for their support.
References
- Akram M., Ahmed R., Shakir I. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014;49(4):1461‒1475. [Google Scholar]
- Angervall L., Berger S., Röckert H. A microradiographic and X-ray crystallographic study of calcium in the pineal body and in intracranial tumours. Acta Pathol. Microbiol. Scand. 2009;44(2):113–119. doi: 10.1111/j.1699-0463.1958.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Anthony J., Bideaux R., Bladh K., Nichols M. Mineralogical Society of America; Chantilly, VA, US: 2000. “Hydroxylapatite,” Handbook of Mineralogy (PDF). IV (Arsenates, Phosphates, Vanadates) [Google Scholar]
- Antonietti M., Breulmann M., Göltner C.G., Cölfen H., Wong K.K.W., Walsh D., Mann S. Inorganic/organic mesostructures with complex architectures: precipitation of calcium phosphate in the presence of double-hydrophilic block copolymers. Chem. Eur J. 1998;4:2493–2500. [Google Scholar]
- Ağaoğullari D., Kel D., Gökçe H., Duman I., Öveçoglu M.L., Akarsubaşi A.T., Bilgic D., Oktard F.N. Bioceramic production from sea urchins. Act. Phy. Pol. A. 2012;121:23. [Google Scholar]
- Beevers C.A. “The crystal structure of dicalcium phosphate dihydrate, CaHPO4.2H20,” department of chemistry, university of Edinburgh, scotland. Acta Crystallogr. 1958;11(273) [Google Scholar]
- Boonyang U., Chaopanich P., Wongchaisuwat A., Senthongkaew P., Siripaisarnpipat S. Effect of phosphate precursor on the production of hydroxyapatite from crocodile eggshells. J. Biomim. Biomater. Tissue Eng. 2010;5:31–37. [Google Scholar]
- Boutinguiza M., Pou J., Comesana R., Lusquinos F., Ade Carlos, Leon B. Biological hydroxyapatite obtained from fish bones. Mat. Sci. Eng. C-Mater. 2012;32:478–486. [Google Scholar]
- Bouyer E., Gitzhofer F., Boulos M.I. Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 2000;11(8):523–531. doi: 10.1023/a:1008918110156. [DOI] [PubMed] [Google Scholar]
- Brown W.E., Epstein E.F. Crystallography of tetracalcium phosphate. J. Res. Natl. Bur. Stand. A. Phys. Chem. 1965;69(6) doi: 10.6028/jres.069A.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcia C.R., Scibek J.S. Causation and management of calcific tendonitis and periarthritis. Curr. Opin. Rheumatol. 2013;25(2):204–209. doi: 10.1097/BOR.0b013e32835d4e85. [DOI] [PubMed] [Google Scholar]
- Carrodeguas R.G. Aza SD a-tricalcium phosphate: synthesis, properties, and biomedical applications. Acta Biomater. 2011;7:3536–3546. doi: 10.1016/j.actbio.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Chen B.H., Chen K.I., Ho M.L., Chen H.N., Chen W.C., Wang C.K. Synthesis of calcium phosphates and porous hydroxyapatite beads prepared by emulsion method. Mater. Chem. Phys. 2009;113:365–371. [Google Scholar]
- Coelho T.M., Nogueira E.S., Steimacher A., Medina A.N., Weinand W.R., Lima W.M., Baesso M.L., Bento A.C. Characterization of natural nanostructured hydroxyapatite obtained from the bones of Brazilian river fish. J. Appl. Phys. 2006;100:94312–94316. [Google Scholar]
- Daculsi G., Passuti N., Martin S., Deudon C., LeGeros R.Z., Raher S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. J. Biomed. Mater. Res. 1990;17:769–784. doi: 10.1002/jbm.820240309. [DOI] [PubMed] [Google Scholar]
- De-Groot K. Bioceramics consisting of calcium phosphate salt. Biomaterials. 1980;1:47–50. doi: 10.1016/0142-9612(80)90059-9. [DOI] [PubMed] [Google Scholar]
- Driessens F.C.M. In: “Formation and Stability of Calcium Phosphates in Relation to the Phase Composition of the Mineral in Calcified Tissues,” Bioceramics of Calcium Phosphate. de Groot K., editor. CRC Press; Boca Raton, FL: 1983. pp. 1–31. [Google Scholar]
- Elizondo-Villarreal N., Martínez-De-La-Cruz A., Guerra R.O., Gómez-Ortega J.L., Torres-Martínez L.M., Castaño V.M. Biomaterials from agricultural waste: eggshell-based hydroxyapatite. Water Air Soil Pollut. 2012;223(7):3643–3646. [Google Scholar]
- Ellinger R., Nery E.B., Lynch K.L. Histological assessment of periodontal osseous defects following implantation of HA and biphasic calcium phosphate ceramics: a case report. Int. J. Periodontics Restor. Dent. 1986;3:23–33. [PubMed] [Google Scholar]
- Elliott J.C. Elsevier Amsterdam; London/New York/Tokyo: 1994. Studies in Inorganic Chemistry 18: Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; pp. 9–29. [Google Scholar]
- Ferraz M.P., Monteiro F.J., Manuel C.M. Hydroxyapatite nanoparticles: a review of preparation methodologies. J. Appl. Biomat. Biomechan. JABB. 2004;2(2):74–80. PMID 20803440. [PubMed] [Google Scholar]
- Ganachari S.V., Bevinakatti A.A., Yaradoddi J.S. Rapid synthesis, characterization, and studies of hydroxyapatite nanoparticles. Adv. Mater. Sci. Res. 2016;1(1):9–13. [Google Scholar]
- Guo X., Yan H., Zhao S., Zhang L., Li Y., Liang X. Effect of calcining temperature on the particle size of hydroxyapatite synthesized by solid-state reaction at room temperature. Adv. Powder Technol. 2013;24:1034–1038. [Google Scholar]
- Habibah T.U., Salisbury H.G. 2018. Biomaterials, Hydroxyapatite. PMID 30020686. [Google Scholar]
- Hall B.K. second ed. Academic Press; 2005. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. [Google Scholar]
- Hiller J.C., Thompsom T.J.U., Evison M.P. “Bone mineral change during experimental heating: an X‒ray scattering investigation. Biomaterials. 2003;24(28):5091–5097. doi: 10.1016/s0142-9612(03)00427-7. [DOI] [PubMed] [Google Scholar]
- Huang Y.C., Hsiao P.C., Chai H.J. Hydroxyapatite extracted from fish scale: effects on MG63 osteoblast-like cells. Ceram. Int. 2011;37:1825–1831. [Google Scholar]
- Idris Abdulrahman, Hamzat Ibiyeye, Tijani, Bashir Abubakar, Mohammed, Haruna Saidu, Hindatu Yusuf, Mohammed Ndejiko, Jibrin, Sulaiman Mohammed. From Garbage to Biomaterials: An Overview on Egg Shell Based Hydroxyapatite. J. Mat. 2014;2014 [Google Scholar]
- Ivankovic H., Tkalcec E., Orlic S., Ferrer G.G., Schaupererl Z. Hydroxyapatite formation from cuttlefish bones: kinetics. J. Mater. Sci. Mater. Med. 2010;21:2711–2722. doi: 10.1007/s10856-010-4115-4. [DOI] [PubMed] [Google Scholar]
- Jarcho M. Calcium phosphate ceramics as hard tissue prosthesis, Clin. Orthop. Relat. Res. 1981;157:259–278. [PubMed] [Google Scholar]
- Ji H.L., Meisha L.S. Copolymer-mediated synthesis of hydroxyapatite nanoparticles in an organic solvent. Am. Chem. Soc. Langmuir. 2013;29:10940–10944. doi: 10.1021/la402434v. [DOI] [PubMed] [Google Scholar]
- Jojor L.M., Bambang S., Decky J.I. Characterization of hydroxyapatite derived from bovine. Asian J. Appl. Sci. 2015;3(4):758. www.ajouronline.com ISSN: 2321 – 0893. Asian Online Journals. [Google Scholar]
- Junqueira L., José C., Foltin J., Lebowitz H., Boyle P.J., editors. Basic Histology, Text & Atlas. tenth ed. Vol. 144. ” McGraw-Hill Companies; 2003. [Google Scholar]
- Kim S., Sun P.M., Jeon O., Yong C.C., Kim B. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Cho I.S., Kim J.Y., Jang H.L., Han G.S., Ryu H.S., Shin H., Jung H.S., Kim H., Hong K.S. “Simple large-scale synthesis of hydroxyapatite nanoparticles: in situ observation of crystallization process,” pubs.acs.org/Langmuir American chemical society. Langmuir. 2010;26(1):384–388. doi: 10.1021/la902157z. [DOI] [PubMed] [Google Scholar]
- Kivrak N., Tas A.C. “Synthesis of calcium hydroxyapatite–tricalcium phosphate (HA–TCP) composite bioceramic powders and their sintering behavior. J. Am. Ceram. Soc. 1998;81(9):2245–2252. [Google Scholar]
- Kongsri S., Janparadit K., Buapa K., Techawongstien S., Chanthai S. Nanocrystalline hydroxyapatite from fish scale waste: preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013;215–216:522–532. [Google Scholar]
- Krishna D.S.R., Siddharthan A., Seshadri S.K., Kumar T.S.S. A novel route for the synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007;18(9):1735–1743. doi: 10.1007/s10856-007-3069-7. [DOI] [PubMed] [Google Scholar]
- Kusmanto F., Walker G., Gan Q., Walsh P., Bushanan F., Dickson G., McCaigue M.C., Dring M. Development of composite tissue scaffolds containing naturally sourced mircoporous hydroxyapatite. Chem. Eng. J. 2008;139:398–407. [Google Scholar]
- LeGeros R.Z. Calcium phosphate materials in restorative dentistry: a review. Adv. Dent. Res. 1988;2:164–180. doi: 10.1177/08959374880020011101. [DOI] [PubMed] [Google Scholar]
- LeGeros R.Z., Daculsi G., Nery E.B., Lynch K.L., Kerebel B. 2B. 1988. In vivo studies of biphasic calcium phosphates of varying b-TCP:HA ratios: ultrastructural characterization; pp. 1–35. (Transactions of the Third World Biomaterials Congress). [Google Scholar]
- Li-Chan E.C., Kim H.-O. Structure and chemical composition of eggs. Egg Biosci. Biotechn. 2008 [Google Scholar]
- Marentette J., Norwig J., Stöckelmann E., Meyer W.H., Wegner G. Crystallization of CaCO3 in the presence of PEO-block- PMAA copolymers. Adv. Mater. 1997;9:647–651. [Google Scholar]
- Masaki K., Keiichi M., Waite D.E., Hiroshi N., Toru O. In vitro stability of biphasic calcium phosphate ceramics. Biomaterials. 1993;14:299–304. doi: 10.1016/0142-9612(93)90122-i. [DOI] [PubMed] [Google Scholar]
- Mathai M., Shozo T. Structures of biological minerals in dental research. J. Res. Natl. Inst. Stand. Technol. 2001;106:1035–1044. doi: 10.6028/jres.106.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh D.P., Lyczko N., Sebei H., Nzihou A., Sharrock P. Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: a comparative study. Mater. Sci. Eng. 2012;177:1080–1089. [Google Scholar]
- Mobasherpour I., Soulati H.M., Kazemzadeh A., Zakeri M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007;430:330–333. [Google Scholar]
- Monmaturapoj N. Nano-size hydroxyapatite powders preparation by wet-chemical precipitation route. J. Met. Mater. Miner. 2008;18:15–20. [Google Scholar]
- Moore D.C., Chapman M.W., Manske D. The evaluation of biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defects. J. Orthop. Res. 1987;5:356–365. doi: 10.1002/jor.1100050307. [DOI] [PubMed] [Google Scholar]
- Mulye N.V., Turco S.J. Use of dicalcium phosphate dehydrate for sustained release of highly water-soluble drugs. Drug Dev. Ind. Pharm. 1994;20:2621–2632. [Google Scholar]
- Mwambungu A., Siulapwa N., Mubbunu L. Comparison of mineral composition of commercial hen egg shells to fresh water crocodile egg shells. Int. J. Res. Agr. Food Sci. 2014;2(7) http://www.ijsk.org/ijrafs.html ISSN:2311-2476. [Google Scholar]
- Nayak A.K. Hydroxyapatite synthesis methodologies: an overview. Int. J. Chem. T. Res. 2010;2:903–907. [Google Scholar]
- Neira I.S., Guitian F., Taniguchi T., Watanabe T., Yoshimura M. Hydrothermal synthesis of hydroxyapatite whiskers with sharp faceted hexagonal morphology. J. Mater. Sci. 2008;43:2171–2178. [Google Scholar]
- Nery E.B., Lynch K.L., Hirthe W.M., Mueller K.H. Bioceramic implants in surgically produced infrabony defects. J. Periodontol. 1975;46:328–339. doi: 10.1902/jop.1975.46.6.328. [DOI] [PubMed] [Google Scholar]
- Nosrati H., Le D.Q.S., Emameh R.Z., Bunger C.E. Characterization of the precipitated dicalcium phosphate dehydrate on the graphene oxide surface as a bone cement reinforcement. J. Tiss. Mat. 2019;2(1):33–46. [Google Scholar]
- Núñez D., Elgueta E., Varaprasad K., Oyarzún P. Hydroxyapatite nanocrystals synthesized from calcium-rich bio-wastes. Mater. Lett. 2018;230:64–68. [Google Scholar]
- Öner M., Norwig J., Meyer W.H., Wegner G. Control of ZnO crystallization by a PEO-b-PMAA diblock copolymer. Chem. Mater. 1998;10:460–463. [Google Scholar]
- Ofudje E.A., Rajendran A., Adeogun A.I., Idowu M.A., Kareem S.O., Pattanayak D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2018;29(1):1–8. [Google Scholar]
- Oladele I.O., Akinola O.S., Agbabiaka O.G., Omotoyinbo J.A. Mathematical model for the prediction of impact energy of organic material based hydroxyapatite (HAp) reinforced Epoxy composites fibers and polymers. 2018;19(2):452–459. ISSN 1229-9197. ISSN 1875-0052. [Google Scholar]
- Oladele I.O., Agbabiaka O.G., Olasunkanmi O.G., Balogun A.O., Popoola M.O. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018;5(2):88–95. [Google Scholar]
- Oladele Isiaka, Oluwole, Agbabiaka Okikiola Ganiu, Adediran Adeolu Adesoji, Akinwekomi Akeem Damilola, Balogun Augustine Olamilekan. Structural performance of poultry eggshell derived hydroxyapatite based high density polyethylene bio-composites. Heliyon. 2019;5(2405–8440) doi: 10.1016/j.heliyon.2019.e02552. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouerfelli N., Zid M.F. New polymorph of CaHPO4 (monetite): synthesis and crystal structure. J. Struct. Chem. 2016;57(3):628–631. [Google Scholar]
- Pandharipande S.L., Sondawale S.S. Review on the characterization methods of hydroxyapatite and its bio-composites. Int. J. Sci. Eng. Technol. Res. (IJSETR) 2016;5(7):2416–2425. ISSN: 2278 – 7798. [Google Scholar]
- Pandharipande S.L., Sondawale S.S. Review on the characterization methods of hydroxyapatite and its bio-composites. Int. J. Sci. Eng. Technol. Res. (IJSETR) 2016;5(17):3410–3416. [Google Scholar]
- Piccirillo C., Silva M.F., Pullar R.C., Cruz I.B., Jorge R., Pintado M.M.E., Castro P.M.L. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from codfish bones. Mat. Sci. Eng. C-Mater. 2013;33:103–110. doi: 10.1016/j.msec.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Raynaud S., Champion E., Bernache-Assollant D., Thomas P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials. 2002;23(4):1065–1072. doi: 10.1016/s0142-9612(01)00218-6. PMID 11791909. [DOI] [PubMed] [Google Scholar]
- Rejda B.V., Peelen J.G.J., De Groot K. Tri-calcium phosphate as bone substitute. J. Bioeng. 1977;1:93–97. [PubMed] [Google Scholar]
- Renooij W., Hoogendoorn H.A., Visser W.J., Lentferink Janssen W.M., Akkermans L.M.A., Wittebol P. Bioresorption of ceramic strontium-85-labeled calcium phosphate implants in dog femora, Clin. Orthop. Relat. Res. 1985;197:272–285. [PubMed] [Google Scholar]
- Rey C., Combes C., Drouet C., Grossin D. Vol. 1. Elsevier; 2011. pp. 187–281. (“Bioactive Ceramics: Physical Chemistry,” in Ducheyne,” Paul. Comprehensive Biomaterials). [Google Scholar]
- Rivera E.M., Araiza M., Brostow W., Castaño V.M., D´ıaz-Estrada J.R., Herna´ndez R., Rodr´ıguez J.R. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999;41(3):128–134. www.elsevier.com/locate/matlet [Google Scholar]
- Sanosh K.P., Chu M.C., Balakrishnan A., Kim T.N., Cho S.J. “Preparation and characterization of nano-hydroxyapatite powder using sol–gel technique. Bull. Mater. Sci. 2009;32(5):465–470. [Google Scholar]
- Shaltout A.A., Allam M.A., Moharram M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta A. 2011;83:56–60. doi: 10.1016/j.saa.2011.07.036. [DOI] [PubMed] [Google Scholar]
- Shih W.J., Wang M.C., Hon M.H. Morphology and crystallinity of the nanosized hydroxyapatite synthesized by hydrolysis using cetyltrimethylammonium bromide (CTAB) as a surfactant. J. Cryst. Growth. 2005;275:2339–2344. [Google Scholar]
- Singh A., Purohit K.M. Chemical synthesis, characterization and bioactivity evaluation of hydroxyapatite prepared from garden snail (Helix aspersa) J. Bioprocess. Biotech. 2010;1:104. [Google Scholar]
- Sono-synthesis of nano-hydroxyapatite. 2019. www.hielscher.com
- Takagi S., Chow L.C., Yamada E.M., Brown W.E. Enhanced enamel F uptake by monocalcium phosphate monohydrate gels. J. Dent. Res. 1987;66:1523–1526. doi: 10.1177/00220345870660100201. [DOI] [PubMed] [Google Scholar]
- Takeishi A., Hayashi H., Kamatsubara H., Yokoyama A., Kohri M., Kawasaki T., Miki K., Kohgo T. Implant of calcium phosphate ceramics altering Ca/P ratio in bone. J. Dent. Res. 1989;68:680–684. [Google Scholar]
- Tampieri A., Spiro S., Ruffini A., Celotti G., Lesci I.G., Roveri G. From wood to bone: multi-step process to convert wood hierarchical structures into biomimetic hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Chem. 2009;19:4973–4980. [Google Scholar]
- Tanner K.E. Small but extremely tough. Science. 2012;336(6086):1237–1238. doi: 10.1126/science.1222642. Bibcode: 2012Sci...336.1237T. [DOI] [PubMed] [Google Scholar]
- Tjandra W., Yao J., Ravi P., Tam K.C., Alamsjah A. Nanotemplating of calcium phosphate using a double-hydrophilic block copolymer. Chem. Mater. 2005;17:4865–4872. [Google Scholar]
- Valletregi M. Synthesis and characterization of calcium-deficient apatite. Solid State Ionics. 1997;101–103:1279–1285. [Google Scholar]
- Walsh P.J., Buchanan F.J., Dring M. “Low‒pressure synthesis and characterization of hydroxyapatite derived from mineralize red algae. Chem. Eng. J. 2008;137(1):173–179. [Google Scholar]
- Weaver J.C., Milliron G.W., Miserez A., Evans-Lutterodt K., Herrera S., Gallana I., Mershon W.J., Swanson B., Zavattieri P., Dimasi E., Kisailus D. The stomatopod dactyl club: a formidable damage-tolerant biological hammer. Science. 2012;336(6086):1275–1280. doi: 10.1126/science.1218764. Bibcode: 2012Sci...336.1275W. [DOI] [PubMed] [Google Scholar]
- Webmineral . 2019. Hydroxylapatite.http://www.webmineral.com/data/Hydroxylapatite.shtml [Google Scholar]
- Wu T., Shu T., Kang L. “Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow‒ and human adipose tissue-derived mesenchymal stem cells. Int. J. Mol. Med. 2017;39(4):984‒992. doi: 10.3892/ijmm.2017.2906. [DOI] [PubMed] [Google Scholar]
- Yeong K.C.B., Wang J., Ng S.C. Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials. 2001;22:2705–2712. doi: 10.1016/s0142-9612(00)00257-x. [DOI] [PubMed] [Google Scholar]
- Zhang C., Yang J., Quan Z. Hydroxyapatite nano- and microcrystals with multiform morphologies: controllable synthesis and luminescence properties. Cryst. Growth Des. 2009;9(6):2725–2733. [Google Scholar]