Abstract
Treponema pallidum infections have been primarily known as slightly contagious mucocutaneous infections called yaws (tropical Africa and America) and bejel (subtropical North Africa). T. pallidum emerged as a highly infectious venereal syphilis agent in South America, probably about 500 years ago, and because of its venereal transmission, it quickly caused a worldwide pandemic. The disease manifests as lesions, including a chancre; then antibodies become detectable when or slightly after the chancre appears, and before the development of a rash and other systemic manifestations. Venereal diseases are poorly known in monkeys. During fieldwork in Senegal, we discovered an epizootic outbreak of venereal disease that we explored. We detected a venereal form of T. pallidum subsp. pertenue infection in green monkeys (Chlorocebus sabaeus), then observed an epizootic outbreak in Senegal and its spread among baboons a year later. Comparative analysis of T. pallidum genomes from the monkeys' chancres and other Treponema genomes showed an acceleration of the number of single nucleotide polymorphisms, comparable to that observed in syphilis. Identified T. pallidum clones seem to be epizootic through the acceleration of their mutation rate, which is linked to their larger diffusion.
Keywords: Baboon, Green monkey, Senegal, Syphilis, Treponema, West Africa
Introduction
Treponema pallidum infections have been primarily presented as slightly contagious mucocutaneous infections, called yaws (tropical Africa and America) and bejel (subtropical North Africa). Overall, the origin of treponematoses and relations between the pandemic venereal syphilis and endemic treponematoses (yaws, bejel and pinta) are not yet clear; even palaeontologic evidence is not confirmative [1]. Although endemic treponematoses underwent a mass eradication campaign in the 1950s and 1960s [2], all treponematoses are now coming back [3], so there is an urgent need to understand the pathogens that cause these diseases in order to eradicate them.
Moreover, treponemes cannot easily be cultured in traditional laboratory settings [4,5]. This is one reason why we know so little about these bacteria. Comparative genomics is one way to better understand the biology of these bacteria. Whole-genome sequencing of agents of syphilis [6], yaws [7] and bejel [8] show subtle differences (mostly single nucleotide polymorphism (SNP)) among these agents, with almost the same genome structure and less than 0.2% divergence [7]. The current dominant theory is that syphilis appeared as a venereal epidemic caused by a clone derived from the endemic yaws agent, Treponema pallidum subsp. pertenue, in pre-Columbian America [9] and quickly caused a worldwide pandemic after Columbus's arrival.
The first evidence of endemic T. pallidum infection in monkeys, although without genital lesions, was reported in Guinea baboons (Papio papio) in Senegal and Guinea [[10], [11], [12], [13]] via serology. An isolate (Fribourg-Blanc) was also obtained from a popliteal lymph node. This strain is pathogenic in hamsters [12] and humans [14], and is genetically quite similar to human yaws [15]. Since then, monkeys and apes have been found to experience endemic treponematoses comparable to yaws [16]. Genital lesions caused by T. pallidum in nonhuman primates including Papio anubis, Papio cynocephalus, Chlorocebus pygerythrus and Cercopithecus mitis were reported in Tanzania [12,[17], [18], [19]], suggesting sexual transmission. Serologic evidence of treponemal infection without lesions was also reported in Guinea baboons in Senegal in 2013–2014 [18] and in Tanzania [19,20].
During fieldwork in Senegal for the collection of monkey specimens originally planned for a study on simian immunodeficiency viruses, we discovered by chance an epidemic of venereal disease that we explored. Thus, we report here what is to our knowledge the first observed epidemic of venereal treponematosis in wild monkeys.
Materials and methods
Study population
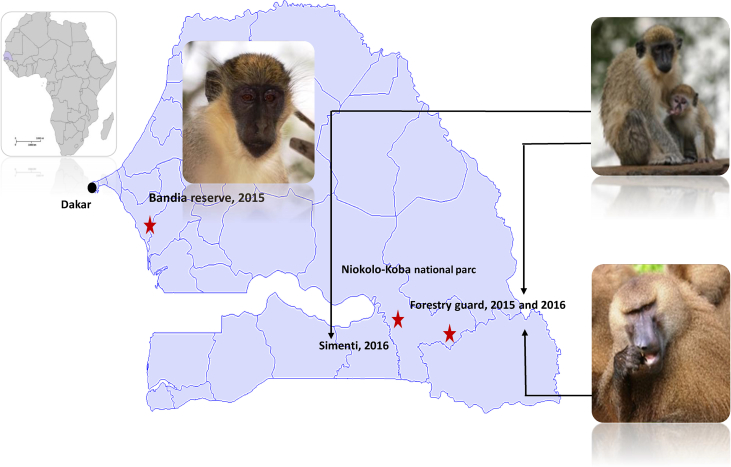
Green monkeys and baboons were studied in two different nature parks in Senegal (West Africa). Niokolo-Koba National Park is a World Heritage Site and one of the oldest natural protected areas in Africa. It is situated in south-eastern Senegal near the border of Guinea. The Bandia reserve is a small private natural reserve situated 65 km south of Dakar, the capital of Senegal. One population in each park was studied in 2015: the green monkey group living in the proximity of the welcome centre of the Bandia natural reserve (14°33′35.5″N 17°00′30.6″W) and the group of monkeys in the Niokolo forestry guardhouse in Niokolo-Koba National Park (13°04′28.6″N 12°43′18.2″W). In 2016 in Niokolo-Koba National Park we studied the same population of green monkeys in the Niokolo forestry guardhouse as well as Guinea baboons living in sympatry and another population of green monkey from the Simenti site (13°01′34.5″N 13°17′40.9″W).
Ethics statement
The study was approved by the Senegalese Ministry of the Environment (Direction of the National Parks, no. 1302, 16 October 2015; and no. 904, 12 July 2016) and was performed in accordance with the recommendations of the Weatherall report.
Sample collection
In November 2015, three clinically healthy male green monkeys (Chlorocebus sabaeus) were captured in the reserve of Bandia, and 13 individuals, 11 male and 2 female monkeys, were captured in Niokolo-Koba National Park. Among animals collected in the park, four male monkeys presented chancre-like lesions on the penis. Neither of the two female green monkeys exhibited clinically evident lesions. Animals were not tagged. In 2016, 21 green monkeys (including 14 males and seven females) were captured in Niokolo-Koba (two populations); among the male monkeys, four presented chancres. In addition, among 14 Guinea baboons (Papio papio; 13 male and 1 female) living in sympatry, six males presented with chancres (Supplementary Table S1). All individuals were anaesthetized using ketamine (10 mg/kg), which was administered intramuscularly via blowpipe or tranquilizer gun.
Cotton swab (Copan Diagnostics, Murrieta, CA, USA) samples were taken from the edge of a lesion in clinically affected individuals and from normal unaffected tissue in healthy individuals. Samples were immediately frozen in liquid nitrogen. Anal swabs were taken from all monkeys. Blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes from the femoral vein. Plasma and erythrocytes were separated by sedimentation during 3 hours, then frozen in liquid nitrogen. Samples were exported to France at −20°C, then transferred to a freezer and stored at −80°C. A tissue biopsy was performed of the chancre-like lesion of the penis of one baboon.
Histologic analysis and immunohistochemical detection of T. pallidum
A formalin-fixed, paraffin-embedded tissue specimen obtained from a baboon was cut to 3 μm thickness and stained routinely with haematoxylin and eosin. In order to detect the spirochetes, serial sections were also obtained for Warthin-Starry staining and immunohistochemical investigations. The immunohistochemical procedure used a rabbit polyclonal antibody specifically directed against T. pallidum diluted 1:100 as recommended by the manufacturer (Biocare Medical, Pacheco, CA, USA) with the Ventana BenchMark autostainer (Ventana, Tucson, AZ, USA). A negative control was performed using an irrelevant rabbit polyclonal antibody.
Western blot analysis
Anti–T. pallidum Western blot analyses (Euroimmun, Lübeck, Germany) were performed according to the manufacturer's instructions. Samples and negative controls (human sera previously tested negative for syphilis) were diluted in serum diluent (1:51), and 1.5 mL of the diluted samples was dispensed in each channel with a strip. After incubation for 30 minutes on a rocking shaker at room temperature, the diluted samples were aspirated and washed 3 times for 5 minutes with 1.5 mL of diluted universal buffer. Strips were then incubated with 1.5 mL of the enzyme conjugate (alkaline phosphatase–conjugated antihuman immunoglobulin G) solution for 30 minutes with agitation, pipetted and washed 3 times for 5 minutes with 1.5 mL of diluted buffer. Strips were further incubated on a rocking shaker with 1.5 mL of substrate solution for 10 minutes at room temperature. The substrate solution was pipetted, and strips were rinsed 3 times with cold deionized or distilled water and air dried.
The development of coloured bands on a strip indicated the presence of anti–T. pallidum immunoglobulin G antibodies. Strips with these bands were compared against a positive control strip with bands identifying TpN15, TpN17, p22 (unspecific), TpN45 and TpN47 protein antigens. A sample was considered positive if the test strip displayed more than one of the four specific bands (TpN15, TpN17, TpN45 and TpN47).
Molecular detection
Genomic DNA was extracted from cotton swabs with the QIAamp Tissue Kit (Qiagen, Hilden, Germany) with the EZ1 apparatus following the manufacturer's protocols. The genomic DNA was stored at −20°C under sterile conditions until the next stage of the investigation. DNA was stored at −20°C until assayed by PCR. The real-time quantitative PCR (qPCR) assays were performed to screen all samples using polA-based and flaA-based primers and probes (Supplementary Table S1) [21,22]. All qPCRs were performed by a CFX96 Real-Time system (Bio-Rad Laboratories, Marnes-La-Coquette, France) and the Eurogentec Master Mix Probe PCR kit (Eurogentec, Seraing, Belgium). We included T. pallidum DNA extracted from a syphilis patient's swab as a positive control and master mixes as a negative control for each assay. Samples were considered positive when the cycle threshold (Ct) was lower than 35 Ct. In order to identify the subspecies of T. pallidum infecting monkeys, we chose two genes (TENDBA_0136 and TENDBA_0859 based on T. pallidum endemicum genome) [8] that would enable us to characterize identified treponema (Supplementary Table S1).
Treponemal genome sequencing by in-solution hybridization capture
DNA extracts were fragmented using a Covaris S220 Focused ultrasonicator (Covaris, Woburn, MA, USA) in a volume of 130 μL low-EDTA TE buffer using settings to generate a modal 400 bp fragment size (intensity = 4, duty cycle = 10%, cycles per burst = 200, treatment time = 55 seconds, temperature = 7°C). Fragmented extracts were concentrated using the MinElute PCR purification kit and eluted into 2 × 10 μL low-EDTA TE buffer (Qiagen). DNA concentrations of all extracts were then measured using a Qubit dsDNA High Sensitivity kit (Thermo Fisher Scientific, Waltham, MA, USA). Libraries were prepared using the Accel-NGS 2S DNA PCR free library kit following the standard protocol, with unique single indexes (Swift Biosciences, Ann Arbor, MI, USA). Libraries were quantified using the KAPA HiFi Library Quantification Kit (Roche, Basel, Switzerland) and, when needed, subsequently amplified using the KAPA Hot Start Library Amplification Kit (Roche), with Illumina (San Diego, CA, USA) adaptor-specific primers, then requantified. Samples were pooled according to species. For baboon libraries, 100 ng per library was used (total pool mass, 700 ng), and for African green monkey libraries, 140 ng per library was used (total pool mass, 700 ng).
RNA baits covering the entire T. pallidum subsp. pertenue Fribourg-Blanc genome were designed with twofold tiling of 120 mer baits. We essentially followed the myBaits Sequence Enrichment for Targeted Sequencing protocol (version 2.3.1), but we only used 125 ng bait per capture round and a hybridization time of 20 hours (Arbor Biosciences, Ann Arbor, MI, USA). After this first round of capture, the capture product was amplified using the KAPA Hot Start Library Amplification Kit for 20 cycles. DNA was concentrated using a MinElute PCR Purification Kit, and 1 μg of DNA was used for the second round of hybridization capture, which was performed under the same conditions as for the first round of capture. The final capture product was amplified using the KAPA Hot Start Library Amplification Kit for 12 cycles, purified using the MinElute PCR Purification Kit, quantified using the KAPA HiFi Library Quantification Kit and diluted to 4 nM as input for an Illumina MiSeq run (version 3; 2 × 300 bp).
Bioinformatic and evolutionary analyses
The draft genome sequences of T. pallidum A10, T. pallidum A12, T. pallidum BNK07 and T. pallidum SNK05 have been deposited at European Bioinformatics Institute (EBI) and European Nucleotide Archive (ENA) respectively under the following accession numbers: OMPM01000001–OMPM01000006; OMHU01000001–OMHU01000103, OMHW01000001–OMHW01000017, OMHZ01000001–OMHZ01000216.
All other Treponema genomes (CP002103, CP016062, CP016065, CP010422, CP007548, CP002375, CP002376 and CP003902) were downloaded from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). After sequencing, reads from BNK07, A10 and A12 were mapped on the reference T. pallidum Fribourg-Blanc using CLC Genomics Workbench 7 (Qiagen) with the following thresholds: 0.7 for coverage and 0.8 for similarity. Consensus sequences were extracted from the mapped reads (>3 read threshold).
SNPs were calculated by CLC Genomics Workbench 7 on the basis of consensus sequences. Two hypervariable regions (positions 230–240 kb and 280–290 kb) were excluded from analysis. Genomes were annotated using Prokka [23] with default parameters. Regarding ortholog detection, we applied Proteinortho with default values [24]. All orthologous genes were aligned using Muscle [25], then concatenated. Phylogenetic reconstruction was performed using RaxML (randomized axelerated maximum likelihood) with the GTRCAT model and a bootstrap value of 100 [26].
In order to investigate the possible relationships among strains, the neighbour-joining network using the Bandelt method was constructed using the Network 5.0 programme (https://www.fluxus-engineering.com/sharenet.htm) using equal weights for all mutations [27].
Results
In November 2015, in order to study simian immunodeficiency viruses, we investigated and sampled two populations of green monkeys in Senegal, one from the Bandia private nature reserve and the other from Niokolo-Koba National Park (Fig. 1). In Niokolo-Koba, we serendipitously discovered typical chancrous lesions that looked like human syphilis on four of 14 monkey penises (Fig. 2 and Supplementary Fig. S1) that were positive for T. pallidum using two different specific qPCR assays (Supplementary Tables S1 and S2). Specific Western blot serology (Fig. 2) was also positive for the four symptomatic monkeys. Among the eight asymptomatic monkeys, only three were positive by Western blot analysis, but two females and three juvenile males were negative. All monkeys from the Bandia reserve tested negative.
Fig. 1.
Map of Senegal with locations of collection sites. Map created from Wikimedia Commons (https://commons.wikimedia.org/wiki/Atlas_of__world).
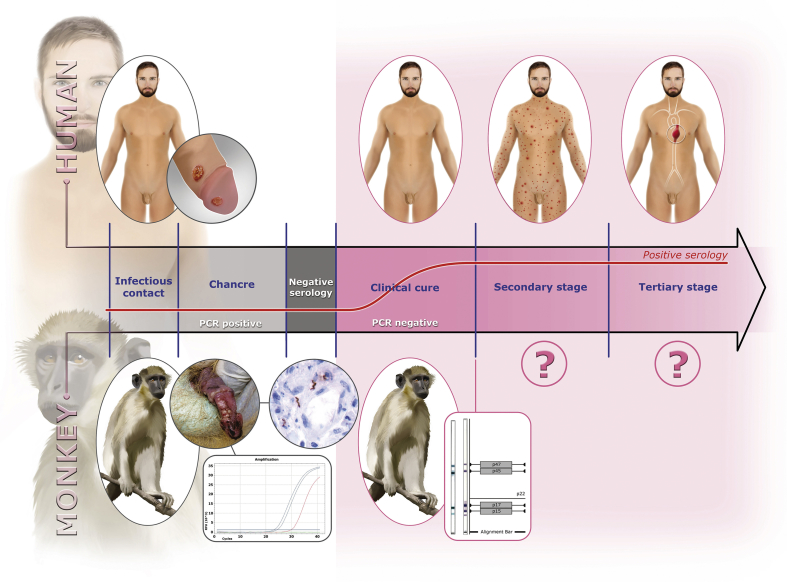
Fig. 2.
Parallel in-clinical course between human syphilis and treponematosis of green monkeys. Chancre was on penis of a green monkey (Niokolo forestry guardhouse) sampled in November 2015 (A09) and was PCR positive for Treponema pallidum. Visualization of T. pallidum (brown spirochetes) using immunohistochemical detection (original magnification ×400) was based on biopsy results of penis lesion found on baboon sampled in August 2016 (BNK14). Positive Western blot serology was obtained from green monkey sampled in August 2016 (SNK21).
During a second mission in August 2016, we observed the development of this venereal disease in various monkey populations, with the natural evolution of this venereal disease mimicking primary syphilis in humans. We studied two populations of green monkeys in Niokolo-Koba, including the same population studied in 2016. All captured monkeys were seropositive in both populations, with a significantly higher prevalence than in 2015 (7/12 in 2015 vs. 21/21 in 2016; Fisher exact test = 0.003, p < 0.05). Moreover, five of 21 monkeys were qPCR positive: three males with chancre-like genital lesions (genital swabbing), one female without lesions (genital swabbing) and one male without genital lesions (anal swabbing) (Supplementary Fig. S1). Although animals captured in 2015 were not tagged, serendipitously in 2016 we captured a remarkable one-eyed male—the same one we had seen the year before. His chancre had spontaneously regressed to a qPCR-negative scar (Supplementary Fig. S1), mimicking the natural history of primary syphilis in humans.
In August 2016, we also captured 14 Guinea baboons living in sympatry with green monkeys. We detected penis chancres on six baboons (Supplementary Fig. S1), all of which were qPCR positive. Moreover, six of 11 sampled serum samples were positive by Western blot analysis, including three without genital lesions. One PCR-positive monkey was seronegative, suggesting very recent infection. Spirochetes were stained in great numbers in the connective tissue of the foreskin using Warthin-Starry stain and by immunohistochemistry (Fig. 2 and Supplementary Fig. S2). Bacteria were mixed with an inflammatory infiltrate composed of lymphocytes, plasma cells and neutrophils (Supplementary Fig. S2). All monkeys with genital lesions observed in 2016 were then treated with injections of prolonged penicillin.
We selected two gene fragments presenting differences among most known T. pallidum subspecies. A 589 bp portion of the TENDBA_0136 gene coding for virulence-associated, Treponema-conserved hypothetical proteins that bind human fibronectin [28] was found to be identical for all qPCR-positive monkeys (both green monkeys and baboons). It was also identical to all strains except SamoaF strains of the yaws agent and Fribourg-Blanc Treponema. A 464 bp portion of the TENDBA_0859 gene coding for a protein of unknown function was identical in all green monkeys sampled in 2015 and 2016 as well as in one baboon (BNK14; Supplementary Table S2) sampled in 2016 and infected by the same Treponema as green monkeys. All other baboons had two identical point mutations differentiating them from all known Treponema strains, thus providing evidence of the circulation of another clone. The closest identity, however, was with Fribourg-Blanc Treponema (462/464) (Supplementary Fig. S3).
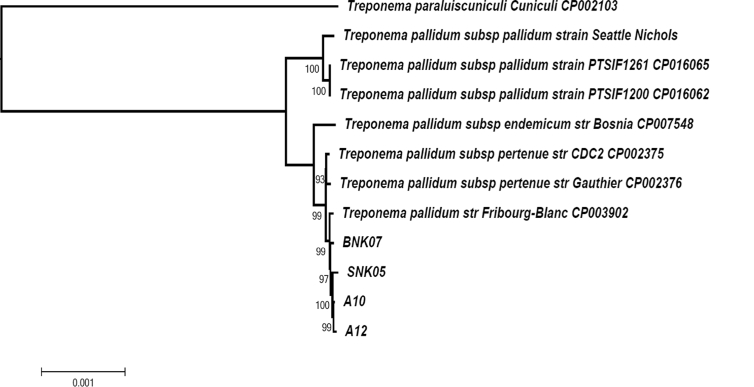
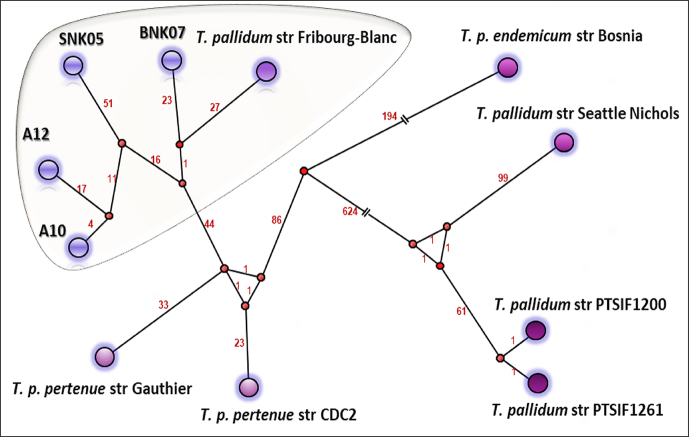
The clinical natural history and dynamics (2015–2016) of the discovered disease resembles that of syphilis: the appearance of a chancre harbouring Treponema before serologic response; the spontaneous healing of the chancre; and finally the appearance and persistence of antitreponemal antibodies (Fig. 2). We faced an epizootic outbreak of a bacterial infection. Such outbreaks are often caused by a single clone [[29], [30], [31], [32]], whereas endemic diseases are caused by several genotypes. To evaluate the evolutionary ratio of this putative clone, we sequenced it. We succeeded in almost entirely sequencing the genomes of Treponema from samples taken from the lesions of baboons (BNK07) and green monkeys (SNK05) sampled in 2016 as well as from two green monkeys (A10 and A12) sampled in 2015, and compared them to other open-source complete genomes. We found that they were nearly identical (Fig. 3 and Table 1). We obtained the sequence of the treponeme genome sequences from the geographical region where Fribourg-Blanc isolated his strain in 1966. This strain is thought not to have evolved much in the laboratory because most of the time since the 1960s, it has remained frozen. The relationships between different strains of subspecies of T. pallidum based on SNPs are also represented on the median joining network (Fig. 4).
Fig. 3.
Phylogenomic tree reconstruction based on core genomes of Treponema isolates including T. pallidum subspecies and monkey T. pallidum from present study.
Table 1.
Pairwise comparison of number of single nucleotide polymorphisms between different strains of Treponema pallidum
| A10 | A12 | BNK07 | SNK05 | T. pallidum str. Fribourg-Blanc CP003902 | T. pallidum subsp. endemicum str. Bosnia CP007548 | T. pallidum subsp. pallidum str. PTSIF1200 CP016062 | T. pallidum subsp. pallidum str. PTSIF1261 CP016065 | T. pallidum subsp. pallidum str. Seattle Nichols | T. pallidum subsp. pertenue str. CDC2 CP002375 | T. pallidum subsp. pertenue str. Gauthier CP002376 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A12 | 53 | ||||||||||
| BNK07 | 64 | 108 | |||||||||
| SNK05 | 112 | 155 | 145 | ||||||||
| T. pallidum str. Fribourg-Blanc CP003902 | 66 | 108 | 63 | 146 | |||||||
| T. pallidum subsp. endemicum str. Bosnia CP007548 | 371 | 397 | 368 | 439 | 372 | ||||||
| T. pallidum subsp. pallidum str. PTSIF1200 CP016062 | 801 | 837 | 797 | 863 | 802 | 848 | |||||
| T. pallidum subsp. pallidum str. PTSIF1261 CP016065 | 798 | 834 | 790 | 853 | 795 | 842 | 3 | ||||
| T. pallidum subsp. pallidum str. Seattle Nichols | 830 | 862 | 822 | 884 | 824 | 857 | 167 | 167 | |||
| T. pallidum subsp. pertenue str. CDC2 CP002375 | 101 | 145 | 105 | 185 | 94 | 342 | 777 | 769 | 799 | ||
| T. pallidum subsp. pertenue str. Gauthier CP002376 | 117 | 158 | 119 | 196 | 111 | 357 | 791 | 781 | 808 | 67 | |
| T. paraluiscuniculi str. Cuniculi CP002103 | 6269 | 6225 | 6237 | 6139 | 628 | 6291 | 6278 | 6281 | 6316 | 627 | 6268 |
Strains (str) A10 and A12 are from green monkeys sampled in November 2015, SNK05 from a green monkey sampled in August 2016 and BNK07 from a baboon sampled in August 2016.
Fig. 4.
Median joining network (MJN) representing relationships between different strains of subspecies of Treponema pallidum based on single nucleotide polymorphisms. Each circled area indicates unique genotype. Number of mutation steps between strains is indicated in red.
Thus, the SNP between Treponema from BNK07 (found in 2016) baboons and the Fribourg-Blanc strain (isolated 50 years ago) of only 63 bp may correspond to the natural evolution of the genome of T. pallidum—that is, 1.26 mutations per year. Interestingly, this is comparable with the generally accepted mutation rate in unicellular eukaryotes and prokaryotes of roughly 1 per 300 mutations (10−6 Mbp generation) per effective genome per cell generation [33] applied to one T. pallidum generation in 30 hours [34]. Moreover, the average number of 2111.7 nucleotide differences between syphilis and yaws strains [7] may be easily obtained by applying this mutation rate for the 500-year-old documented history of treponematoses in the Old World [7,8,15]. Therefore, the forgotten and now-neglected Hudson unitarian hypothesis [35,36], which states that all human treponematoses are caused by the same agent and that differences are due to different climate conditions and social habits of the targeted populations, may have a second life. However, others estimate the evolution time between yaws and syphilis agents to be about 10 000 years [37], although the most recent common ancestor of human syphilis strains evolved after the 15th century—precisely around 1744 [38]. The numbers of SNPs detected between strains isolated during a rapidly evolving outbreak in 2015 (A10, A12) and 2016 (SNK05) were 112 and 155, respectively. Although this may be explained by sampling existing genetic variations in this bacterial population, these SNP numbers are particularly high, while only 1 year separated both samplings, consequently reflecting the epidemic nature of these clones. The long branch regrouping Treponema strains of the current epizooty in monkeys on the phylogenetic tree (Fig. 4) also resembles that of the Treponema of syphilis.
Discussion
We believe that the emergence of an outbreak of venereal clonal treponematosis in monkeys may be a paradigm of human syphilis. A nonvenereal Treponema infection due to the agent of human yaws, T. pallidum subsp. pertenue, is known in monkeys in Western Africa [39]. We occasionally identified in 2015 what we think may be the beginning of an outbreak in green monkeys in West Senegal. The population of green monkeys in Niokolo-Koba is estimated to be 30 000 individuals [40]; in the Bandia reserve, situated about 550 km to the north-west, no estimate is available and may be around several thousand. Within a few months, the tested population of green monkeys in Niokolo-Koba was infected. Moreover, the infection was found in baboons as well, in which we identified two genotypes, including one almost identical to that of the green monkeys. We think that it is compatible with the cross-species transmission of T. pallidum subsp. pertenue already reported in Côte d’Ivoire [41]. Although only a few green monkeys were studied in the Bandia reserve, the absence of venereal treponematosis (both acute and serologic) may mean that the presumptive outbreak has not yet reached this site. We speculate that this outbreak resembles the emergence of syphilis, which started as a local venereal disease stemming from endemic yaws in America and became rapidly pandemic after Columbus's arrival [42].
The difference between yaws and syphilis is that yaws is slightly contagious mucocutaneous infection and syphilis is a highly contagious venereal disease. Syphilis develops in three stages [43]: an inoculation chancre where treponema is found and where antibodies are not found; spontaneous healing of the chancre; and antibodies found for life. We first detected these antibodies in monkeys by Western blot test; then we saw a dramatic extension of syphilis in a year from part of the population to the entire population (based on serology). The late clinical manifestations, secondary (rash) and tertiary syphilis (gum, neurologic and vascular involvements), are currently poorly described in monkeys [39,41] and must be investigated. The rash, however, may be invisible in monkeys.
Genomic studies showed that the bacterium we studied was quite close to the agent of yaws (once endemic in Senegal), with a number of SNPs comparable to a rapid and epidemic spread of syphilis observed elsewhere. In addition, we hypothesize that this clone was transferred to baboons (on the basis of the identification of a close strain found in one baboon). This suggests that the disease could spread rapidly to different primates. Thus, this strain of Treponema may become epidemic; an outbreak may originate from the emergence of a clone. In treponemes, this has already happened once in humans with the global syphilis epidemic.
The genomic era allows a paradigm change. It has become clear that in most cases (such as the case described here, but also for tuberculosis with the Beijing clone [29], with Clostridium difficile clones [30], with typhoid [31] and with cholera [32]) epidemics are caused by clones emerging among numerous endemic strains after usually just a few genetic modifications. Currently, genomic studies carried out on these bacteria have made it possible to show, with respect to endemic strains, an increase in the number of SNPs, which makes it possible to identify the epidemic clones because their multiplication number is greater than that of the endemic species. We believe that we documented the beginning of an epizooty of treponematosis. Its risk of dissemination to other populations of monkeys and apes will require active surveillance of these primate populations.
These results lead to at least two important questions regarding the possible emergence of an epidemic of treponematoses. Firstly, could this zoonosis threaten to become the reservoir of a new disease in humans, as has already happened with HIV [44]? Secondly, could prevention be considered via the systematic treatment of monkey populations in which one or more cases were found to be positive—for example, by administering penicillin in food?
Conflict of interest
None declared.
Acknowledgements
We are deeply grateful to S. Calvignac-Spencer and F. H. Leendertz (Robert Koch Institute, Berlin, Germany) for hosting and supervising hybridization capture experiments, performing part of the sequencing efforts and carefully reading of the article. We are also grateful to C. Michelle (Aix Marseille Univ, MEФI) for her technical help. Supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, France, the National Research Agency (ANR), France under the programme ‘Investissements d'avenir,’ reference ANR-10-IAHU-03, Région Provence Alpes Côte d’Azur; and European funding from FEDER-PRIMI, European Regional Development Fund (ERDF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2020.100670.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Meyer C., Jung C., Kohl T., Poenicke A., Poppe A., Alt K.W. Syphilis 2001—a palaeopathological reappraisal. Homo. 2002;53:39–58. doi: 10.1078/0018-442x-00037. [DOI] [PubMed] [Google Scholar]
- 2.Guthe T., Willcox R.R. Treponematoses: a world problem. Chron World Health Organ. 1954;8:37–114. [Google Scholar]
- 3.Smajs D., Strouhal M. Uncultivable pathogenic treponemes. In: Tang Y.W., Sussman M., Liu D., Poxton I., Schwartzman J., editors. Molecular medical microbiology. 2nd ed. Academic Press; London: 2015. pp. 1421–1436. [Google Scholar]
- 4.Edmondson D.G., Hu B., Norris S.J. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. MBio. 2018;9(3) doi: 10.1128/mBio.01153-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fieldsteel A.H., Cox D.L., Moeckli R.A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981;32:908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser C.M., Norris S.J., Weinstock G.M., White O., Sutton G.G., Dodson R. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281(5375):375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 7.Cejkova D., Zobanikova M., Chen L., Pospisilova P., Strouhal M., Qin X. Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl Trop Dis. 2012;6:e1471. doi: 10.1371/journal.pntd.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudova B., Strouhal M., Zobanikova M., Cejkova D., Fulton L.L., Chen L. Whole genome sequence of the Treponema pallidum subsp. endemicum strain Bosnia A: the genome is related to yaws treponemes but contains few loci similar to syphilis treponemes. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacani L., Lukehart S.A. The endemic treponematoses. Clin Microbiol Rev. 2014;27:89–115. doi: 10.1128/CMR.00070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fribourg-Blanc A., Niel G., Mollaret H.H. Note on some immunological aspects of the African cynocephalus. 1. Antigenic relationship of its gamma globulin with human gamma globulin 2. Guinean endemic focus of treponematosis. Bull Soc Pathol Exot Filiales. 1963;56:474–485. [PubMed] [Google Scholar]
- 11.Fribourg-Blanc A., Mollaret H.H., Niel G. Serologic and microscopic confirmation of treponemosis in Guinea baboons. Bull Soc Pathol Exot Filiales. 1966;59:54–59. [PubMed] [Google Scholar]
- 12.Fribourg-Blanc A., Mollaret H.H. Natural treponematosis of the African primate. Primates Med. 1969;3:113–121. [PubMed] [Google Scholar]
- 13.Baylet R., Thivolet J., Sepetjian M., Bert J. Seroepidemiological studies on primate treponematosis in Senegal. Bull Soc Pathol Exot Filiales. 1971;64:836–841. [PubMed] [Google Scholar]
- 14.Smith J.L., David N.J., Indgin S., Israel C.W., Levine B.M., Justice J., Jr. Neuro-ophthalmological study of late yaws and pinta. II. The Caracas project. Br J Vener Dis. 1971;47:226–251. doi: 10.1136/sti.47.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zobanikova M., Strouhal M., Mikalova L., Cejkova D., Ambrozova L., Pospisilova P. Whole genome sequence of the Treponema Fribourg-Blanc: unspecified simian isolate is highly similar to the yaws subspecies. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knauf S., Liu H., Harper K.N. Treponemal infection in nonhuman primates as possible reservoir for human yaws. Emerg Infect Dis. 2013;19:2058–2060. doi: 10.3201/eid1912.130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knauf S., Batamuzi E.K., Mlengeya T., Kilewo M., Lejora I.A., Nordhoff M. Treponema infection associated with genital ulceration in wild baboons. Vet Pathol. 2012;49:292–303. doi: 10.1177/0300985811402839. [DOI] [PubMed] [Google Scholar]
- 18.Knauf S., Barnett U., Maciej P., Klapproth M., Ndao I., Frischmann S. High prevalence of antibodies against the bacterium Treponema pallidum in Senegalese Guinea baboons (Papio papio) PLoS One. 2015;10 doi: 10.1371/journal.pone.0143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuma I.S., Batamuzi E.K., Collins D.A., Fyumagwa R.D., Hallmaier-Wacker L.K., Kazwala R.R. Widespread Treponema pallidum infection in nonhuman primates, Tanzania. Emerg Infect Dis. 2018;24:1002–1009. doi: 10.3201/eid2406.180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman D.M., Hardgrove E.H., von Fricken M.E., Kamau J., Chai D., Mutura S. Endemicity of yaws and seroprevalence of Treponema pallidum antibodies in nonhuman primates, Kenya. Emerg Infect Dis. 2019;25:2147–2149. doi: 10.3201/eid2511.190716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie D.E., Azzato F., Karapanagiotidis T., Leydon J., Fyfe J. Development of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay’s performance by comparison with serological testing. J Clin Microbiol. 2007;45:93–96. doi: 10.1128/JCM.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar J.C., Rathi A., Michael N.L., Radolf J.D., Jagodzinski L.L. Assessment of the kinetics of Treponema pallidum dissemination into blood and tissues in experimental syphilis by real-time quantitative PCR. Infect Immun. 2007;75:2954–2958. doi: 10.1128/IAI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. [Google Scholar]
- 24.Lechner M., Findeiss S., Steiner L., Marz M., Stadler P.F., Prohaska S.J. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinform. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar R.C. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandelt H.J., Forster P., Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 28.Brinkman M.B., McGill M.A., Pettersson J., Rogers A., Matejkova P., Smajs D. A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect Immun. 2008;76:1848–1857. doi: 10.1128/IAI.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merker M., Blin C., Mona S., Duforet-Frebourg N., Lecher S., Willery E. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M., Miyajima F., Roberts P., Ellison L., Pickard D.J., Martin M.J. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt K.E., Parkhill J., Mazzoni C.J., Roumagnac P., Weill F.X., Goodhead I. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet. 2008;40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weill F.X., Domman D., Njamkepo E., Tarr C., Rauzier J., Fawal N. Genomic history of the seventh pandemic of cholera in Africa. Science. 2017;358(6364):785–789. doi: 10.1126/science.aad5901. [DOI] [PubMed] [Google Scholar]
- 33.Drake J.W., Charlesworth B., Charlesworth D., Crow J.F. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaFond R.E., Lukehart S.A. Biological basis for syphilis. Clin Microbiol Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudson E.H. Treponematosis and anthropology. Ann Intern Med. 1963;58:1037–1048. doi: 10.7326/0003-4819-58-6-1037. [DOI] [PubMed] [Google Scholar]
- 36.Hudson E.H. Treponematosis in perspective. Bull World Health Organ. 1965;32:735–748. [PMC free article] [PubMed] [Google Scholar]
- 37.Strouhal M., Mikalova L., Havlickova P., Tenti P., Cejkova D., Rychlik I. Complete genome sequences of two strains of Treponema pallidum subsp. pertenue from Ghana, Africa: identical genome sequences in samples isolated more than 7 years apart. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arora N., Schuenemann V.J., Jager G., Peltzer A., Seitz A., Herbig A. Origin of modern syphilis and emergence of a pandemic Treponema pallidum cluster. Nat Microbiol. 2016;2:16245. doi: 10.1038/nmicrobiol.2016.245. [DOI] [PubMed] [Google Scholar]
- 39.Knauf S., Gogarten J.F., Schuenemann V.J., De Nys H.M., Dux A., Strouhal M. Nonhuman primates across sub-Saharan Africa are infected with the yaws bacterium Treponema pallidum subsp. pertenue. Emerg Microbe. Infect. 2018;7:157. doi: 10.1038/s41426-018-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groves C. 3rd ed. Johns Hopkins University Press; Baltimore, MD: 2005. Mammal species of the world. [Google Scholar]
- 41.Mubemba B., Gogarten J.F., Schuenemann V.J., Dux A., Lang A., Nowak K. Geographically structured genomic diversity of non-human primate–infecting Treponema pallidum subsp. pertenue. bioRXiv. 2019 doi: 10.1099/mgen.0.000463. https://www.biorxiv.org/content/10.1101/848382v1 [preprint] Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Melo F.L., de Mello J.C., Fraga A.M., Nunes K., Eggers S. Syphilis at the crossroad of phylogenetics and paleopathology. PLoS Negl Trop Dis. 2010;4:e575. doi: 10.1371/journal.pntd.0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peeling R.W., Mabey D.C. Syphilis Nat Rev Microbiol. 2004;2:448–449. doi: 10.1038/nrmicro914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keele B.F., Van H.F., Li Y., Bailes E., Takehisa J., Santiago M.L. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313(5786):523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.