Abstract
Objective
The aim of this study is to evaluate mitral valve hemodynamics after mitral valve repair for degenerative disease, and seek the impact of type/size of annuloplasty prosthesis on resting cardiac hemodynamics.
Methods
Between October 2012 and June 2019, 301 patients underwent isolated mitral valve repair for degenerative disease were enrolled. Correlation between postoperative mitral hemodynamics and type/size of annuloplasty prosthesis was evaluated.
Results
There were significant correlations between annuloplasty size and peak velocity (r = −0.41, p < 0.001), peak transmitral pressure gradient (TMPG) (r = −0.40, p < 0.001), mean TMPG (r = −0.41, p < 0.001), effective orifice area (EOA) (r = 0.26, p < 0.001), and pulmonary artery systolic pressure (r = −0.15, p = 0.010). In patients with larger annuloplasty prostheses (≥30 mm), the type of annuloplasty prosthesis (band or ring) did not influence the mitral hemodynamics, however, mean TMPG was significantly greater in patients with a full ring (2.9 mmHg [2.1–3.7] vs. 4.0 mmHg [2.8–5.0], p < 0.001) in patients with smaller annuloplasty (<30 mm). Left ventricular ejection fraction and stroke volume were significantly associated with an increase of TMPG (r = 0.14, p = 0.016 and r = 0.24, p < 0.001).
Conclusions
A larger partial band had the potential to improve mitral hemodynamics after mitral repair for degenerative disease. However, echocardiographic mitral hemodynamics was influenced by LV function. Therefore, a more accurate method is required to elucidate the true impact of mitral repair on hemodynamics.
Keywords: Mitral valve repair, Degenerative disease, Hemodynamics, Functional mitral stenosis
1. Introduction
Mitral valve repair is a standard procedure for functional and degenerative mitral regurgitation (MR). Recurrence of MR is the main concern and research is focused on long-term success of surgical repair. At the same time, functional mitral stenosis (FMS), according to the reduction of the mitral effective valve orifice area (EOA), and elevation of the transmitral pressure gradient (TMPG), has been emphasized. A high incidence of FMS with a mitral valve area less than 1.5 cm2 or a mean TMPG greater than 5 mmHg has recently been reported in patients who underwent surgical mitral annuloplasty for ischemic MR [1], [2], [3]. Additionally, TMPG after mitral valve repair for degenerative mitral regurgitation is reported to cause lower exercise capacity and atrial fibrillation [4], [5], [6].
The size and type of annuloplasty prosthesis and technique of repair were reported as predictors of FMS, following valve repair. Compared to a partial band, the postoperative TMPG was greater in patients with a full ring prosthesis [5]. On the other hand, a larger annuloplasty prosthesis is expected to reduce TMPG. It is difficult to assess FMS after mitral valve repair as various techniques and annuloplasty products are used, depending on surgical preferences. Additionally, the relationship between type and size of annuloplasty prosthesis and mitral valve hemodynamics is still unclear, and there are few reports on hemodynamic difference between various products. Based on these backgrounds, the aim of this study is to evaluate mitral valve hemodynamics after mitral valve repair for degenerative disease, and seek the impact of type/size of annuloplasty prosthesis on resting cardiac hemodynamics.
2. Patients and methods
2.1. Patient population
This study is a retrospective evaluation of mitral valve hemodynamic status measured by resting echocardiogram at 2 weeks after surgery. Three hundred and six patients underwent isolated mitral valve repair for degenerative disease with annuloplasty prosthesis at the Sakakibara Heart Institute of Okayama, Japan, between October 2012 and June 2019. After excluding 5 patients with residual mitral regurgitation ≥ moderate, the remaining 301 patients were enrolled. The overall mean patient age was 60 (48–68) years, of which 35% (106/301) were female. The mean body surface area was 1.66 m2 (1.50–1.78). The cohort included 52 (17%) paroxysmal and 32 (11%) chronic atrial fibrillation. Patient characteristics are presented in Supplementary Table 1. The goal was to evaluate the correlation between type/size of annuloplasty prosthesis and mitral valve hemodynamics. This study was approved by the institutional ethics committee in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments (No. A201908-01, September 26, 2019). Consent for using patients’ data was obtained from all patients.
2.2. Surgical technique of mitral valve repair
In this cohort, 76.1% of patients underwent minimally invasive mitral repair via a right mini-thoracotomy. The main technique of mitral repair included resection in 115 patients (38%), artificial chorda in 165 patients (55%), folding plasty in 18 patients (6%), and augmentation in 3 patients (1%). The prolapse lesion included 49 anterior (16%), 213 posterior (71%) and 39 bi-leaflet (13%) (Supplementary Table 2).
2.3. Quantitative echocardiography
Standard transthoracic echocardiography was performed on all patients in the left-lateral decubitus position by experienced sonographers before and after symptom-limited exercise, using a commercially available ultrasound machine (Aplio Artida, Toshiba Medical Systems Corporation, Tochigi, Japan). Standard data, such as left ventricular ejection fraction (LVEF), end-diastolic and systolic dimensions, and tricuspid regurgitation pressure gradient (TRPG) were obtained from the official echocardiographic report [7]. Stroke volume (SV) was measured as follows: the LV outflow tract (LVOT) area × LVOT velocity time integral (VTI), by the pulse wave Doppler method. To evaluate the hemodynamic status of the mitral valve, the EOA, which was calculated with the continuity equation, the TMPG, the Doppler velocity index (DVI = VTIMV/VTILVOT), and the peak flow velocity were measured. The pulmonary artery systolic pressure (PASP) was estimated from the maximal velocity of the tricuspid regurgitant jet using continuous-wave Doppler with the simplified Bernoulli equation and adding the right atrial pressure (RAP), which was estimated by the diameter and collapsibility of the inferior vena cava (IVC). For an IVC with a diameter < 2.1 cm that collapses ≥ 50% with a sniff, the RAP value of 3 mmHg was used; an IVC with a diameter ≥ 2.1 cm that collapses < 50% suggests a RAP of 15 mmHg. If the IVC diameter and collapse did not fit this scenario, an intermediate value of 8 mmHg was used [8], [9], [10], [11].
2.4. Statistical analysis
Continuous data are presented as median and interquartile values (the 1st to the 3rd quartile). Categorical variables are given as a count and percentage of patients. Continuous data were compared with a Mann-Whitney U test. Categorical variables are given as a count and percentage of patients and were compared using the χ2 test. When any expected frequency was less than 1, or 20% of expected frequencies were less than or equal to 5, the Fisher’s exact test was used. The correlation between annuloplasty size and mitral hemodynamics was assessed using Spearman’s correlation coefficient. All data were analyzed using the Statistical Analysis Systems software JMP 12.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Implanted annuloplasty prostheses
A partial band was used for 158 (52.5%) patients and a full ring for 143 (47.5%) patients. There was no significant difference in the mean size of band and ring (32 mm [30–33] vs. 32 mm [28–32], p = 0.14). For the partial band group, CG future (Medtronic Inc., Minneapolis, MN, USA), Cosgrove (Edwards Lifesciences, Irvine, CA, USA), Duran (Medtronic Inc., Minneapolis, MN, USA), and Tailor (Abbott Laboratories, Abbott Park, IL, USA) bands were implanted in 23, 82, 20, and 33 patients, respectively. For the full ring group, 68 Carpentier-Edwards Physio/Physio II (Edwards Lifesciences, Irvine, CA, USA) and 75 Memo 3D (LivaNova, Saluggia, Italy) rings were implanted.
3.2. Comparison of hemodynamic status between different partial band products
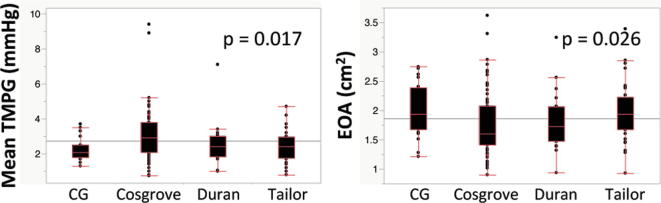
The median of the implanted size of the respective partial band was as follows: CG future band (32 mm [28–32]), Cosgrove band (32 mm [30–32]), Duran band (29 mm [29–31]), and Tailor band (31 mm [31–33]). There were significant differences in mean TMPG and EOA between respective partial bands (p = 0.017 and 0.026). The median of the TMPG of respective bands was 2.3 mmHg (1.8–2.5) in CG future band, 2.9 mmHg (2.1–3.8) in Cosgrove band, 2.4 mmHg (1.9–3.0) in Duran band, and 2.4 mmHg (1.8–3.0) in Tailor band. The median of the EOA of respective bands was 1.93 cm2 (1.68–2.40) in CG future band, 1.60 cm2 (1.41–2.07) in Cosgrove band, 1.72 cm2 (1.47–2.07) in Duran band, and 1.93 cm2 (1.68–2.23) in Tailor band (Supplementary Figure 1).
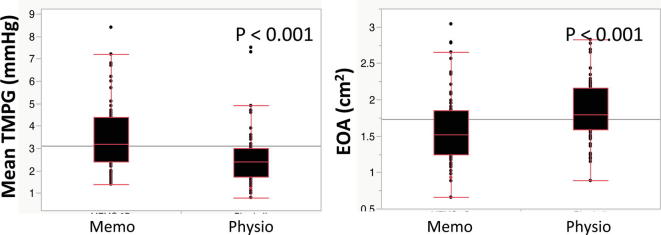
3.3. Comparison of hemodynamic status between different full ring products
The median implanted size of the respective full rings were as follows: Physio ring (32 mm [31–34]), and Memo 3D ring (30 mm [28–32]), (p < 0.001). There were significant differences in mean TMPG and EOA between Physio and Memo 3D rings (2.4 mmHg [1.7–3.0] vs. 3.2 mmHg [2.4–4.4], p < 0.001, and 1.80 cm2 [1.59–2.17] vs. 1.52 cm2 [1.24–1.85], p < 0.001) (Supplementary Figure 2).
3.4. Correlation between annuloplasty size and mitral hemodynamic parameters
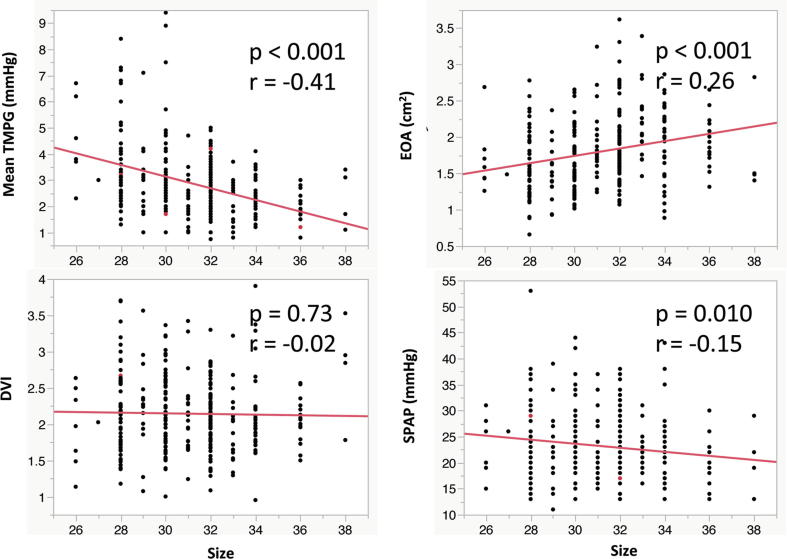
There were significant correlations between annuloplasty size and peak velocity (r = −0.41, p < 0.001), peak TMPG (r = −0.40, p < 0.001), mean TMPG (r = −0.41, p < 0.001), EOA (r = 0.26, p < 0.001), and PASP (r = −0.15, p = 0.010). DVI had no significant correlation with annuloplasty size (r = −0.02, p = 0.73) (Fig. 1).
Fig. 1.
Correlation between annuloplasty size and mitral hemodynamic parameters. TMPG, transmittal pressure gradient, EOA, effective orifice area, DVI, Doppler velocity index, SPAP, systolic pulmonary artery pressure.
3.5. Comparison of hemodynamic status between partial band and full ring
In patients with the partial band, LVEF and LVSV were significantly greater than patients with the full ring (60% [56–65] vs. 57% [53–63], p = 0.003, and 60 ml [52–70] vs. 55 ml [48–67], p = 0.011). There were no significant differences in peak velocity, peak TMPG, DVI, and PASP between the band and ring groups. Mean TMPG was significantly lower and EOA was significantly greater in patients with the partial band (2.5 mmHg [1.9–3.2] vs. 2.7 mmHg [2.0–3.7], p = 0.016, and 1.75 cm2 [1.49–2.15] vs. 1.71 cm2 [1.39–2.08], p = 0.026) (Table 1).
Table 1.
Comparison of echocardiographic data.
| Variables | Partial band (n = 158) | Full ring (n = 143) | p-value |
|---|---|---|---|
| LVDD (mm) | 45 (42–49) | 46 (42–49) | 0.53 |
| LVDS (mm) | 30 (28–34) | 31 (29–35) | 0.05 |
| LVEF (%) | 60 (56–65) | 57 (53–63) | 0.003 |
| LAD (mm) | 36 (32–40) | 36 (31–41) | 0.49 |
| LVSV (ml) | 60 (52–70) | 55 (48–67) | 0.011 |
| MR | 1 (1–2) | 1 (1–2) | 0.33 |
| DVI | 2.1 (1.7–2.4) | 2.1 (1.8–2.6) | 0.22 |
| EOA (cm2) | 1.75 (1.49–2.15) | 1.71 (1.39–2.08) | 0.06 |
| Peak velocity (m/s) | 1.26 (1.08–1.50) | 1.31 (1.08–1.57) | 0.28 |
| Peak TMPG (mmHg) | 6.4 (4.7–9.0) | 6.7 (4.6–9.9) | 0.35 |
| Mean TMPG (mmHg) | 2.5 (1.9–3.2) | 2.7 (2.0–3.7) | 0.039 |
| TRPG (mmHg) | 19 (16–23) | 18 (15–22) | 0.22 |
| PASP (mmHg) | 22 (19–27) | 22 (19–26) | 0.52 |
LVDD, left ventricular diastolic dimension; LVDS, left ventricular systolic dimension; LVEF, left ventricular ejection fraction; LAD, left atrial dimension; LVSV, left ventricular stroke volume; MR, mitral regurgitation; DVI, Doppler velocity index; MVPG, mitral valve pressure gradient; TRPG, tricuspid regurgitation pressure gradient; PASP, pulmonary artery systolic pressure.
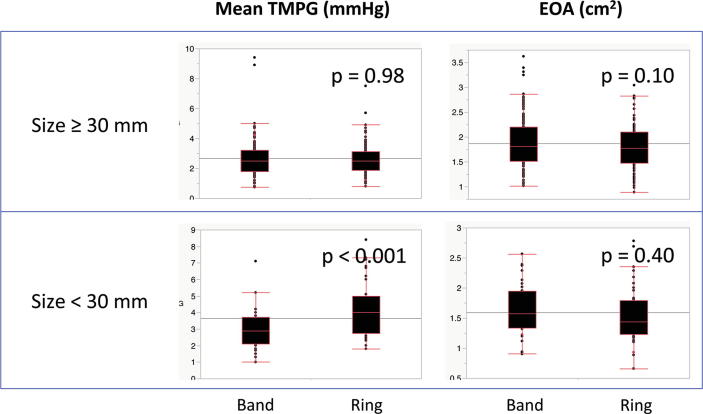
In patients with larger annuloplasty prostheses (≥30 mm), there were no significant differences in mean TMPG and EOA between the band and ring (2.5 mmHg [1.8–3.2] vs. 2.5 mmHg [1.9–3.1], p = 0.98, and 1.82 cm2 [1.51–2.20] vs. 1.78 cm2 [1.48–2.10], p = 0.10). In patients with smaller annuloplasty (<30 mm), there was no significant difference in EOA between the band and ring (1.57 cm2 [1.34–1.95] vs. 1.44 cm2 [1.23–1.79], p = 0.40); however, mean TMPG was significantly greater in patients with a full ring (2.9 mmHg [2.1–3.7] vs. 4.0 mmHg [2.8–5.0], p < 0.001) (Fig. 2).
Fig. 2.
Influence of annuloplasty size on the comparison of transmittal pressure gradient (TMPG) and effective orifice area (EOA) between band and ring.
3.6. Correlation between cardiac and mitral hemodynamic parameters
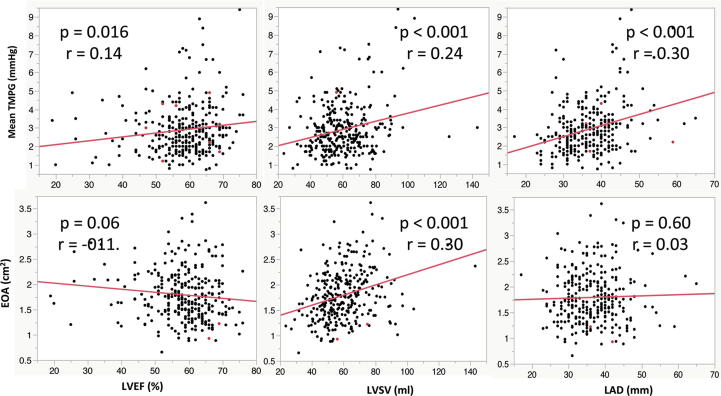
LVEF, LVSV and left atrial dimension (LAD) were significantly associated with an increase of TMPG (r = 0.14, p = 0.016, r = 0.24, p < 0.001, and r = 0.30, p < 0.001). EOA had a significantly positive correlation with LVSV (r = 0.30, p < 0.001) (Fig. 3).
Fig. 3.
Correlation between cardiac and mitral hemodynamic parameters. TMPG, transmittal pressure gradient, EOA, effective orifice area, LVEF, left ventricular ejection fraction, LVSV, left ventricular stroke volume, LAD, left atrial dimension.
4. Discussion
The major findings of this study are as follows: (1) there were significant differences in mean TMPG and EOA between different products of bands and rings; (2) greater annuloplasty size had a potential to improve not only mitral hemodynamics, but also pulmonary hypertension obtained by resting echocardiogram; (3) compared to a full ring, a partial band can reduce the mean TMPG and increase the EOA and LV output; (4) in patients with larger annuloplasty prostheses (≥30 mm), the type of annuloplasty prosthesis (band or ring) did not influence the mitral hemodynamics; and, (5) TMPG and EOA were influenced by LV function.
Several reports have compared full rings and partial bands for repair of degenerative MR. Spiegelstein, et al. compared the Physio ring and Cosgrove band. They concluded that a full ring provided a longer coaptation and durability while the partial band preserved motion of the aortic curtain [12]. Chan, et al. reported a slight elevation of mean TMPG (>3 mmHg) which can lead to worse intracardiac hemodynamics, as was observed in 98% of patients (41/42) with full ring [5]. Mesana, et al. compared outcomes of patients following mitral valve repair with either a complete ring or a partial band, measured by stress echocardiogram, and they confirmed greater mitral gradients and pulmonary pressures in patients repaired with a complete ring [13]. In this study, we obtained the same findings; however, we used a full ring for an anterior leaflet lesion. We found that different products had different geometric orifice areas and there were significant differences in mitral hemodynamic status among a variety of annuloplasty products, even if their sizes were equivalent. Also, in other studies, a smaller annuloplasty ring was associated with increased TMPG [14], [15]. We obtained similar findings, and that greater annuloplasty size improved not only mitral hemodynamics, but also LV output and pulmonary hypertension, measured by resting echocardiogram. However, to prevent FMS, the recommended size for each patient is still undetermined. According to our results, in patients with larger annuloplasty prostheses (≥30 mm), the mitral hemodynamic parameters were equivalent between bands and rings. A full ring with size ≥ 30 mm may be recommended to avoid FMS after mitral repair for Japanese with small BSA in this cohort. Therefore, this may not be applied for Western larger people.
On the other hand, there was a different issue which should be discussed regarding FMS after mitral repair. Mitral hemodynamic indices (TMPG and EOA) can be affected by transmitral flow provided by the left ventricular (LV) and atrial functions. Greater LV output can increase transmitral flow, and elevate TMPG. Therefore, a correlation between flow and gradient should be considered when evaluating FMS, such as high/low flow – high/low gradient aortic stenosis, which has been investigated in the field of transcatheter aortic valve replacement. Although EOA is a parameter calculated by reducing the influence of transmitral flow, EOA was correlated with LVSV (EOA = LVSV/MVVTI). Transmitral flow has less influence on the DVI, and the DVI had no significant correlation with size/type of annuloplasty prostheses and LV function. However, there was no significant correlation between the DVI and PASP; therefore, we may see left ventricular diastolic function and left atrial boost function when mitral hemodynamics are evaluated using EOA/TMPG; or, the DVI may not be appropriate for the accurate evaluation of mitral hemodynamics. In either case, it is still difficult to detect the influencing factors on mitral hemodynamics after mitral repair using various techniques and products. Stress echocardiogram is a more effective option to obtain detailed information under loading status [16], [17], [18]. Exercise-induced pulmonary hypertension (EIPH) is a cause of exercise intolerance and exertional dyspnea, and worsens clinical outcomes for left ventricular dysfunction and MR [19], [20], [21], [22]. Nevertheless, stress echocardiogram cannot completely eliminate the influence of LV function and exercise capacity, depending on non-cardiac factors. Additionally, a stress echocardiogram cannot be performed routinely for all patients in all institutes. Therefore, a simple and reproducible method is still required to know the impacts of mitral repair on hemodynamics.
4.1. Study limitations
There were several limitations. First, this study was a retrospective observational study in a single center. Therefore, there was selection bias in deciding the techniques and products for mitral repair. Atrial fibrillation may influence on mitral hemodynamics, since left atrial boost function reduced. Second, this cohort included physically small Japanese patients, and therefore annuloplasty size selection may be different with a variant population. The geometric orifice area is a more accurate measure than size, but the geometric orifice area of the partial band cannot be estimated perfectly and obtained as commercial data. Lastly, resting echocardiogram may not be appropriate to evaluate mitral hemodynamics.
5. Conclusions
Resting echocardiogram revealed that a larger partial band had the potential to improve not only mitral hemodynamics, but also pulmonary hypertension after mitral repair for degenerative disease. However, echocardiographic parameters on mitral hemodynamics were influenced by LV function. Therefore, a more accurate method is required to elucidate the true impact of mitral repair on hemodynamics.
Funding statement
None.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100517.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Comparison of transmittal pressure gradient (TMPG) and effective orifice area (EOA) between different partial band products.
Supplementary figure 2.
Comparison of transmittal pressure gradient (TMPG) and effective orifice area (EOA) between different full ring products.
References
- 1.Williams M.L., Daneshmand M.A., Jollis J.G., Horton J.R., Shaw L.K., Swaminathan M. Mitral gradients and frequency of recurrence of mitral regurgitation after ring annuloplasty for ischemic mitral regurgitation. Ann. Thorac. Surg. 2009;88:1197–1201. doi: 10.1016/j.athoracsur.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Kainuma S., Taniguchi K., Daimon T., Sakaguchi T., Funatsu T., Kondoh H. Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation. 2011;124:S97–S106. doi: 10.1161/CIRCULATIONAHA.110.013037. [DOI] [PubMed] [Google Scholar]
- 3.Ma W., Shi W., Wu W., Ma X., Kong Y., Zhu D., Zhang W. Patient-prosthesis mismatch in mitral annuloplasty for degenerative mitral regurgitation: an ignored issue. Eur. J. Cardiothorac. Surg. 2019 doi: 10.1093/ejcts/ezz086. [DOI] [PubMed] [Google Scholar]
- 4.Magne J., Sénéchal M., Mathieu P., Dumesnil J.G., Dagenais F., Pibarot P. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J. Am. Coll. Cardiol. 2008;51:1692–1701. doi: 10.1016/j.jacc.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 5.Chan K.L., Chen S.Y., Chan V., Hay K., Mesana T., Lam B.K. Functional significance of elevated mitral gradients after repair for degenerative mitral regurgitation. Circ. Cardiovasc. Imaging. 2013;6:1041–1047. doi: 10.1161/CIRCIMAGING.112.000688. [DOI] [PubMed] [Google Scholar]
- 6.Ma W., Shi W., Wu W., Ye W., Kong Y., Zhu D., Zhang W. Elevated gradient after mitral valve repair: The effect of surgical technique and relevance of postoperative atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2019;157:921–927. doi: 10.1016/j.jtcvs.2018.07.107. [DOI] [PubMed] [Google Scholar]
- 7.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Shim C.Y., Kim S.A., Choi D., Yang W.I., Kim J.M., Moon S.H. Clinical outcomes of exercise-induced pulmonary hypertension in subjects with preserved left ventricular ejection fraction: implication of an increase in left ventricular filling pressure during exercise. Heart. 2011;97:1417–1424. doi: 10.1136/hrt.2010.220467. [DOI] [PubMed] [Google Scholar]
- 9.Gargani L., Pignone A., Agoston G., Moreo A., Capati E., Badano L.P. Clinical and echocardiographic correlations of exercise-induced pulmonary hypertension in systemic sclerosis: a multicenter study. Am. Heart J. 2013;165:200–207. doi: 10.1016/j.ahj.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Chemla D., Humbert M., Sitbon O., Montani D., Herve P. Systolic and mean pulmonary artery pressures: are they interchangeable in patients with pulmonary hypertension? Chest. 2015;147:943–950. doi: 10.1378/chest.14-1755. [DOI] [PubMed] [Google Scholar]
- 11.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 12.Spiegelstein D., Moshkovitz Y., Sternik L., Fienberg M.S., Kogan A., Malachy A. Midterm results of mitral valve repair: closed versus open annuloplasty ring. Ann. Thorac. Surg. 2010;90:489–496. doi: 10.1016/j.athoracsur.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 13.Mesana T.G., Lam B.K., Chan V., Chen K., Ruel M., Chan K. Clinical evaluation of functional mitral stenosis after mitral valve repair for degenerative disease: potential effect on surgical strategy. J. Thorac. Cardiovasc. Surg. 2013;146:1418–1423. doi: 10.1016/j.jtcvs.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Doi K., Yamano T., Ohira S., Yamazaki S., Numata S., Yaku H. Annuloplasty ring size determines exercise-induced mitral stenosis severity after valve repair. J. Heart Valve Dis. 2015;24:744-51. [PubMed] [Google Scholar]
- 15.Kawamoto N., Fujita T., Fukushima S., Shimahara Y., Kume Y., Matsumoto Y., Yamashita K., Asakura K., Kobayashi J. Functional mitral stenosis after mitral valve repair for Type II dysfunction: determinants and impacts on long-term outcome. Eur. J. Cardiothorac. Surg. 2018;54:453–459. doi: 10.1093/ejcts/ezy062. [DOI] [PubMed] [Google Scholar]
- 16.Kubota K., Otsuji Y., Ueno T., Koriyama C., Levine R.A., Sakata R., Tei C. Functional mitral stenosis after surgical annuloplasty for ischemic mitral regurgitation: importance of subvalvular tethering in the mechanism and dynamic deterioration during exertion. J. Thorac. Cardiovasc. Surg. 2010;140:617–623. doi: 10.1016/j.jtcvs.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrand P.B., Verbrugge F.H., Verhaert D., Smeets C.J., Grieten L., Mullens W., Gutermann H., Dion R.A., Levine R.A., Vandervoort P.M. Mitral valve area during exercise after restrictive mitral valve annuloplasty: importance of diastolic anterior leaflet tethering. J. Am. Coll. Cardiol. 2015;65:452–461. doi: 10.1016/j.jacc.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samiei N., Tajmirriahi M., Rafati A., Pasebani Y., Rezaei Y., Hosseini S. Pulmonary arterial pressure detects functional mitral stenosis after annuloplasty for primary mitral regurgitation: An exercise stress echocardiographic study. Echocardiography. 2018;35:211–217. doi: 10.1111/echo.13744. [DOI] [PubMed] [Google Scholar]
- 19.Tumminello G., Lancellotti P., Lempereur M., D’Orio V., Pierard L.A. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur. Heart J. 2007;28:569–574. doi: 10.1093/eurheartj/ehl561. [DOI] [PubMed] [Google Scholar]
- 20.Marechaux S., Pincon C., Le Tourneau T., de Groote P., Huerre C., Asseman P. Cardiac correlates of exercise induced pulmonary hypertension in patients with chronic heart failure due to left ventricular systolic dysfunction. Echocardiography. 2008;25:386–393. doi: 10.1111/j.1540-8175.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- 21.Magne J., Lancellotti P., Pierard L.A. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010;122:33–41. doi: 10.1161/CIRCULATIONAHA.110.938241. [DOI] [PubMed] [Google Scholar]
- 22.Lancellotti P., Magne J., Dulgheru R., Ancion A., Martinez C., Pierard L.A. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. Am. J. Cardiol. 2015;115:1454–1461. doi: 10.1016/j.amjcard.2015.02.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.