Abstract
Background
Vorolanib (X-82, CM082) is a multi-target tyrosine kinase inhibitor. This study aimed to evaluate the tolerability, safety, pharmacokinetics and antitumor activities of vorolanib plus everolimus (an inhibitor of mammalian target of rapamycin).
Methods
Patients had histologically or cytologically confirmed advanced RCC and failed with standard therapy were eligible for this study. Dose-escalated combinations of vorolanib (100, 150 or 200 mg once daily) with everolimus (5 mg once daily) were administered on 28-day cycles until disease progression or unacceptable toxicity using a conventional 3 + 3 dose-escalation design.
Findings
22 patients (100 mg n = 4, 150 mg n = 3, 200 mg n = 15) were enrolled. Only one patient experienced dose-limiting toxicity (DLT, grade 4 thrombocytopenia) in the vorolanib 200 mg combination cohort, and the maximum tolerated dose (MTD) was not reached. The most common treatment-related adverse events were proteinuria (100%), leukopenia (77%), hypercholesterolaemia (77%), increased low-density lipoprotein (68%), hypertriglyceridaemia (64%), hyperglycaemia (59%), and fatigue (55%). Most treatment-related adverse events were grade 1 to 2, with grade 3 or higher toxicities mostly seen in the 200 mg cohort. Single dosing of vorolanib demonstrated dose-proportional increases in the Cmax and AUC, and observed short t1/2z ranging from 4.74±1.44 to 12.89±7.49 h. The pharmacokinetic parameters for everolimus were similar among all cohorts. Of 19 evaluable patients, the ORR and DCR was 32% (n = 6, 95% CI, 13–57%) and 100% (95% CI, 82–100%), respectively.
Interpretation
Combination therapy of vorolanib 200 mg plus everolimus 5 mg once daily is potentially effective with potential activity. Further evaluation of the combination in advanced RCC patients is ongoing (NCT03095040).
Funding
Betta Pharmaceutical Co., Ltd., Hangzhou, China.
Keywords: Renal cell carcinoma, Vorolanib, CM082, Everolimus, Combination therapy
Research in context.
Evidence before this study
VEGF and mTOR pathways play key role in the development of renal cell carcinoma (RCC), the combination of agents targeting both VEGF- and mTOR-mediated pathways have been investigated with distinct results. We searched in PubMed with the terms of “combination therapy” and “renal cell carcinoma” up to December, 2019. We restricted our search to clinical trials, and 63 researches explored the effect of anti-angiogenic drugs (most were bevacizumab, sorafenib or sunitinib) on RCC. Moreover, single-agent treatments based on tyrosine kinase inhibitors showed modest efficacy in overall survival and few complete response events. Seven studies specifically discussed the use of everolimus in the combination treatments. Lenvatinib plus everolimus has proven to prolong progression-free survival compared with everolimus alone. However, more grade 3 and 4 AEs occurred in patients assigned combination therapy than those received monotherapy. Vorolanib (CM082) is a potent and selective inhibitor of VEGFR and PDGFR, and the safety and efficacy of the vorolanib in combination therapy remains to be discussed.
Added value of this study
Several immune checkpoint inhibitors have entered clinic as the first-line treatment for renal cell carcinoma (RCC). So far, second-line therapy for RCC included single-agent TKI and immune checkpoint inhibitor with modest efficacy in terms of overall survival and tumor response. Besides, intolerable toxicities were seen in several studies investigating the combination of VEGFR TKIs and mTOR inhibitors. In this study, compared with everolimus alone, efficacy was improved by the combination strategy of vorolanib and everolimus, and with vorolanib alone. More importantly, the safety profile of combination therapy was within the range of the known safety profiles of each agent. The toxicities observed in the present study were manageable with dose adjustments and supportive medication.
Implications of all the available evidence
The results from this phase 1 study show the efficacy and safety profile of the combination of vorolanib and everolimus for patients with metastatic renal cell carcinoma. A phase II/III study (NCT03095040) is ongoing to assess the efficacy and safety of vorolanib, everolimus, or their combination as second-line treatment in patients with metastatic renal cell carcinoma.
Alt-text: Unlabelled box
1. Introduction
Renal cell carcinoma (RCC) is the most common form of kidney cancer, in which clear cell RCC (ccRCC) accounts for 70–75% of all RCC cases, and associated with alterations in the von Hippel–Lindau (VHL) gene [[1], [2], [3]]. Understanding in the essential role of the VHL gene and its regulation of cellular levels of hypoxia-inducible factor 1 alpha (HIF-1a) and HIF-2a in ccRCC has led to the development of agents that could inhibit the downstream mediators of HIF [[4], [5], [6]], including multi-targeted tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) inhibitors. The VHL inactivation and VEGF overexpression have been demonstrated to correlate with tumor aggressiveness and poor survival in RCC [7]. VEGF regulates its activity through the interaction with transmembrane tyrosine-kinase receptors that include VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2), and Neuropilin-1 (NRP1) [8], which are expressed mainly in vascular endothelial cells. VEGF plays a vital role in both normal and tumor-associated angiogenesis via regulating the proliferation and migration of endothelial cells [9]. Moreover, mTOR contributes in the hypoxic tumor response by stabilizing HIF-1α [10]. Recently, mTOR inhibitors have been proved to reduce VEGF expression and act as antiangiogenic agents [11]. mTOR inhibition induces apoptosis in tumor-associated endothelial cells, leading to significant reductions in microvessel density and tumor growth. In addition, mTOR inhibitors can also suppress the production of pro-angiogenic factors in tumors and tumor-associated stromal cells [12]. These agents showed longer progression-free survival (PFS) or trends of improved overall survival (OS) compared with cytokine-based immunotherapy in advanced and metastatic RCC [[13], [14], [15], [16], [17], [18]].
Multi-targeted TKIs, including sunitinib, pazopanib, cabozantinib, have been standard for first-line treatment of RCC. However, the landscape for treatment of metastatic RCC keeps evolving as new data with immune checkpoint inhibitors and novel TKI therapies are presented. Emerging data revealed improved PFS/OS with combination regimens which include phase III studies of atezolizumab plus bevacizumab, ipilimumab plus nivolumab, and avelumab/pembrolizumab plus axitinib, when compared with sunitinib or sorafenib as initial therapy [19,20]. Paradigm shift in the first-line setting brought new question about standard care for the second line since there are no data supporting sequential use of multiple immune checkpoint inhibitors. Currently used second-line options for metastatic RCC include axitinib, cabozantinib, nivolumab, and everolimus alone, which was associated with a median PFS of 3.8–7.4 months [[21], [22], [23], [24]]. Attempts have also been made on the combination of VEGFR TKIs (e.g., sunitinib or sorafenib) and mTOR inhibitors (e.g., everolimurs or temsirolimus) based on the concepts that drug resistance develops through feedback mechanisms which could be inhibited by different agents acting on two or more different levels of the same pathway, such as suppressing HIF-a by blocking its translation and inhibiting HIF-a – induced gene products and their functions [25]. Intolerable toxicities were seen in several phase I studies evaluating combination of temsirolimus/everolimus and sunitinib or temsirolimus and sorafenib, respectively, while everolimus plus sorafenib showed favorable toxicity profile and preliminary antitumor activity [[26], [27], [28], [29]]. More recently, the combination of lenvatinib plus everolimus is approved for patients with advanced RCC following one previous antiangiogenic therapy based on phase II data showing an impressive median PFS of 14.6 months versus 5.5 with everolimus and 7.4 months with lenvatinib alone [30]. However, it should be noted that fewer grade 3 and 4 events occurred in patients allocated everolimus alone (50%) compared with those assigned lenvatinib plus everolimus (71%) or lenvatinib alone (79%) [30]. Moreover, substantial toxicity has been a recurrent observation with combination studies including bevacizumab plus temsirolimus, both in the phase 2 TORAVA [31] and phase 3 INTORACT [32] studies, or bevacizumab plus sunitinib or any mTOR inibitors with sunitinib. Therefore, new agents for combination therapies with favorable safety and improved efficacy will provide more options for clinicians.
Vorolanib (X-82, CM082) is a highly potent VEGFR/PDGFR TKI with a small volume of distribution and limited tissue accumulation, which designed to minimize side effects while maintaining target effect. The earlier-generation small-molecule VEGFR inhibitors, such as sunitinib [33], sorafenib [34], and pazopanib [35] suffer from poor kinase selectivity. In fact, many of them inhibit more than 10 kinases with similar potency. As a consequence, the drug exposures at the maximum tolerated dose (MTD) are limited due to inhibition of multiple targets, resulting in less optimal and/or short duration of inhibition of any targets, in particular VEGFR. Axitinib is a more selective tyrosine kinase inhibitor because it only shows activity against VEGFR-1, VEGFR-2, and VEGFR-3 with higher potency in vitro than that of pazopanib and the first-generation VEGFR inhibitors such as sunitinib and sorafenib [[36], [37], [38]]. Vorolanib is a novel indolinone-based kinase inhibitor that targets several receptors including the VEGFR, PDGFR, and Colony Stimulating Factor 1 Receptor (CSF1R) [39,40], with low substantial inhibitory effect on RET or AMPK, which might contribute to less adverse effects and a wide therapeutic window. Vorolanib was found to inhibit VEGFR2 (KDR), PDGFR, Flt3 and c-Kit with an IC50 of 0.13–1.12 nmol/L (unpublished data). In a phase 1, first-in-human study, vorolanib was well tolerated across a dose range from 20 to 400 mg daily, without achieving the most tolerated dose (MTD) [41]. The most treatment-related adverse events (TRAEs) were fatigue, nausea, diarrhea, anorexia, and vomiting [41]. Similarly, favorable safety profile of vorolanib was also seen in another phase 1 study in China [42]. No dose-limited toxicity (DLT) was observed, and the most common TRAEs were leukopenia, fatigue, diarrhea, neutropenia, and hypertension [42]. Sequential combination of vorolanib with docetaxel had been explored, and favorable safety and enhanced pharmacodynamic effects of the combination therapy were observed [43]. In this study, we hypothesized that vorolanib in combination with everolimus might effectively enhance antitumor activity by inhibiting VEGFR and mTOR signaling pathways with tolerable toxicities. Therefore, we performed this study to evaluate the safety and determine the MTD of vorolanib administered with everolimus.
2. Materials and methods
2.1. Patients
Patients aged 18 to 70 years with a histologically or cytologically confirmed diagnosis of advanced ccRCC, disease progression after targeted therapy, an ECOG performance status of 0–1, and an expected survival time of more than 3 months were eligible for the study. Additionally, eligible patients were required to have adequate hematologic, hepatic, and renal function (hemoglobin ≥9.0 g/dL, ALT/AST ≤1.5 × ULN or ≤5 × ULN when having liver metastases, total bilirubin ≤1.5 × ULN, and serum creatinine ≤1.5 × ULN); and measurable disease according to Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1, and should not have received any chemotherapy, radiotherapy, biotherapy or endocrine therapy within 4 weeks before study treatment. Patients were excluded if they have poorly controlled blood pressure (150/90 mm Hg or higher with or without antihypertensive drugs); severe vascular disease including cardiovascular, cerebrovascular, or peripheral vascular disease; cardiac dysrhythmias or QTc prolongation (>450 ms); or brain metastases.
2.2. Study design
We designed a single-center, open-label, phase 1 study with a standard 3 + 3 dose escalation plan to assess the tolerability, safety, PK and antitumor activity and establish the RP2D of vorolanib plus fixed dose of everolimus in Chinese patients with ccRCC. Three to six patients of each cohort were enrolled sequentially to receive concurrent vorolanib and everolimus orally in 4-week continuous cycles. The dose-escalation schedule for vorolanib was set as 100, 150, and 200 mg. The decision to proceed to the next dose cohort was based on events in the first cycle. Everolimus was administered on a fixed dose of 5 mg daily, while vorolanib was administered 4 h later with a starting dose of 100 mg daily. The primary endpoint was safety and tolerability. Dose limited toxicity (DLT) was assessed during the first 28-day cycle. The maximum tolerated dose (MTD) was defined as the highest dose level at which less than 2 of 6 patients experienced a DLT. The recommended dose of vorolanib would be 200 mg daily if no more than 1 DLT occurred, otherwise the MTD would be the recommended dose. After determination of the MTD and recommended dose, additional patients (10–15) were enrolled in the expansion part of the study, vorolanib at recommended dose and everolimus 5 mg daily were administered until disease progression or unacceptable toxicity.
This study was performed in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. The protocol was approved by the ethic committee of Peking University School of Oncology and all patients provided written informed consent. This study was registered with ClinicalTrials.gov (No. NCT02577458).
2.3. Safety assessment
Safety assessment consisted of monitoring and recording all AEs, including periodic laboratory tests, vital signs, 12-lead electrocardiograms, and physical examination. AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs version 4.03. DLT was defined as treatment-related failure to administer ≥75% of the planned dosage of vorolanib/everolimus combination or any clinically significant treatment-related adverse event that met any of the following criteria: (a) escalation of ALT/AST ≥5 × ULN or ≥2 × baseline value (the baseline value was abnormal) for 2 weeks, or elevated ALT/AST ≥3 × baseline value together with elevated bilirubin ≥2 × ULN or international normalized ratio (INR) ≥1.5; (b) uncontrolled grade 3 hypertension by medication within 7 days; (c) grade 3 or higher proteinuria or grade 2 proteinuria lasting >7 days, (d) grade 3 neutropenia with fever or grade 4 neutropenia; (e) grade 3 thrombocytopenia lasting >7 days or grade 3 thrombocytopenia with bleeding; (f) grade 3 nausea or vomiting with symptomatic treatment; (g) other grade 4 hematologic AEs and grade 3 or higher non-hematologic toxicities.
Dose modification was permitted among patients who experience grade 3 and 4 adverse events according to pre-specified plan. Stepwise dose reductions of vorolanib from 200 mg/day to 150 mg/day and 100 mg/day were allowed for patients with grade 3 non-hematological toxicity, or grade 4 hematological toxicity without fever. If patients had everolimus-related toxicities (grade ≥2 non-infective pneumonia), dose reduction of everolimus to 5 mg every other day was allowed. A 4-week dose interruption was allowed for the management of toxicity.
2.4. Pharmacokinetics
Serial blood samples were collected at pre-dose, and 0.5, 1, 1.5, 2, 3.5, 4.5 (0.5 h after vorolanib administration), 5, 6, 7, 8, 10, 12,16, 24 and 28 h after the first dose of everolimus on day 1 of cycle 1 (Supplementary Fig. 1); and at pre-dose, and 4 h after multiple dosing on day 14 of cycle 1. The concentration of everolimus, vorolanib, and X-297 (the main metabolite of vorolanib) in plasma was determined by using a liquid chromatography-mass spectrometry (LC-MSMS) method. Pharmacokinetic parameters in plasma were calculated by non-compartmental analysis and included the maximum drug concentration (Cmax), the time to Cmax (Tmax), the terminal half-life (t1/2), and the area under the plasma concentration-time curve (AUC).
2.5. Antitumor assessment
Tumor assessment was carried out by investigators using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 at screening and every 2 cycles (8 weeks) after initiation of the study treatment. Computerized tomography was used for scans of chest, abdomen, and pelvis, while gadolinium-enhanced MRI was used for brain scans, at screening and subsequent assessments. Complete response (CR) and partial response (PR) should be confirmed by re-evaluation at least 4 weeks after initial response.
2.6. Statistical analysis
Patients included in the safety analysis set (SS) should have received at least one dose of the study drugs and has at least one post-baseline safety evaluation. Efficacy analyses were conducted in the full analysis set (FAS), which included patients who received at least one dose the study drugs and has at least one post-baseline tumor assessment. Descriptive analyses of baseline status, medical history, laboratory examinations, safety indices, etc., were used to compare qualitative and quantitative data. The primary study objectives were to evaluate safety and tolerability and to identify MTD for the combination of everolimus and vorolanib. Toxicities were described by frequency and graded with the maximum grade over all cycles used as the summary measure per patient. The analyses were conducted with SAS 9.4 software (SAS Institute, Cary, NC). PK analyses were conducted in patients with evaluable PK concentrations using non-compartmental methods with SPSS17.0.
3. Results
3.1. Patient characteristics
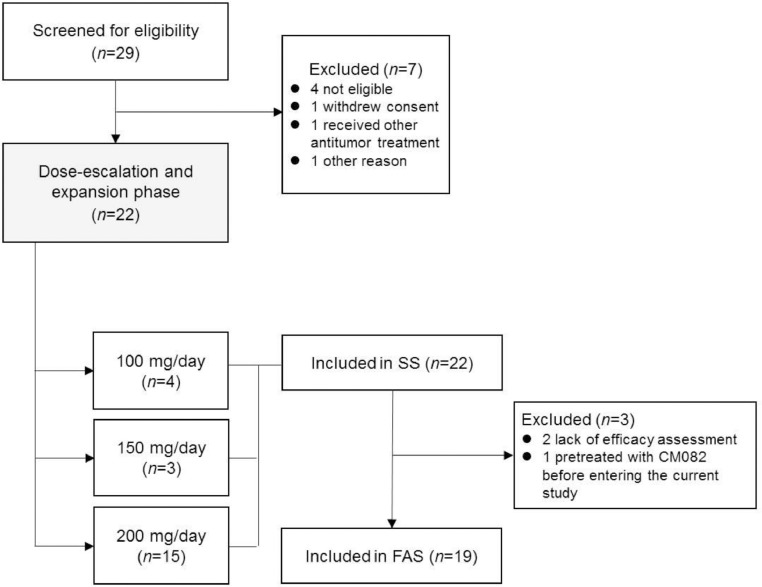
A total of 29 patients were screened in which 22 patients were enrolled from September 2015 to July 2016 in China (100 mg n = 4, 150 mg n = 3, 200 mg n = 15, Fig. 1). Demographics and baseline characteristics were summarized in Table 1. The mean age was 54.8 years, and 73% of the patients were men. All patients had metastatic ccRCC and most patients (95.4%) were pretreated with at least one VEGFR TKI, including first-line sunitinib (9), sorafenib (9), pazopanib (1), anlotinib (1) and famitinib (1) as well as second-line anlotinib (1), sorafenib (1) and sunitinib (2). All patients received at least one dose of combination of vorolanib and everolimus and were included in the safety analysis set, while 3 patients lack of post-dose tumor assessment, leaving 19 patients were included in the full analysis set (2 patients were excluded due to lack of post-dose tumor assessment and 1 were pretreated with vorolanib before entering the current study).
Fig. 1.
Trial profile.
Table 1.
Baseline characteristics.
| Vorolanib 100 mg | Vorolanib 150 mg | Vorolanib 200 mg | Total | |
|---|---|---|---|---|
| (n = 4) | (n = 3) | (n = 15) | (n = 22) | |
| Age (years) | 62.8 ± 7.5 | 59.7 ± 6.8 | 51.7 ± 9.5 | 54.8 ± 9.7 |
| Sex | ||||
| Men | 3 (75%) | 2 (67%) | 11 (73%) | 16 (73%) |
| Women | 1 (25%) | 1 (33%) | 4 (27%) | 6 (27%) |
| BMI (kg/m2) | 24.5 ± 2.0 | 22.2 ± 3.5 | 25.3 ± 2.6 | 24.7 ± 2.7 |
| ECOG performance status | ||||
| 0 | 1 (25%) | 0 (0) | 8 (53%) | 9 (41%) |
| 1 | 3 (75%) | 3 (100%) | 7 (47%) | 13 (59%) |
| Prior antitumor therapy | ||||
| Prior nephrectomy | 4 (100%) | 2 (67%) | 15 (100%) | 21 (95%) |
| Prior radiotherapy | 0 | 1 (33%) | 4 (27%) | 5 (23%) |
| Prior chemotherapy | 0 | 0 | 1 (7%) | 1 (5%) |
| Prior targeted therapy | 4 (100%) | 3 (100%) | 14 (93%) | 21 (95%) |
3.2. Safety and tolerability
The median overall duration of treatment (range) was 168.5 (19–602) days across all cohorts, and was 127.0 (19–208), 168.0 (71–396), and 172.0 (20–602) days for cohorts 1, 2, and 3, respectively. DLT was observed in one patient allocated to vorolanib 200 mg (grade 4 thrombocytopenia) and the MTD was not reached. vorolanib 200 mg and everolimus 5 mg once daily was set as the recommended dose, and was further evaluated in the expansion part.
All patients experienced at least one treatment-related adverse event (TRAE), in which 64% (14/22) were grade 3 or higher. The primary treatment-related toxicity was proteinuria with an incidence of 100%. Other common treatment-related toxicities included leukopenia (77%), hypercholesterolaemia (77%), increased low-density lipoprotein (68%), hair color change (68%), hypertriglyceridaemia (64%), and hyperglycaemia (59%), and fatigue (55%). Most treatment-related adverse events were grade 1 to 2, while grade 3 or higher toxicities were mostly seen in the vorolanib 200 mg cohort. Seven (31.8%) patients experienced grade 4 TRAEs, including 2 hypertriglyceridaemia, 2 thrombocytopenia, 1 creatine phosphokinase elevation, 1 hyponatremia, and 1 dyspnea. The most frequent TRAEs of grade 3/4 were hypertriglyceridemia (27.3%), hypertension (22.7%), and leukopenia (18.1%). Table 2 summarizes TRAEs reported by 20% or more of patients. No interstitial pneumonia that probably related to everolimus occurred in the study. Dose reduction and interruption of vorolanib was seen in four and fourteen patients, respectively, while eight patients had experienced dose interruption of everolimus.
Table 2.
Treatment-related adverse events.
| Any grade, n (%) | Grade 3, n (%) | Grade 4, n (%) | |
|---|---|---|---|
| Proteinuria | 22 (100.0) | 1 (4.5) | 0 (0) |
| Leukopenia | 17 (77.3) | 4 (18.2) | 0 (0) |
| Hypercholesterolaemia | 17 (77.3) | 1 (4.5) | 0 (0) |
| Increased low-density lipoprotein | 15 (68.2) | 0 (0) | 0 (0) |
| Hair color change | 15 (68.2) | 0 (0) | 0 (0) |
| Hypertriglyceridaemia | 14 (63.6) | 4 (18.2) | 2 (9.1) |
| Neutropenia | 14 (63.6) | 2 (9.1) | 0 (0) |
| Raised blood glucose | 13 (59.1) | 0 (0) | 0 (0) |
| Fatigue | 12 (54.5) | 0 (0) | 0 (0) |
| Hypertension | 11 (50.0) | 4 (18.2) | 1 (4.5) |
| Creatine phosphokinase elevation | 11 (50.0) | 0 (0) | 1 (4.5) |
| AST elevation | 10 (45.5) | 0 (0) | 0 (0) |
| Diarrhea | 10 (45.5) | 1 (4.5) | 0 (0) |
| Thrombocytopenia | 9 (40.9) | 1 (4.5) | 2 (9.1) |
| Decreased hemoglobin | 9 (40.9) | 0 (0) | 0 (0) |
| Mucosal inflammation | 9 (40.9) | 1 (4.5) | 0 (0) |
| Lid edema | 8 (36.4) | 0 (0) | 0 (0) |
| Anemia | 7 (31.8) | 3 (13.6) | 0 (0) |
| Mouth ulceration | 7 (31.8) | 0 (0) | 0 (0) |
| ALT elevation | 5 (22.7) | 0 (0) | 0 (0) |
| Decreased appetite | 5 (22.7) | 1 (4.5) | 0 (0) |
| Peripheral edema | 5 (22.7) | 0 (0) | 0 (0) |
| Dyspnea | 3 (13.6) | 0 (0) | 1 (4.5) |
Data are number (%). Treatment-related adverse events with an incidence of 20% or greater are listed in this table. All adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0). AST: aspartate aminotransferase; ALT: alanine aminotransferase.
3.3. Pharmacokinetics
Pharmacokinetic analyses were performed for both single dose administration (all cohorts, n = 9) and continuous dose administration (only vorolanib 200 mg cohort, n = 7) of combined vorolanib and everolimus (Supplementary Tables 1 and 2). After the first single dose of vorolanib (100–200 mg) in combination with everolimus, the mean Cmax of vorolanib ranged from 417.35±134.45 to 883.45±538.14 ng/mL, and the median time to Cmax ranged from 4.15 to 7.03 h. The plasma concentration of vorolanib declined rapidly after Tmax, fitting a single-compartment model, and the mean t1/2z (half-life) ranged from 4.74 to 12.89 h. The AUC0−t was found ranging from 4303.80±2170.82 to 10628.10±7569.13 h*ng/mL, and a trend of increasing exposure was seen with increasing vorolanib doses. After multiple doses, the mean Cmax of vorolanib (200 mg, once daily) was 591.50±358.15 ng/mL and the median Tmax was 2.02 h. The t1/2z was 11.85±7.17 h and the mean AUC0−t was 6705.18±5320.53 h*ng/mL. X-297 was the main active metabolite of vorolanib identified in preclinical study, exhibiting a mean Cmax of 127.80±51.85 ng/mL and a median Tmax of 2.02 h in the current study after multiple doses. Additionally, X-297 showed a smaller AUC0−t of 1757.49±1011.73 h*ng/m, but a longer t1/2z of 11.85±7.17 h, compared with vorolanib.
After the first dose in combination with vorolanib, the mean Cmax of everolimus ranged from 41.11±24.04 to 52.61±12.06 ng/mL and the median Tmax ranged from 0.75±0.35 to 0.87±0.26 h. The median T1/2z ranged from 13.94±7.28 to 20.77±16.95 h and the mean AUC0-t ranged from 202.83±83.04 to 267.79±89.54 h*ng/mL. After multiple doses, the mean Cmax of everolimus at day 14 of cycle 1 was 47.86±9.80 ng/mL and the median Tmax was 0.5 h, which was similar to those after single dose administration.
3.4. Antitumor activity
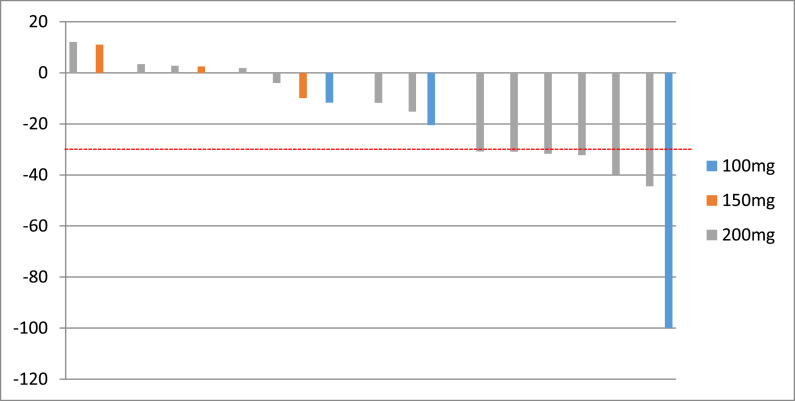
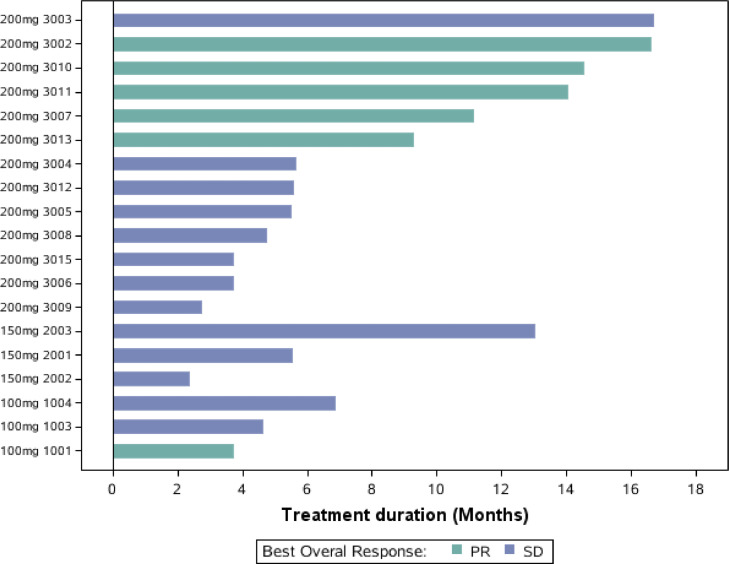
Overall, 19 of 22 patients had evaluable post-treatment tumor assessments, and tumor burden was reduced from baseline in 68.4% of patients (Fig. 2). One patient in the vorolanib 100 mg cohort and 5 patients in the vorolanib 200 mg cohort displayed confirmed PR and 13 patients had stable disease. The ORR was 32% (95% CI: 13–57%, Table 3) and the DCR was 100% (95% CI: 82–100%, Table 3). Fig. 3 illustrated the swimmers plot of the duration of treatment.
Fig. 2.
Waterfall plot of the best overall response. The bars indicate the largest percentage change in target lesions from baseline. The colors represent different dose of vorolanib with the same dose of everolimus. The dotted lines at –30% represent the cutoffs for partial response.
Table 3.
Tumor response based on investigator assessments.
| Objective response | 100 mg | 150 mg | 200 mg | Total |
|---|---|---|---|---|
| (n = 3) | (n = 3) | (n = 13) | (n = 19) | |
| CR | 0 | 0 | 0 | 0 |
| PR | 1 (33%) | 0 | 5 (39%) | 6 (32%) |
| SD | 2 (67%) | 3 (100%) | 8 (62) | 13 (68) |
| PD | 0 | 0 | 0 | 0 |
| ORR (95%CI) | 33% (1−91%) | 0 (0–71%) | 39% (14−68%) | 32% (13−57%) |
| DCR (95%CI) | 100% (29−100%) | 100% (29−100%) | 100.0 (75−100%) | 100.0 (82−100%) |
| PFS (month, 95%CI) | 4.6 (3.7-NA) | 5.5 (2.6–13.0) | 5.7 (4.8–16.7) | 5.6 (4.6–13) |
Fig. 3.
Swimmers plot of the duration of treatment.
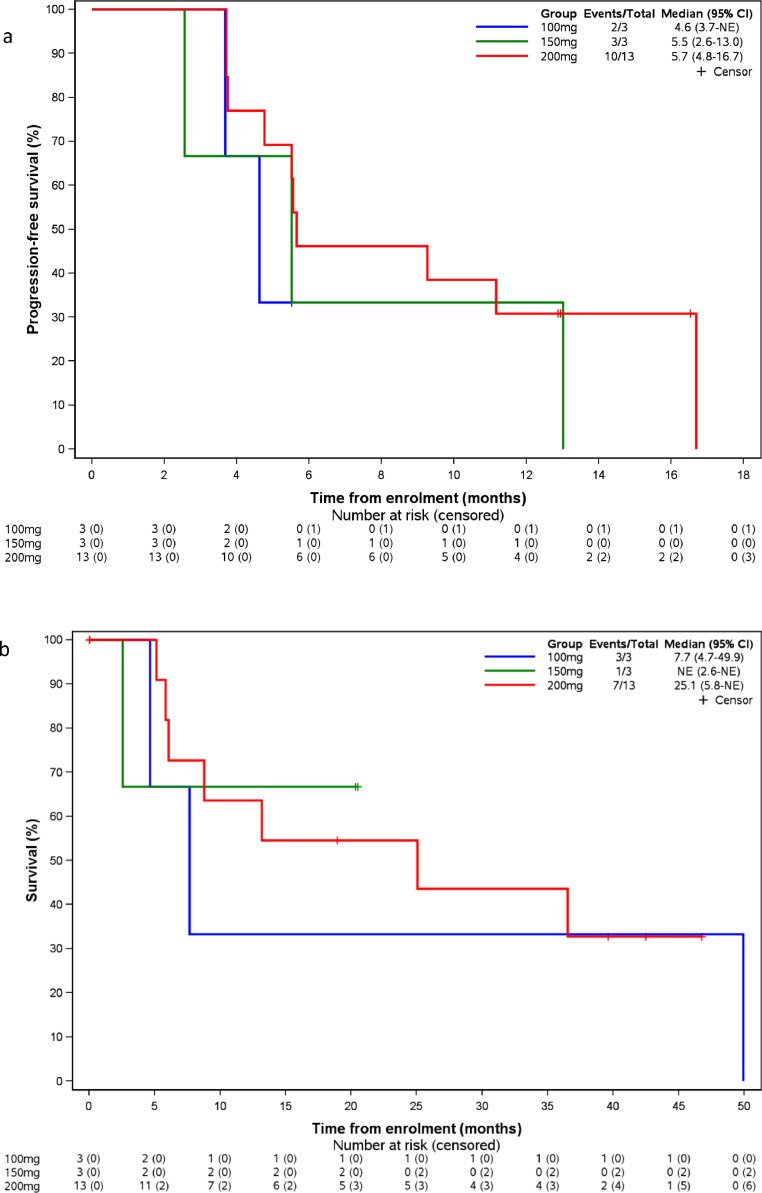
Fifteen patients had disease progression (n = 13) or death (n = 2), and the median progression-free survival was 5.6 months (95% Cl: 4.6–13.0). For patients in the 200 mg cohort (n = 13), the ORR and DCR was 38.5% (95% CI: 14−68%) and 100% (95% CI: 75−100%), respectively, and the median PFS was 5.7 months (95% Cl: 4.8–16.7) (Fig. 4(a)). Among the 8 patients treated with only one prior VEGFR TKI, the median progression-free survival was 10.2 months (95%CI: 3.7−16.7%). By November 30th, 2019, eleven patients had OS events, the median OS was 25.1 months (95% CI 5.9, 49.9) and 25.1 months (95% CI 5.9, NA) for all patients and those in the 200 mg cohort, respectively (Fig. 4(b)).
Fig. 4.
Progression-free survival (PFS, 4a) and overall survival (OS, 4b) analysis according to the dose level of CM082 with everolimus (n = 19).
4. Discussion
This phase I dose-finding study demonstrated that vorolanib in combination with everolimus were generally well tolerated in patients with advanced ccRCC who had progressed after targeted therapy and/or chemotherapy. The treatment-related AEs were well managed with dose adjustment or supportive medication. Only 1 patient experienced DLT and the MTD of vorolanib in combination with everolimus was not reached.
Since VHL mutations and the activation of VEGF and PDGF have been discovered, the treatment of advanced renal cell carcinoma (RCC) has undergone a major change with the development of potent angiogenesis inhibitors and targeted agents, including sorafenib, sunitinib, pazopanib, axitinib, everolimus, and tersirolimus. More recently, novel agents like cabozantinib and the introduction of immunotherapy has significantly changed the frontline treatment paradigm for RCC owing to improved PFS and/or OS in randomized, phase 3 trials as compared with sunitinib or sorafenib [19,20,44,45]. Nivolumab/ipilimumab, as well as axitinib/pembrolizumab resulted in significantly longer OS, PFS, and a higher ORR in untreated RCC patients, while atezolizumab/bevacizumab and axitinib/avelumab could also be relevant first-line options on the basis of prolonged PFS. However, there is no consensus on optimal treatment sequencing in RCC, which raised the question about treatment selection for second-line setting after progression on immunotherapy. Established second-line monotherapies include axitinib, cabozantinib, nivolumab, and everolimus alone, which were associated with a median PFS around 4.4–8.3 months. Notable, cabozantinib has been shown to be effective after treatment with immunotherapy [[46], [47], [48]]. In addition, combining therapies with VEGF pathway and mTOR inhibition were also explored. Most early investigations failed due to lack of clinical activity and greater toxicities than with single-agent treatments [[26], [27], [28], [29]]. In the randomized, phase 2 Study, patients were randomized to three treatment arms: lenvatinib-everolimus, lenvatinib alone, and everolimus alone, which showed the longest median PFS at 14.6 months in the lenvatinib-everolimus arm in the second-line setting [30]. Moreover, median PFS achieved 16.9 (95% CI: 12.1–20.6) in unselected patients with metastatic renal cell carcinoma (mRCC) treated with lenvatinib with everolimus [49]. These findings suggested that the validity of targeted strategies should not be overshadowed by immunotherapies, combinations can achieve good results. Nevertheless, the data from different clinical trials must be interpreted with caution partly due to the ethnicity, sample size, population, etc. Safety and tolerability remains a major concern for combinations of targeted treatment. Lenvatinib-everolimus combination resulted in an increased rate of dose reductions (71%) and discontinuations due to AEs (24%), and there was one treatment-related death with the combination and one with single-agent lenvatinib [30]. Vorolanib is a small molecule indolinone inhibitor of VEGFRs-1, −2, −3, platelet derived growth factor (PDGFR α and β), stem cell factor (c-kit), and ligand for FLT-3. The first-in-human phase 1 study of vorolanib tablet (dose range 50–800 mg once daily) in patients with solid tumors was conducted in the USA, which showed favorable safety profile with fatigue, diarrhea, nausea and vomiting being the most common adverse events. The most common treatment-related grade 3 adverse event (AE) being proteinuria (4%), no grade 4 AEs or deaths related to vorolanib was reported. MTD was not achieved, and enrollment was stopped because of the apparent saturation of absorption at 400–800 mg. The recommended dose of vorolanib monotherapy in patients with advanced cancer is 400 mg once daily [50]. A subsequent phase 1 study was conducted in China to evaluate the safety and preliminary antitumor activities of monotherapy of vorolanib in patients with solid tumors. Consistent with previous study, vorolanib also shown acceptable safety profile and preliminary activities over the dose range of 50–250 mg once daily in Chinese patients [42]. Proteinuria, leukopenia, fatigue, hypertension and diarrhea were the most common AEs with vorolanib, grade 3 or higher events were seen in 7 out of 19 patients across 100 to 250 mg dose cohort. MTD was not achieved, and 200 mg was established as the recommended dose for monotherapy in further studies.
In a phase 2 study of lenvatinib (an oral inhibitor of multiple VEGFRs), everolimus and the combination in patients with metastatic RCC, the most common treatment-emergent AEs with everolimus (10 mg once daily) were stomatitis, diarrhea, fatigue or asthenia, and cough [30]. The RECORD-4 study of everolimus in the second-line setting reported the most common grade 3 and 4 AEs were anemia, stomatitis, proteinuria, hyperglycemia, hypertriglyceridemia and hypercholesterolemia [51]. Moreover, the AEs in our study were consistent with those previously reported for everolimus [52]. Compared with previous studies on monotherapy of vorolanib or everolimus, the present study did not show any unexpected AEs, and the safety profile was within the range of the known safety profiles of each agent. Furthermore, the safety profile observed in the present study was also very similar to that of combination therapy of lenvatinib plus everolimus, which was expected to inhibit the VEGFR, FGFR and mTOR signaling pathways simultaneously [53]. Additionally, although 18 patients (82%) experienced TRAEs that need supportive medication, and 12 patients (55%) experienced dose interruption or reduction of this combination therapy due to AEs in the present study, most patients could continue to receive the treatment and only one patient discontinue the treatment. Therefore, the toxicities observed in the present study were manageable with dose adjustments and supportive medication. TRAEs in this study were consistent with the typical class effects of VEGFR and mTOR inhibitors without new safety signals. The grade 3/4 AEs for sunitinib included increased lipase (13%), lymphopenia (12%), neutropenia (11%), hypertension (8%), fatigue (7%), diarrhea (5%), hand-foot syndrome (5%), vomiting (4%) [54], renal AEs were not common in everolimus plus sunitinib [26]. With similar structural features of sunitinib, renal toxicity was also not frequently seen in our study with the exception of proteinuria. Besides, we hypothesized that inhibition of TKI and downstream mTOR modulation may induce off-target effects, leading to the increased toxicity, which limited clinical application of combination of effective regimens. Vorolanib has a relatively short half-life and a low volume of distribution, which may contribute to the reduced incidence of adverse effects [41].
The PK analysis demonstrated that the parameter for everolimus were similar among the three cohorts, indicating the concurrent administration of vorolanib might not affect the PK of everolimus substantially. Nevertheless, the Cmax and AUC of vorolanib increased with the dose escalation, which indicated the plasma exposure of vorolanib increased dose proportionally. The Cmax and AUC0-t was 591.50±358.15 ng/mL and 6705.18±5320.53 h*ng/mL, respectively, for patients receiving 200 mg vorolanib plus everolimus after multiple doses, which seemed higher than that reported in the western population who received vorolanib monotherapy [33]. Ethnic factors, and various baseline characteristics like age and weight should be considered when integrating this difference.
Although second-line everolimus can produce objective response and extend PFS and OS in patients with advanced RCC, it is not curative and the benefits must be weighed against the burden of toxicity [24]. Previous trials have proven that enough dosage of targeted agents could produce favorable therapeutic effect but also enough AEs [26,27]. To balance the clinical benefit with the toxicity, antiangiogenic TKIs at safe dose in combination with low dose of everolimus were expected to enhance antitumor activity with tolerated toxicities. Lenvatinib plus everolimus (18 mg/day and 5 mg/day, respectively) has proven to prolong progression-free survival compared with everolimus alone. Higher ORR of 43% was achieved in the combination treatment group than that of 6% in the everolimus monotherapy group. However, grade 3 and 4 AEs occurred in more patients allocated lenvatinib plus everolimus compared with those assigned single-agent everolimus (10 mg/day), with diarrhea, fatigue or asthenia, hypertension being the most common [30]. In the present study, vorolanib combined with low doses of everolimus (5 mg/day) showed good response in treating advanced clear-cell RCC, achieving an ORR of 32% and a DCR of 100%. Even though the overall median PFS in the present study was 5.6 months, 6 of 13 patients in the expansion cohort achieved a PFS of more than 9 months and 3 of them are still under treatment with a PFS of more than 12 months. Therefore, vorolanib plus everolimus possessed the potential to treat advanced clear-cell RCC with good efficacy.
In conclusion, the combination of vorolanib and everolimus was well tolerated with an overall response rate outcomes indicate the combined regime was convincing in therapeutic effect. We regard 200 mg vorolanib plus 5 mg everolimus as the recommended regimen for further study. A phase 2/3 trial (NCT03095040) is ongoing and has completed enrollment to assess vorolanib, everolimus, or their combination as second-line treatment in patients with metastatic RCC.
Declaration of Competing Interest
Li Mao and Lieming Ding are employee and stock owner of Betta Pharmaceuticals. All remaining authors have declared no conflicts of interest.
Funding sources
This study was sponsored by Betta Pharmaceutical Co., Ltd., which was involved in design and conduct of the study; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Acknowledgments
We would like to acknowledge the patients participating in the study, and the investigators and institutions involved in this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102755.
Appendix. Supplementary materials
References
- 1.Barata P.C., Rini B.I. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–524. doi: 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3.Nickerson M.L., Jaeger E., Shi Y. Improved identification of von Hippel–Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latif F., Tory K., Gnarra J. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 5.Kim W.Y., Kaelin W.G. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 6.Kaelin W.G., Jr. The von Hippel–Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13:680s–684s. doi: 10.1158/1078-0432.CCR-06-1865. [DOI] [PubMed] [Google Scholar]
- 7.Patard J.J., Rioux-Leclercq N., Masson D. Absence of VHL gene alteration and high VEGF expression are associated with tumour aggressiveness and poor survival of renal-cell carcinoma. Br J Cancer. 2009;101:1417–1424. doi: 10.1038/sj.bjc.6605298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 9.Pal K., Madamsetty V.S., Dutta S.K. Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. NPJ Precis Oncol. 2019;3:1–11. doi: 10.1038/s41698-019-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iommarini L., Porcelli A.M., Gasparre G. Non-canonical mechanisms regulating hypoxia-inducible factor 1 alpha in cancer. Front Oncol. 2017;7:286. doi: 10.3389/fonc.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost P., Berlanger E., Mysore V. Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis. J Oncol. 2013;2013:1–12. doi: 10.1155/2013/897025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conciatori F., Bazzichetto C., Falcone I. Role of mTOR signaling in tumor microenvironment: an overview. Int J Mol Sci. 2018;19:2453. doi: 10.3390/ijms19082453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer R.J., Hutson T.E., Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 14.Motzer R.J., Hutson T.E., Tomczak P. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil S., Figlin R.A., Hutson T.E. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2011;22:295. doi: 10.1093/annonc/mdq342. [DOI] [PubMed] [Google Scholar]
- 16.Melichar B., Bracarda S., Matveev V., Alekseev B., Ivanov S., Zyryanov A. A multinational phase II trial of bevacizumab with low-dose interferon-α2a as first-line treatment of metastatic renal cell carcinoma: BEVLiN. Ann Oncol. 2013;24:2396–2402. doi: 10.1093/annonc/mdt228. [DOI] [PubMed] [Google Scholar]
- 17.Escudier B., Szczylik C., Hutson T.E., Demkow T., Staehler M., Rolland F. Randomized phase II trial of firstline treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. Erratum in: J Clin Oncol. 2009; 27:2305. [DOI] [PubMed] [Google Scholar]
- 18.Hudes G., Carducci M., Tomczak P. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 19.Motzer R.J., Tannir N.M., McDermott D.F., Aren Frontera O., Melichar B., Choueiri T.K. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rini B.I., Powles T., Atkins M.B., Escudier B., McDermott D.F., Suarez C. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 21.Rini B.I., Escudier B., Tomczak P., Kaprin A., Szczylik C., Hutson T.E. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 22.Choueiri T.K., Escudier B., Powles T., Tannir N.M., Mainwaring P.N., Rini B.I. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 23.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 25.Sosman J.A., Puzanov I., Atkins M.B. Opportunities and obstacles to combination targeted therapy in renal cell cancer. Clin Cancer Res. 2007;13(2 Pt 2):764s–769s. doi: 10.1158/1078-0432.CCR-06-1975. [DOI] [PubMed] [Google Scholar]
- 26.Molina A.M., Feldman D.R., Voss M.H. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118(7):1868–1876. doi: 10.1002/cncr.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel P.H., Senico P.L., Curiel R.E. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7:24–27. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patnaik A., Ricart A., Cooper J. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. J Clin Oncol. 2007;25(Suppl 18):3512. [Google Scholar]
- 29.Harzstark A.L., Small E.J., Weinberg V.K. A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer. 2011;117(18):4194–4200. doi: 10.1002/cncr.25931. [DOI] [PubMed] [Google Scholar]
- 30.Motzer R.J., Hutson T.E., Glen H. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 31.Negrier S., Gravis G., Perol D. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12:673–680. doi: 10.1016/S1470-2045(11)70124-3. [DOI] [PubMed] [Google Scholar]
- 32.Rini B.I., Bellmunt J., Clancy J. Randomized phase III trial of temsirolim us and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014;32:752–759. doi: 10.1200/JCO.2013.50.5305. [DOI] [PubMed] [Google Scholar]
- 33.Grandinetti C.A., Goldspiel B.R. Sorafenib and sunitinib: novel targeted therapies for renal cell cancer. Pharmacotherapy. 2007;27:1125–1144. doi: 10.1592/phco.27.8.1125. [DOI] [PubMed] [Google Scholar]
- 34.Keating G.M., Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 35.van Geel R.M., Beijnen J.H., Schellens J.H. Concise drug review: pazopanib and axitinib. Oncologist. 2012;17:1081–1089. doi: 10.1634/theoncologist.2012-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rini B.I., Escudier B., Tomczak P. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 37.Scagliotti G., Govindan R. Targeting angiogenesis with multitargeted tyrosine kinase inhibitors in the treatment of non-small cell lung cancer. Oncologist. 2010;15:436–446. doi: 10.1634/theoncologist.2009-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhargava P., Robinson M.O. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep. 2011;13:103–111. doi: 10.1007/s11912-011-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng X., Yan X., Tang B. A phase I clinical trial of CM082 (X-82) in combination with everolimus for treatment of metastatic renal cell carcinoma. J Clin Oncol. 2017;35(15_suppl):4575. 4575. [Google Scholar]
- 40.Yan X.Q., Sheng X.N., Tang B.X. Anti-VEGFR, PDGFR, and CSF1R tyrosine kinase inhibitor CM082 (X-82) in combination with everolimus for treatment of metastatic renal cell carcinoma: a phase 1 clinical trial. Lancet Oncol. 2017;18:S8. S8. [Google Scholar]
- 41.Moore K.N., Jones S.F., Kurkjian C. Phase I, first-in-human trial of an oral VEGFR tyrosine kinase inhibitor (TKI) x-82 in patients (pts) with advanced solid tumors. J Clin Oncol. 2012;30(15_Suppl):3041. 3041. [Google Scholar]
- 42.Song Y., Wang J., Zhou A.P. Vorolanib (CM082) in Chinese patients with advanced solid tumor: a phase 1, open-label, dose escalation study. J Clin Oncol. 2018;36(15_Suppl):2580. 2580. [Google Scholar]
- 43.Scarpelli M., Rampurwala M., Eickhoff J., Carmichael L., Heideman J., Binger K. Pharmacodynamic study using FLT PET/CT in advanced solid malignancies treated with a sequential combination of X-82 and docetaxel. Cancer Chemother Pharmacol. 2018;82(2):211–219. doi: 10.1007/s00280-018-3599-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motzer R.J., Penkov K., Haanen J., Rini B., Albiges L., Campbell M.T. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 46.Derosa L., Rouche J., Colomba E., Baciarello G., Routy B., Albiges L. Efficacy of cabozantinib (C) after PD-1/PD-L1 checkpoint inhibitors in metastatic renal cell carcinoma (mRCC): the Gustave Roussy experience. Proceedings of the annual congress of the European Society for medical oncology; Madrid, Spain; 2017. [Google Scholar]
- 47.Shah A.Y., Lemke E., Gao J., Chandramohan A., Campbell M.T., Zurita A.J. Outcomes of patients (pts) with metastatic clear-cell renal cell carcinoma (mCCRCC) treated with second-line (2 L) vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI) after first-line (1 L) immune checkpoint inhibitors (ICI) J Clin Oncol. 2018;36:682. Abstract. [Google Scholar]
- 48.Tannir N.M., Pal S.K., Atkins M.B. Second-line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist. 2018;23:540–555. doi: 10.1634/theoncologist.2017-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkova M., Abdelgafur A., Amoev Z. Efficacy and safety of lenvatinib in combination with everolimus in metastatic renal cell carcinoma resistant to antiangiogenic targeted therapy: Russian multicenter observational study ROSLERCM. J Clin Oncol. 2020;38(6_suppl):644. 644. [Google Scholar]
- 50.Bendell J.C., Patel M.R., Moore K.N., Chua C.C., Arkenau H.T., Dukart G. Phase I, first-in-human, dose-escalation study to evaluate the safety, tolerability, and pharmacokinetics of vorolanib in patients with advanced solid tumors. Oncologist. 2019;24(4):455–e121. doi: 10.1634/theoncologist.2018-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motzer R.J., Alyasova A., Ye D. Phase II trial of second-line everolimus in patients with metastatic renal cell carcinoma (RECORD-4) Ann Oncol. 2016;27(3):441–448. doi: 10.1093/annonc/mdv612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodnar L., Stec R., Cierniak S. Clinical usefulness of PI3K/Akt/mTOR genotyping in companion with other clinical variables in metastatic renal cell carcinoma patients treated with everolimus in the second and subsequent lines. Ann Oncol. 2015;26:1385–1389. doi: 10.1093/annonc/mdv166. [DOI] [PubMed] [Google Scholar]
- 54.Matsubara N., Naito Y., Nakano K. Lenvatinib in combination with everolimus in patients with advanced or metastatic renal cell carcinoma: a phase 1 study. Int J Urol. 2018;25(11):922–928. doi: 10.1111/iju.13776. [DOI] [PubMed] [Google Scholar]
- 53.Butz H., Ding Q., Nofech-Mozes R. Elucidating mechanisms of sunitinib resistance in renal cancer: an integrated pathological-molecular analysis. Oncotarget. 2018;9:4661–4674. doi: 10.18632/oncotarget.23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.