Abstract
Fms-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) is a key predictive factor for the prognosis of acute myeloid leukemia (AML). We compared the detection sensitivity of fragment analysis with that of PCR-electrophoresis using MV4-11 (FLT3-ITD) and NKM-1 (FLT3-wild type) cell lines. DNA of these cells was mixed at different ratios and subjected to PCR-electrophoresis or fragment analysis. PCR-electrophoresis was found to have an FLT3-ITD allelic ratio (AR) detection limit of 0.034–0.072. Visual inspection of the PCR-electrophoresis revealed a lower detection sensitivity than that of fragment analysis. Therefore, it is essential to conduct fragment analysis when screening for FLT3-ITD.
Keywords: FLT3-ITD, Allelic ratio, Detection method, Sensitivity, Acute myeloid leukemia
1. Introduction
Complete remission can be achieved in 60–80% of cases with acute myeloid leukemia (AML) by using effective induction therapy [1]. Since, however, the five-year survival rate remains around 40%, both a finer classification of prognosis and the development of new treatment methods have been strongly called for. In recent years, developments such as the Fms-like tyrosine kinase 3 (FLT3) inhibitor, the isocitrate dehydrogenase 1/2 (IDH1/2) inhibitor, the B-cell lymphoma/leukemia-2 (Bcl-2) inhibitor venetoclax, and the ability to select a therapeutic agent based on concurrent gene abnormalities have added greatly to the field [2], [3], [4], [5], [6].
FLT3-internal tandem duplication (FLT3-ITD) mutations are observed in approximately 25–30% of AML cases and have become a key predictive factor for poor prognosis in AML [7,8]. Clinicians have proposed allogeneic hematopoietic stem cell transplantation (allo-HSCT) as a proactive intervention for AML patients during their first complete remission if they are FLT3-ITD-positive [9]. Recently, a classification system for patients has been proposed using the FLT3-ITD allelic ratio (AR), in which nucleophosmin (NPM1) positivity coupled with a low FLT3-ITD AR indicates a favorable prognosis. It has been suggested that such patients need not undergo allogeneic hematopoietic stem cell transplantation during the first complete remission [2,10]. However, given that FLT3-ITD has long been regarded as an unfavorable prognosis factor, several reports that are skeptical of this recommendation have been published. [11,12] With the introduction of FLT3 inhibitors, FLT3-ITD has become an even-more important indicator for determining subsequent treatment modalities.
In clinical settings, peripheral blood (PB) samples can be used to screen for FLT3-ITD if, for any reason, bone marrow (BM) specimens cannot be tested. It is thus hypothesized that similar levels of tumor cells are in the BM as in PB. One study has suggested that FLT3-ITD expression is greater in PB than in BM samples; another describes an exceedingly rare case in which FLT3-ITD was detected in only PB [13,14]. These reports indicate potentially different FLT3-ITD test results depending on the type of specimen examined.
FLT3-ITD detection methods include agarose gel electrophoresis following polymerase chain reaction (PCR-electrophoresis) and fragment analysis using capillary sequencing. The aim of this study was to compare the sensitivity of these FLT3-ITD detection methods as well as to analyze results between BM and PB samples.
2. Materials and methods
2.1. Patients
[Case 1]: a 39-year-old female, WBC 4000/μL, PB myeloblasts 13.0%, BM myeloblasts 22.6%, Hb 6.2 g/dL, platelets 11.4 × 104/μL, LDH 220 IU/L, normal karyotype, and FAB class M2. [Case 2]: a 62-year-old female, WBC 10,540/μL, PB myeloblasts 51.0%, BM myeloblasts 40.0%, Hb 9.0 g/dL, platelets 21.5 × 104/μL, LDH 481 IU/L, normal karyotype, and FAB class M2.
FLT3-ITD testing was conducted using samples taken at disease onset to determine the course of treatment. Case 1 provided a BM sample at our institution and PB sample at an outside lab, while Case 2 provided a PB sample at our institution and BM sample at an outside lab.
All samples were obtained at diagnosis after written informed consent in accordance with the Declaration of Helsinki. All the experiments were approved by the Ethics Committee at each institution.
2.2. FLT3-ITD detection methods
Following a previously reported method [15, 11], a 5′-GCAATTTAGGTATGAAAGCCAGC-3′ forward primer and 5′-CTTTCAGCATTTTGACGGCAAC-3′ reverse primer were used for PCR. For fragment analysis, a fluorescent marker was added at the 5′ end of the primers. Approximately 25 ng of DNA was added to a mixture of 0.2 mM of each primer with TaKaRa Taq (Takara Bio, Shiga, Japan) (0.25 µL TaKaRa Ex Taq polymerase, 4 µL dDNP mixture, and 5 µL Ex Taq Buffer) and the entire mixture was brought to an overall volume of 50 µl with sterile purified water. The resulting mixture was subjected to polymerase chain reaction amplification at 95 ℃ for 3 min, followed by 35 cycles at 98 ℃ for 5 s, 64 ℃ for 30 s, 72 ℃ for 1 min, and 72 ℃ for 7 min. The amplified products were electrophoresed through 3% agarose gels and visualized under UV light using ethidium bromide staining. Cases with an additional higher molecular weight band were identified as FLT3-ITD–positive. The outside laboratory used a TaKaRa PCR FLT3/ITD Mutation Detection Set (Takara Bio, Shiga, Japan) to perform gel electrophoresis in a similar fashion. The FLT3-ITD AR was analyzed by fragment analysis using Applied Biosystems 3130 and 3130xl Genetic Analyzers (Thermo Fisher, Carlsbad, CA). FLT3-ITD AR was calculated as the ratio of the area under the curve (AUC) of mutant to wild-type alleles (FLT3-ITD/FLT3wt). FLT3-ITD allelic frequency was calculated as the AUC of mutant alleles as a percentage of mutant and wild-type alleles. If there was more than one mutant, the AUCs were added together unless there were no cases associated with this scenario.
2.3. Sensitivity analysis of FLT3-ITD detection methods
MV4-11 cells were purchased from the American Type Culture Collection (ATCC) and NKM-1 cells were purchased from the JRBC cell bank. DNA was extracted from MV4-11 (FLT3-ITD) and NKM-1 (FLT3wt) cell lines, mixed at different ratios (Table 1), and subjected to PCR-electrophoresis or fragment analysis. Analyses were performed in triplicate for both DNA extracted from these cell lines and the patient samples. For each patient, the differences in the averages of the AR between BM and PB were assessed using an unpaired t-test. The resulting data was used to assess the relationship between PCR-electrophoresis sensitivity and fragment analysis.
Table 1.
Results of fragment analysis.
| Mixture sample no. | DNA | Calculated value | Actual value (fragment analysis) | ||
|---|---|---|---|---|---|
| MV4-11 (%) | NKM-1 (%) | Allele frequency (%) | Allele ratio (AR) | Allele ratio | |
| 1 | 100 | 0 | 100 | ∞ | ∞ |
| 2 | 33.3 | 66.7 | 33.3 | 0.499 | 0.511 ± 0.005 |
| 3 | 16.7 | 83.3 | 16.7 | 0.200 | 0.222 ± 0.002 |
| 4 | 13.3 | 86.7 | 13.3 | 0.153 | 0.172 ± 0.013 |
| 5 | 10 | 90 | 10 | 0.111 | 0.126 ± 0.007 |
| 6 | 6.7 | 93.3 | 6.7 | 0.072 | 0.080 ± 0.001 |
| 7 | 3.3 | 96.7 | 3.3 | 0.034 | 0.035 ± 0.001 |
| 8 | 1.7 | 98.3 | 1.7 | 0.017 | 0.022 ± 0.006 |
| 9 | 0.7 | 99.3 | 0.7 | 0.007 | 0 |
| 10 | 0.2 | 99.8 | 0.2 | 0.002 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 |
FLT3-ITD allele ratio (AR) was calculated as the ratio of the area under the curve (AUC) of mutant to wild-type alleles (FLT3-ITD/FLT3wt).
FLT3-ITD allele frequency was calculated as the AUC of mutant alleles as a percentage of mutant and wild-type alleles.
3. Results
3.1. Sensitivity comparison between PCR-electrophoresis and fragment analysis
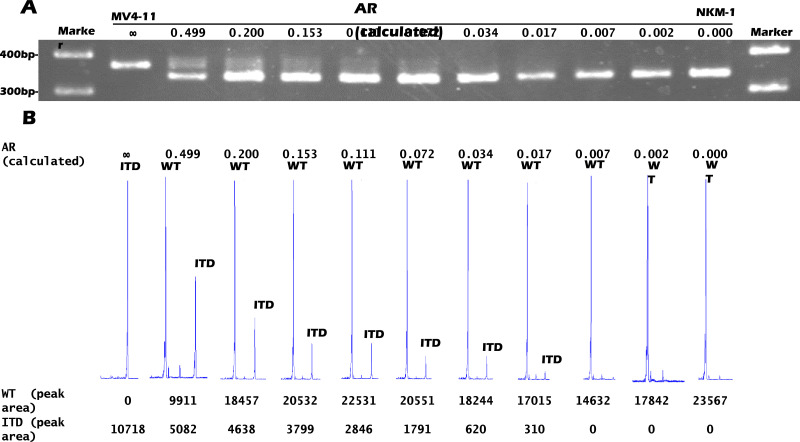
Results from PCR-electrophoresis sensitivity analysis using mixed MV4-11 and NKM-1 samples are shown in Fig. 1A. FLT3-ITD AR is reduced, and bands indicating abnormal FLT3-ITD are faint. Detection sensitivity of PCR-electrophoresis ranged between 0.034 and 0.072 for the AR. Results from the AR sensitivity analysis using mixed MV4-11 and NKM-1 samples are shown in Fig. 1B and Table 1. The fragment analysis was shown to be more sensitive than PCR-electrophoresis, seemingly detecting FLT3-ITD at ARs of approximately 0.017.
Fig. 1.
Results from PCR-electrophoresis and fragment analysis. DNA was extracted for PCR using mixtures of MV4-11 and NKM-1 cells in different proportions. (A) Results from agarose gel electrophoresis. Reduction in AF was accompanied by fainter FLT3-ITD bands. Bands indicating ITD were visible at a 0.072 AR but were difficult to confirm at lower ARs. Detection limit for FLT3-ITD was between AR = 0.034–0.072. (B) Results from fragment analysis. AR was calculated using WT and ITD waveform area. Detection limit for FLT3-ITD was AR = 0.017. AF: allelic frequency; WT: FLT3wt; ITD: FLT3-ITD; bp: base pair.
3.2. Detection comparison by FLT3-ITD analysis method for BM and PB samples
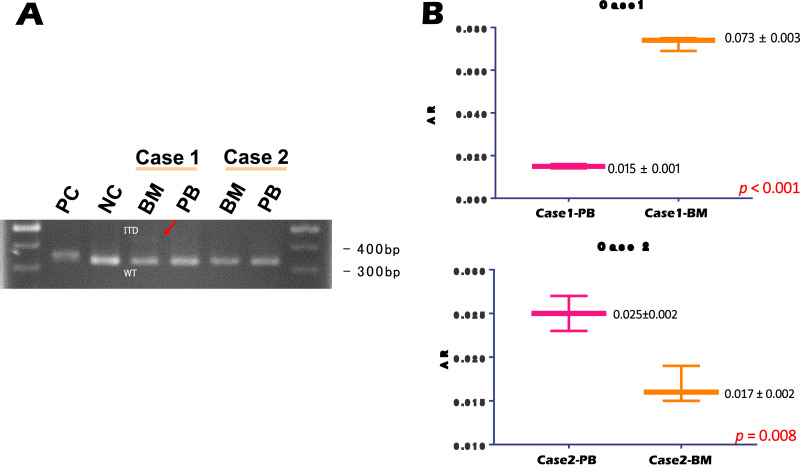
For Case 1, BM was positive for FLT3-ITD as analyzed by PCR-electrophoresis equipment at our institution, but PB samples were negative when analyzed by an outside lab. In contrast, Case 2 was negative for FLT3-ITD based on PB analysis using our in-house PCR-electrophoresis; however BM samples were positive upon analysis by the outside lab.
Since we did not observe particularly high PCR-electrophoresis sensitivity compared to fragment analysis in the previous analysis, each sample was also analyzed using both methods. PCR-electrophoresis resulted in positive FLT3-ITD in the BM from Case 1, but other samples were negative for FLT3-ITD (Fig. 2A). Alternatively, fragment analysis resulted in positive detection of FLT3-ITD for both BM and PB samples in both cases. However, the FLT3-ITD AR was lower in PB than in BM for Case 1, which was below the detection sensitivity for PCR-electrophoresis (Fig. 2A). Similarly, for Case 2, AR values in the BM and PB samples were both lower than the FLT3-ITD detection sensitivity for PCR-electrophoresis (Fig. 2B). The above results suggest that PCR-electrophoresis does not provide high enough sensitivity when detecting FLT3-ITD; thus, samples with low FLT3-ITD AR may provide false negative results when analyzed via PCR-electrophoresis-based tests alone.
Fig. 2.
Results from PCR-electrophoresis and fragment analysis. (A) Results from PCR-electrophoresis. Case 1-BM exhibited a faint double band indicating FLT3-ITD. There were no clear double bands observed for Case 1-PB, Case 2-BM, or Case 1-PB. (B) Results from fragment analysis. In Case 1, the BM AR was near the detection limit of PCR-electrophoresis. Moreover, FLT3-ITD AR was present at lower levels in PB compared to those in BM. In Case 2, AR was observed at levels lower than the PCR-electrophoresis sensitivity range in both BM and PB. PC: positive control; NC: negative control; BM: bone marrow; PB: peripheral blood; bp: base pair; AR: allelic ratio.
4. Discussion
We found that the detection sensitivity of PCR-electrophoresis for AR is between 0.034 and 0.072, whereas the detection sensitivity of fragment analysis for AR is approximately 0.017. We can conclude that fragment analysis is more sensitive and results in fewer false negatives than PCR-electrophoresis. For Case 1, in which the percentage of myeloblasts in PB was approximately half that observed in BM, FLT3-ITD was undetectable by PCR-electrophoresis of PB. In similar cases with low myeloblast percentages, clinicians should be aware that low FLT3-ITD AR may cause a false negative result when employing PCR-electrophoresis. To clarify the present results, a comparison between the PB and BM from a larger number of cases would be beneficial. Unfortunately, we were only able to examine two cases in this report, but are planning to carry out an analysis of a larger number of cases in the future.
Cases like ours, in which the AR varies between BM and PB, are exceedingly rare. This may warrant caution when interpreting FLT3-ITD test results. Determinations based on PCR-electrophoresis testing rely on visual confirmation of detection bands, meaning the potential for interpretation error is always present. In Case 2, FLT3-ITD was positive in the BM sample as analyzed by the outside lab, but negative in the same sample analyzed by PCR-electrophoresis in-house. When the PCR-electrophoresis band is very light, it is difficult to make an accurate visual judgment on whether it is indicative of FLT3-ITD. Moreover, there is concern that abnormal bands may be difficult to visualize due to low quantities of tumor cells in a given sample. It is therefore essential to perform fragment analysis in order to augment detection sensitivity.
Previously, we analyzed pairs of samples from AML patients taken at initial onset/diagnosis and relapse for genetic changes. Three of the 11 (23.7%) cases initially positive for FLT3-ITD were negative in subsequent testing, whereas 2/28 (8.7%) cases initially negative for FLT3-ITD tested positive after relapse [16]. Unlike primary AML, ‘early relapse’ cases with low myeloblast counts in the BM are not uncommon. In such cases, the more sensitive fragment analysis method is recommended in the event of a relapse, given that lower-sensitivity PCR-electrophoresis may result in a false negative.
European LeukemiaNet (ELN), and National Comprehensive Cancer Network (NCCN) guidelines advise that patients positive for mutant NPM1 with a FLT3-ITD AR of less than 0.5—corresponding to a favorable prognosis—should not undergo allo-HSCT treatment during the first complete remission [2,10]. According to these standards, since the prognosis differs at the boundary of 0.5, highly accurate measurement of AR is necessary for cases with ARs near 0.5. As shown in Fig. 2, fragment analysis appeared to be a reliable detection method, with the measurement error for this technique being approximately 0.002. In addition, we have reported that some cases with the same mutation profiles did not achieve a favorable prognosis, and therefore required allo-HSCT during the first complete remission [11]. As such, FLT3-ITD screening is an important factor when determining patient suitability for allo-HSCT.
Fragment analysis improves sensitivity and accuracy when screening for FLT3-ITD. However, challenges associated with high costs and labor-intensive practices cause clinical testing laboratories as well as many other facilities to employ PCR-electrophoresis, a simpler and cheaper method. However, it is important for clinicians to be aware of the low detection sensitivity of FLT3-ITD tests using PCR-electrophoresis.
With the emergence of FLT3 inhibitors, FLT3-ITD positive AML patients are expected to exhibit better prognoses [17]. Appropriate administration of this drug requires high-quality companion diagnostics. Clinicians must be aware that testing either PB or BM samples alone might lead to FLT3-ITD being overlooked. PCR-electrophoresis is cheap and simple to perform; however, its sensitivity and measurement error are inferior to those of fragment analysis, which can be used to avoid false negatives for FLT3-ITD and to measure AR more accurately. However, according to a retrospective analysis reported by the European Society for Blood and Marrow Transplantation (EBMT) in 2019, most institutions performing transplants were not stratifying results according to AR [18]. It is therefore recommended that future approaches to FLT3-ITD screening utilize fragment analysis.
Acknowledgments
Author contribution statement
MS, NN and HY were the principal investigators and take primary responsibility for the paper. MS, NN, KT, MM, KA, and TK performed the laboratory work for the study. MS, HY, UN, KS, AM, IO, YF, SY, SW, ND, KO and KI recruited the patients. MS, NN, HY, MS, and KI analyzed the data and wrote the paper. MS, NN and HY contributed equally to the study.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors thank the physicians who cared for patients and collected clinical data during this study.
References
- 1.Ohtake S., Miyawaki S., Fujita H., Kiyoi H., Shinagawa K., Usui N. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117(8):2358–2365. doi: 10.1182/blood-2010-03-273243. Feb 24. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Buchner T. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thol F., Gabdoulline R., Liebich A., Klement P., Schiller J., Kandziora C. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132(16):1703–1713. doi: 10.1182/blood-2018-02-829911. Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson D.R., Foster M.C., Coombs C.C., Zeidner J.F. Advances in genomic profiling and risk stratification in acute myeloid leukemia. Semin. Oncol. Nurs. 2019;35(6) doi: 10.1016/j.soncn.2019.150957. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlenk R.F., Weber D., Fiedler W., Salih H.R., Wulf G., Salwender H. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851. doi: 10.1182/blood-2018-08-869453. Feb 21. [DOI] [PubMed] [Google Scholar]
- 6.Dohner K., Thiede C., Jahn N., Panina E., Gambietz A., Larson R.A. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European Leukemianet in patients with acute myeloid leukemia. Blood. 2020;135(5):371–380. doi: 10.1182/blood.2019002697. Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaemmanuil E., Gerstung M., Bullinger L., Gaidzik V.I., Paschka P., Roberts N.D. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016;374(23):2209–2221. doi: 10.1056/NEJMoa1516192. Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakita S., Yamaguchi H., Ueki T., Usuki K., Kurosawa S., Kobayashi Y. Complex molecular genetic abnormalities involving three or more genetic mutations are important prognostic factors for acute myeloid leukemia. Leukemia. 2016;30(3):545–554. doi: 10.1038/leu.2015.288. Mar. [DOI] [PubMed] [Google Scholar]
- 9.O'Dnnell M.R., Tallman M.S., Abboud C.N., Altman J.K., Appelbaum F.R., Arber D.A. NCCN clinical practice guidelines in oncology (NCCN guidelines) Acute Myeloid Leuk. Version. 2014;2:2014. [Google Scholar]; Available at: http://williams.medicine.wisc.edu/aml.pdf. Accessed 1 April 2017.
- 10.O'Donnell M.R., Tallman M.S., Abboud C.N., Altman J.K., Appelbaum F.R., Bhatt V.R. NCCN clinical practice guidelines in oncology (NCCN guidelines®) acute myeloid leukemia version. 2018;3:2018. [Google Scholar]; https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed 15 Oct 2018.
- 11.Sakaguchi M., Yamaguchi H., Najima Y., Usuki K., Ueki T., Oh I. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018;2(20):2744–2754. doi: 10.1182/bloodadvances.2018020305. Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straube J., Ling V.Y., Hill G.R., Lane S.W. The impact of age, NPM1mut, and FLT3ITD allelic ratio in patients with acute myeloid leukemia. Blood. 2018;131(10):1148–1153. doi: 10.1182/blood-2017-09-807438. Mar 8. [DOI] [PubMed] [Google Scholar]
- 13.Jilani I., Estey E., Manshuri T., Caligiuri M., Keating M., Giles F. Better detection of FLT3 internal tandem duplication using peripheral blood plasma DNA. Leukemia. 2003;17(1):114–119. doi: 10.1038/sj.leu.2402743. Jan. [DOI] [PubMed] [Google Scholar]
- 14.Rogers A., Joe Y., Manshouri T., Dey A., Jilani I., Giles F. Relative increase in leukemia-specific DNA in peripheral blood plasma from patients with acute myeloid leukemia and myelodysplasia. Blood. 2004;103(7):2799–2801. doi: 10.1182/blood-2003-06-1840. Apr 1. [DOI] [PubMed] [Google Scholar]
- 15.Gale R.E., Green C., Allen C., Mead A.J., Burnett A.K., Hills R.K. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. Mar 1. [DOI] [PubMed] [Google Scholar]
- 16.Wakita S., Yamaguchi H., Omori I., Terada K., Ueda T., Manabe E. Mutations of the epigenetics-modifying gene (DNMT3a, TET2, IDH1/2) at diagnosis may induce FLT3-ITD at relapse in de novo acute myeloid leukemia. Leukemia. 2013;27(5):1044–1052. doi: 10.1038/leu.2012.317. Apr. [DOI] [PubMed] [Google Scholar]
- 17.Stone R.M., Mandrekar S.J., Sanford B.L., Laumann K., Geyer S., Bloomfield C.D. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazarbachi A., Labopin M., Battipaglia G., Djabali A., Forcade E., Arcese W. Allogeneic stem cell transplantation for FLT3-mutated acute myeloid leukemia: in vivo T-cell depletion and posttransplant sorafenib maintenance improve survival. A retrospective acute leukemia working party-European society for blood and marrow transplant study. Clinical Hematology International. 2019;1(1):58–74. doi: 10.2991/chi.d.190310.001. eISSN: 2590. [DOI] [PMC free article] [PubMed] [Google Scholar]