Abstract
Objective
Research has shown that polycystic ovary syndrome (PCOS) is a common cause of infertility in women. The drugs used to treat PCOS tend to manage the symptoms rather than cure the disease. Furthermore, these drugs have severe side-effects and influence the quality of life for the patients. There is therefore a need for natural medicine that can effectively treat PCOS without side-effects.
Method
PCOS was induced in adult female Wistar rats by daily oral administration of letrozole (1 mg/kg) for 21 days. From day 22 until the end of the experiment (day 36), these rats were given a daily oral dose of either Prunus dulcis (walnut) or Salvia hispenica (chia seed) alone, or in combination. Animals were subsequently examined for morphological, biochemical, and histopathological changes.
Result
When compared with the control and standard groups, rats who had consumed P. dulcis and S. hispenica, either as individual agents or in combination, had significantly lower body and ovarian weights, and hormone concentrations were maintained at healthy levels. The presence of polyphenolic compounds in these substances induced ovulation in the PCOS model animals.
Conclusion
This study demonstrated that animals fed with P. dulcis and S. hispenica either individually or in combination were able to overcome infertility. Hormone levels and metabolism were restored in these animals. Therefore, P. dulcis and S. hispenica can be used as therapeutic agents to treat patients who are infertile due to suboptimal oocyte competence and anovulation.
Keywords: Clomiphene citrate, Letrozole, Polyphenolic compounds, Polycystic ovary morphology, Polycystic ovary syndrome
الملخص
أهداف البحث
أظهرت الأبحاث أن معظم السيدات يعانين من العقم بسبب متلازمة المبيض المتعدد الأكياس. الأدوية المستخدمة لعلاج متلازمة المبيض المتعدد الأكياس تميل أساسا لعلاج الأعراض بدلا من علاج المرض. إضافة إلى ذلك، تؤدي الأدوية إلى آثار جانبية حادة. هذه الآثار الضارة لها تأثير على جودة الحياة للمريضات أيضا. مما يستلزم الحاجة إلى دواء طبيعي يعالج بفاعلية متلازمة المبيض المتعدد الأكياس من دون آثار جانبية.
طرق البحث
تم تحريض فئران ويستر البالغة بمتلازمة المبيض المتعدد الأكياس عن طريق إعطائهم ليتروزول ١ مجم⁄ كجم لمدة ٢١ يوما عن طريق الفم. كما تم إعطاء برقوق دولسيز(الجوز) والقصعين الإسباني (بذور الشيا)، بشكل فردي ومعا، لفئران التجارب لمدة ١٥ يوما. أي من يوم ٢٢ إلى ٣٦ بعد إحداث متلازمة المبيض المتعدد الأكياس. بعد ذلك، تم فحص الفئران للخصائص الشكلية، والبيوكيميائية والمرضية.
النتائج
كان هناك انخفاض كبير في وزن الجسم ووزن المبيضين. وتم الحفاظ على الهرمونات على مستويات مطلوبة بعد استهلاك برقوق دولسيز والقصعين الإسباني بشكل فردي ومع بعضهم. حثت هذه المواد على الإباضة لدى حيوانات التجارب بواسطة منع ضعف خلايا المبيض بسبب وجود مركبات البوليفينول. تمت مقارنة هذه النتائج بمجموعات التحكم والمجموعات القياسية.
الاستنتاجات
تشير هذه الدراسة إلى أن الحيوانات التي تم تغذيتها ببرقوق دولسيز والقصعين الإسباني بشكل فردي ومعا منعا العقم. كما تمت استعادة مستويات الهرمون واضطرابات الأيض عند حيوانات التجارب. لذلك، يمكن استخدام برقوق دولسيز والقصعين الإسباني كعوامل علاجية لعلاج مرضى العقم بسبب ضعف البويضة والإباضة.
الكلمات المفتاحية: متلازمة المبيض المتعدد الأكياس, تشكل المبيض المتعدد الأكياس, ليتروزول, سترات الكلوميفين, مركبات البوليفينول
Introduction
Polycystic ovary syndrome (PCOS) is a leading cause of infertility in women worldwide and is an endocrine disorder that affects 15–20% of the female population at reproductive age. It is caused by an imbalance in the female reproductive system, which manifests as an excess of androgen, irregular menstrual cycles, ovulatory dysfunction, hirsutism, subfertility, anovulatory infertility, polycystic ovary morphology, and weight gain.1, 2, 3 First described by Stein and Leventhal in 1935,4 there is no solid evidence to indicate the cause of PCOS, but scientist believes that a high level of androgen and endogenous hormones could be the reason behind this disease.5 In PCOS, oestrogen dominance can arise from hyperandrogenism.6, 7, 8, 9, 10 Research studies have revealed that women with PCOS have metabolic abnormalities such as insulin resistance, dyslipidemia, obesity, type II diabetes mellitus, cardiovascular dysfunction, oxidative stress, and genetics problems.11, 12, 13 Ovarian function is affected by elevated luteinising hormone levels and hyperandrogenism, which leads to premature granulosa cell luteinisation and meiosis. Consequentially, the growth of antral follicles is arrested, and oocyte quality degrades.14,15 Mild PCOS is associated with normal ovulation and hyperandrogenism. This can progress to full PCOS over time when progesterone biosynthesis is affected and follicular oestrogen is produced.16.
Current treatments for PCOS have substantial side-effects such as joint and muscle pain, and arthritis, which decreases the quality of life for patients, and there is no definite cure for this disease.17,18 Furthermore, most of these treatments focused on managing the symptoms instead of curing the disease.19 Therefore, there is a need to find effective treatment in natural products, which can have fewer side-effects.20,21 In the last two decades, armed with extensive knowledge of the pathogenesis of this disease, researchers worldwide have focused on identifying PCOS treatments from natural medicines.
Prunus dulcis is a dry fruit that is used by a diverse number of ethnic groups for various ailments.22 People from 7000 BC have often used this as one of the key ingredients in traditional and alternative medicine.23 It contains secondary metabolites, including polyphenolic compounds such as terpenes, tannins, and essential oils,24 which have a variety of biological activities against inflammation,25 atherosclerosis,26 and oxidative damage.27 Salvia hispenica is an oilseed plant that belongs to the Lamiaceae family.28 The plant is native to Bolivia, Guatemala, Mexico, Ecuador, Argentina, and Australia,29 and contains many health-related substances such as phenolic compounds, dietary fibre, alpha-linolenic acid, vitamins, and proteins.30 As S. hispenica has a number of different secondary metabolites, it is known to have significant antimicrobial,31 antioxidant,32 anti-cancer,33 and pro-neurological properties.34 To the best of our knowledge, this is the first study to demonstrate that P. dulcis and S. hispenica, as either single agents or in combination, have an effect against the PCOS induced by letrozole in a rat model. This report suggests that these compounds are effective treatments for PCOS with fewer side-effects and is an important milestone in the development of naturally-derived PCOS treatments.
Materials and Methods
Collection of materials
Good quality P. dulcis and S. hispenica were procured from a local Ayurvedic supplier in Rajahmundry, Andhra Pradesh in February 2018.
Drugs and chemicals
Letrozole (Novartis Pharmaceuticals, India) and clomiphene citrate (Aventis, India) tablets were purchased from a local pharmacy in Rajahmundry.
Experimental animals
Virgin adult female Wistar albino rats (160–200 g) were procured from the Sainath Agencies, Hyderabad, Andhra Pradesh, India. The animals were housed and maintained under standard laboratory conditions: in controlled temperature (22 ± 3 °C), humidity (55 ± 5%), and a 12 h light/dark cycle. The animals had ad libitum access to a standard rat pellet diet and water.35,36 The experiment protocol was approved by the Institutional Animal Ethics Committee at GIET School of Pharmacy in Rajahmundry, Andhra Pradesh, India (GSP/IAEC/2017/03/03). All experimental animals were sacrificed using diethyl ether, and their ovaries were harvested.
Experimental method
Experimental animals that were induced to undergo PCOS by letrozole were fed P. dulcis and S. hispenica. Animals were initially divided into six groups (n = 6): a negative control, a positive control (placebo), a standard, and three treatment groups. Group 1 (negative control) was given daily oral doses of 1 mg/kg in 0.5% carboxymethyl cellulose (CMC) for 21 days. The remaining animals were administered with 1 mg/kg letrozole daily for 21 days to induce PCOS,37 and were further divided into five groups. Group 2 rats were the positive control group and were administered with 40 g of placebo. Groups 3–5 were the treatment groups, with group 3 receiving an oral dose of S. hispenica (40 g/day); group 4, an oral dose of P. dulcis (40 g/day); and group 5, an oral combination of P. dulcis and S. hispenica (20 g each). A sixth group was also administered with an oral dose of clomiphene citrate at 1 mg/kg in 0.5% CMC (standard group). Other than group 1, all groups were treated with their respective treatments for 15 days (from day 22–36). The animals were anaesthetised and sacrificed with diethyl ether at the end of the experiment, and the organs were extracted to assess for various morphological, biochemical, histopathological investigations.38
Morphological parameters
Body weight measurements
Animal body weight was measured by placing the animal on a weighing scale. The body weight of individual rats was recorded on day 1 and 37 as a difference in weight between these two days.39
Ovarian weight measurements
The ovaries were removed after the rats were euthanised and dissected, and were washed thoroughly with 0.9% saline solution to remove the uterine tissue. The ovaries were then weighed with a weighing balance.40
Biochemical analysis
Blood samples were collected from the experimental rats by retro-orbital puncture 24 h after the last dose of treatment and stored in Eppendorf tubes containing heparin sodium for further processing. The plasma samples for hormone assays were separated by centrifugation at 3000 rpm/min for 15 min and stored in a freezer at 20 °C. The oestradiol and testosterone levels were determined by a chemiluminescent immunoassay method using a commercially available kit (Siemens, New York, NY, USA, mean inter- and intra-assay coefficient of variation (CV) of 4.4% and 6.2%, respectively). To measure the plasma progesterone and cholesterol levels, we used a microparticle enzyme immunoassay and a method published by Azeez et al.,41 using a commercially available kit (Court-Acount progesterone; Los Angles, California, USA, mean inter- and intra-assay coefficient of variation (CV) of 14.4% and 4.9%, respectively). All blood sample measurements were recorded twice: 21 days after letrozole induction (that is, on the 22nd day of the study) and 15 days following treatment with P. dulcis and S. hispenica (that is, on the 37th day of study).42
Histopathological examinations
All rats were sacrificed at the end of the experiment according to the Committee for the Purpose of Control and Supervision of Experiments on Animals as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, and the ovaries were extracted, cleansed to remove fat, and weighed. Histopathological examination of these ovaries was carried out as per the standard procedure. Ovary sections were prepared from paraffin-embedded blocks and were stained with haematoxylin-eosin. Light microscopy was used to examine these sections.43
Statistical analysis
Results were statistically assessed by paired t-test and one-way ANOVA. The results of the treatment groups were compared to control and standard groups, respectively.44,45
Results
Body weight
Animals were weighed at the beginning of, and upon completion of the experiment (on the 37th day of the experiment), and expressed as a difference in body weight between these two days. The body weights of experimental animals decreased significantly after single or combination treatment with P. dulcis and/or S. hispenica. The body weight results are summarized in Table 1.
Table 1.
The body weight of experimental animals before and after administration of Prunus dulcis and Salvia hispenica.
| S.No | Group | Initial body weight Mean ± SEM (gms) n = 6 | Final body weight Mean ± SEM (gms) n = 6 | Difference in body weight Mean ± SEM (gms) n = 6 |
|---|---|---|---|---|
| 1 | Negative control | 198.50 ± 2.06 | 207.17 ± 2.44 | 8.67 ± 0.38 |
| 2 | Positive control | 193.17 ± 3.85∗∗ | 231.0 ± 3.01∗ | 37.83 ± 0.84∗ |
| 4 | Prunus Dulcis | 211.95 ± 3.86∗∗ | 227.33 ± 2.80∗∗ | 15.38 ± 1.06∗∗ |
| 5 | Salvia Hispenica | 198.94 ± 3.92∗ | 213.00 ± 5.89∗∗ | 14.06 ± 1.97∗ |
| 6 | Prunus Dulcis and Salvia Hispenica | 199.83 ± 4.55∗ | 212.83 ± 4.80∗∗ | 13.00 ± 0.25∗∗ |
| 3 | Standard (Clomiphene citrate) | 199.33 ± 2.99∗∗ | 211.50 ± 2.83∗ | 12.17 ± 0.16∗∗ |
∗∗P < 0.005, ∗P < 0.001 as compared to negative control. Statistical analysis-One way ANOVA.
Measuring ovarian weight
The weight of both ovaries extracted from the treatment groups (rats treated with P. dulcis and/or S. hispenica as single agents or in combination) was compared to those extracted from the control groups. These results are summarised in Table 2.
Table 2.
The effects of Prunus dulcis and Salvia hispenica on the weight of ovaries extracted from Wistar albino rats induced to undergo PCOS.
| S.No | Group | Left ovary (n = 6) gms | Right ovary (n = 6) gms |
|---|---|---|---|
| 1 | Negative control | 54.8950 ± 1.4316 | 55.7933 ± 1.3547 |
| 2 | Positive control | 61.8417 ± 1.3490∗∗ | 62.8267 ± 1.3754∗ |
| 3 | Prunus Dulcis | 60.0033 ± 1.2965∗∗ | 60.1827 ± 1.0246∗ |
| 4 | Salvia Hispenica | 58.7350 ± 1.3951∗ | 58.8217 ± 1.2856∗∗ |
| 5 | Prunus Dulcis and Salvia Hispenica | 57.5067 ± 1.1324∗∗ | 57.4583 ± 1.0989∗∗ |
| 6 | Standard (Clomiphene citrate) | 55.8583 ± 1.51300∗∗ | 56.9600 ± 1.0246∗ |
∗∗P < 0.005, ∗P < 0.001 as compared to blank and standard respectively. Statistical analysis-One way ANOVA.
Hormone profile
The experimental rats treated with P. dulcis and/or S. hispenica, and these rats produced testosterone and oestradiol within the normal ranges. Whereas, progesterone levels were lower in the letrozole-treated PCOS groups (groups 2–6) than in the negative control group. These natural products also affected the cholesterol level in these animals. The hormone levels of all animals tested are summarised in Table 3.
Table 3.
The effects of Prunus dulcis and Salvia hispenica on hormone levels in Wistar albino rats induced to undergo PCOS.
| S.No | Group | Testosterone (ng/dl) n = 6 | Progesterone (ng/dl) n = 6 | Estradiol (pg/dl) n = 6 |
|---|---|---|---|---|
| 1 | Negative control | 54.8500 ± 0.8164 | 32.4867 ± 0.5192 | 22.7867 ± 0.4189 |
| 2 | Positive control | 73.6167 ± 0.5944∗∗ | 21.2583 ± 0.6855∗ | 85.2833 ± 0.3952∗∗ |
| 4 | Prunus Dulcis | 63.1983 ± 0.8079∗ | 22.4900 ± 0.5210∗∗ | 35.1700 ± 0.4473∗ |
| 5 | Salvia Hispenica | 59.0417 ± 0.8618∗∗ | 24.6600 ± 0.3916∗ | 29.1150 ± 0.3968∗∗ |
| 6 |
Prunus Dulcis and Salvia Hispenica and Chia |
57.4800 ± 0.6058∗∗ | 25.4500 ± 0.2837∗∗ | 26.4767 ± 0.3856∗∗ |
| 3 | Standard (Clomiphene citrate) | 56.2733 ± 0.7069∗ | 28.2667 ± 0.5136∗ | 25.7417 ± 0.4434∗∗ |
∗∗P < 0.005, ∗P < 0.001 as compared to blank and standard respectively. Statistical analysis-One way ANOVA.
Histopathological studies
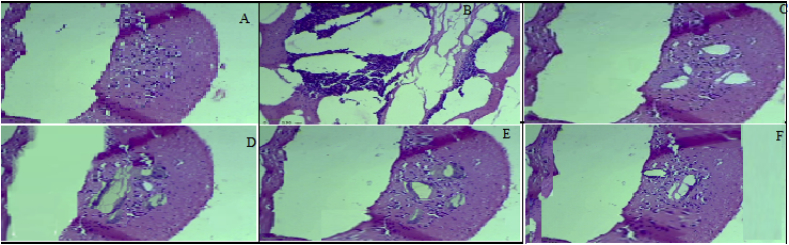
Transverse sections of the left and right ovaries were prepared and were histopathologically studied. The ovaries extracted from the negative control group had healthy follicles at various stages of maturation and were surrounded by a thick ovarian stroma and corpus luteum (Fig 1A). Letrozole causes tissue damage, inducing the formation of free radicals, which lead to the development of polycystic ovaries in the experimental animals. In the positive control group, this is demonstrated by the appearance of multiple cystic follicles lined by a thin granulosa layer, follicular atresia, stromal hyperplasia, and vacuolated stroma (Fig 1B). Prunus dulcis treatment reversed some of these follicular changes, with smaller cysts and fewer pyknotic granulosa cells (Fig 1C). In rats treated with Salvia hispenica, the effect of letrozole was completely reversed, with ovaries that had healthy-looking follicles and stroma (Fig 1D). Interestingly, this morphological reversal correlated with that of progesterone and oestrogen levels, which also returned to normal ranges. These effects were comparable to those observed in the rats treated with the standard drug (clomiphene citrate), which is known to mask letrozole's effect (Fig 1E).
Figure 1.
Histopathological sections of ovaries extracted from rats in all experimental groups. The images were captured using light microscopy after the sections were stained with haematoxylin-eosin. A) Negative Control group; B) Positive control (Placebo) group C) Standard clomiphene citrate-treated group D) Prunus dulcis-treated group E) Salvia hispenica-treated group F) Prunus dulcis and Salvia hispenica combined treatment group.
Discussion
This study was designed to identify the effect of P. dulcis and S. hispenica in the management of PCOS in rats. In this study, letrozole was used to induce PCOS in the experimental animals. Clomiphene citrate, which reverses letrozole's effect, was used as a marker to assess the effectiveness of the test compounds. Both letrozole and clomiphene citrate were prepared in 0.5% CMC and administered orally at a dose of 1 mg/kg as per published protocol. To induce PCOS, letrozole was administered to all rats for 21 days except those belonging to the negative control group (group 1). Group 2 animals were the positive controls for this experiment. Groups 3, 4, and 5 were given a daily dose of P. dulcis (40 g/kg), S. hispenica (40 g/kg), or a combination of both (20 g/kg each) respectively, for 15 days. Group 6 animals were treated with clomiphene citrate. After drug treatment, the body and ovarian weights, hormone levels, and the histopathological changes were measured from animals sacrificed on the 37th day of the experiment.
The results revealed that feeding rats with these natural compounds, either individually or in combination, significantly reduced the body weight of the experimental animals. The results revealed that feeding rats with these natural compounds reversed the effect of letrozole when PCOS was induced. This include a reduction of the body and ovarian weight gained from 199.83 ± 4.55, 57.5067 ± 1.1324 and 57.4583 ± 1.0989. The rats that were treated with a combination of both S. hispenica and P. dulcis exhibited a reduction of 13 ± 0.25 g in body weight, which was comparable to the clomiphene citrate-treated group. Furthermore, S. hispenica (Group 3) or P. dulcis (Group 4) treatments alone considerably increased the body weight of these rats by 14.06 ± 1.97 g and 15.38 ± 1.06 g, respectively. These results are summarised in Table 1.
Experimental animals were sacrificed on the 37th day of the experiment, and the left and right ovaries were extracted and weighed. The result showed that ovaries extracted from the combined-treatment animals did not form cysts, therefore the mean ovarian weight was 57.5067 ± 1.1324 (left) and 57.4583 ± 1.0989 (right). These values were similar to those of the negative control group. In animals that were treated with either compound, an ovarian mean weight of 58.7350 ± 1.3951, 58.8217 ± 1.2856 (S. hispenica) and and 60.0033 ± 1.2965, 60.1827 ± 1.0246 (P. dulcis) was observed. The weights of both ovaries are summarised in Table 2.
At the end of the study, hormone levels and histopathological changes were measured when the animals were sacrificed. In the letrozole-treated rats, both testosterone and oestradiol concentrations increased, whereas progesterone levels were reduced. Combination treatment with P. dulcis and S. hispenica restored hormone levels to normal in these animals because these products contained polyphenolic compounds. These plant products protect the ovaries from the harmful effects of letrozole, resulting in healthy ovarian stroma. Both hormone levels and histopathology of experimental animals are summarised in Table 3 and Figure 1. Similar results were also obtained in studies conducted by other scientists, who found that P. dulcis and S. hispenica were responsible for alleviating ovarian dysfunction by improving metabolic and endocrine parameters in tested animals.46, 47, 48, 49 Because of these benefits, natural compounds should be considered as viable treatment options for PCOS and other emerging diseases. These natural compounds should be further researched to investigate their role in the management of PCOS and other hormone-related diseases caused by the modern lifestyle.
Conclusion
Our study demonstrated that individual or combined treatment with P. dulcis and S. hispenica improves ovarian functions in letrozole-induced PCOS rats. Letrozole-induction is an accurate method to induce PCOS in experimental animals. These natural products contain polyphenolic compounds, which are the active ingredients to treat metabolic and reproductive dysfunctions. Therefore, hormone levels are restored in these experimental animals. Thus, P. dulcis and S. hispenica are promising agents for the management of PCOS and other metabolic disorders in women.
Recommendation
We recommend that further studies should be carried out on the therapeutic activities of P. dulcis and S. hispenica as we have shown that they have significant effects against metabolic and reproductive dysfunctions by restoring normal hormone levels without producing side-effects.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
There are no conflicts of interest.
Ethical approval
The experimental protocol was approved by the Institutional Animal Ethics Committee at the GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, India (GSP/IEAC/2017/03/03).
Authors contributions
SR designed the study and proposed the hypothesis that P. dulcis and S. hispenica can manage letrozole-induced PCOS in Wistar rats. CG and JN carried out the experiment and recorded all data from the animals. MDD contributed to the writing of the manuscript, in particular the structure of the manuscript and its pharmacological content. All authors have read and approved the final manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
All authors wish to express their gratitude to the GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, India, for providing the research facilities to carry out their experiments.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Maryse B., Youssef A., Jean-Patrice B. Basic infertility including polycystic ovary syndrome. Med Clin. 2008;92(5):1163–1192. doi: 10.1016/j.mcna.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Kawwass J.F., Loucks T.L., Berga S.L. An algorithm for treatment of infertile women with polycystic ovary syndrome. Middle East Fertil Soc J. 2010;15(4):231–239. [Google Scholar]
- 3.Legro R.S., Arslanian S.A., Ehrmann D.A., Hoeger K.M., Murad M.H., Pasquali R., Welt C.K. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical proactive guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azziz R., Adashi E.Y. Stein and leventhal: 80 years on. Am J Obstet Gynecol. 2016;214(2):e1–e247. doi: 10.1016/j.ajog.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfield R.L., Ehrmann D.A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allahbadia G.N., Merchant R. Polycystic ovary syndrome and impact on health. Middle East Fertil Soc J. 2011;16(1):19–37. [Google Scholar]
- 7.Witchel S.F., Oberfield S.E., Pena A.S. Polycystic ovary syndrome: pathophysiology, presentation and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545–1573. doi: 10.1210/js.2019-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunalan E., Yaba A., Yilmaz B. The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunction: a critical review. J Turk Ger Gynecol Assoc. 2018;19(4):220–232. doi: 10.4274/jtgga.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf W.M., Wattick R.A., Kinkade O.N., Olfert M.D. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Publ Health. 2018;15(11):2589. doi: 10.3390/ijerph15112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajivandi L., Noroozi M., Mostafavi F., Ekramzadeh M. A comprehensive interventional program for promoting eating behaviours in adolescent girls with polycystic ovarian syndrome (PCOS): protocol for a mixed methods study. Reprod Health. 2018;15:197. doi: 10.1186/s12978-018-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faghfoori Z., Fazelian S., Shadnoush M., Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab. 2017;11(1):S429–S432. doi: 10.1016/j.dsx.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metabol. 1998;9(2):58–61. doi: 10.1016/s1043-2760(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee B., Suri J., Suri J.C., Mittal P., Adhikari T. Impact of sleep-disordered breathing on metabolic dysfunctions in patients with polycystic ovary syndrome. Sleep Med. 2014;15(12):1547–1553. doi: 10.1016/j.sleep.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Ajmal N., Khan S.Z., Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol. 2019;3:100060. doi: 10.1016/j.eurox.2019.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minanni S.L., Marcondes J.A.M., Wajchenberg B.L., Ana M.C., Fortes M.A.H.Z., Rego M.A., Vezozzo D.P., Robard D., Giannella-Neto D. Analysis of gonadotropin pulsatility in hirsute women with normal menstrual cycles and in women with polycystic ovary syndrome. Fertil Steril. 1999;71(4):675–683. doi: 10.1016/s0015-0282(98)00514-7. [DOI] [PubMed] [Google Scholar]
- 16.Leo V.D., Musacchio M.C., Cappelli V., Massaro M.G., Morgante G., Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(38):27423183. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitek W., Alur S., Hoeger K.M. Off-label drug use in the treatment of polycystic ovary syndrome. Fertil Steril. 2015;103(3):605–611. doi: 10.1016/j.fertnstert.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Domecq J.P., Prutsky G., Mullan R.J., Sundaresh V., Wang A.T., Erwin P.J., Welt C., Ehrmann D., Montori V.M., Murad M.H. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4646–4654. doi: 10.1210/jc.2013-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Know C.Y., Lee B., Park K.S. Oriental herbal medicine and moxibustion for polycystic ovary syndrome: a meta-analysis. Medicine. 2018;97(43) doi: 10.1097/MD.0000000000012942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates G.W., Legro R.S. Longterm management of polycystic ovarian syndrome (PCOS) Mol Cell Endocrinol. 2013;373:91–97. doi: 10.1016/j.mce.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran S., Sanjay A.S., Dhanaraju M.D. Antiamnesic effect of Piracetam potentiated with Emblicaofficinalis and Curcuma longa in aluminium induced neurotoxicity of Alzheimer's disease. Int J Adv Res. 2013;1:185–196. [Google Scholar]
- 22.Gorji N., Moeini R., Memariani Z. Almond, hazelnut and walnut, three nuts for neuroprotection in Alzheimer's disease: a neuropharmacological review of their bioactive constituents. Pharmacol Res. 2018;129:115–127. doi: 10.1016/j.phrs.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Pollegioni P., Woeste K., Chiocchini F., Lungo S.D., Ciolfi M., Olimpieri I., Tortolano V., Clark J., Hemery G.E., Mapelli S., Malvolti M.E. Rethinking the history of common walnut (Juglansregia L.) in Europe: its origins and human interactions. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0172541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavecchia T., Rea G., Antonacci A., Giardi M.T. Healthy and adverse effects of plant-derived functional metabolites: the need of revealing their content and bioactivity in a complex food matrix. Crit Rev Food Sci Nutr. 2013;53(2):198–213. doi: 10.1080/10408398.2010.520829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulose S.M., Bielinski D.F., Shukitt-Hale B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J Nutr Biochem. 2013;24(5):912–919. doi: 10.1016/j.jnutbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Bhardwaj R., Dod H., Sandhu M.S., Bedi R., Dod S., Konat G., Chopra H.K., Sharma R., Jain A., Nanda N. Acute effects of diets rich in almonds and walnuts on endothelial function. Indian Heart J. 2018;70(4):497–501. doi: 10.1016/j.ihj.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Liao L., Moore J., Wu T., Wang Z. Antioxidant phenolic compounds from walnut kernals (Juglansregia L.) Food Chem. 2009;113(1):160–165. [Google Scholar]
- 28.Grancieri M., Martino H.S.D., Mejia E.G. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: a Review. Compr Rev Food Sci F. 2019;18:480–483. doi: 10.1111/1541-4337.12423. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira-Alves S., Vendramini-Costa D.B., Cazarin C.B.B., Junior M.R.M., Ferreira J.P.B., Silva A.B., Prado M.A., Bronze M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017;232(1):295–305. doi: 10.1016/j.foodchem.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Valdivia-Lopex A., Tecante A. Chapter two-chia (Salvia hispanica): a review of native Mexican seed and its nutritional and functional properties. Adv Food Nutr Res. 2015;75:53–75. doi: 10.1016/bs.afnr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Kobus-Cisowska J., Szymanowska D., Maciejewska P., Kmiecik D., Gramza-Michalowska A., Kulczynski B., Cielecka-Piontek J. In vitro screening for acetyl cholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia (Salvia hispanica) Electron J Biotechnol. 2019;37:1–10. [Google Scholar]
- 32.Marineli R.S., Moraes E.A., Lenquiste S.A., Godoy A.T., Eberlin M.N., Marostica M.R. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (salvia hispanica L) LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2014;2(2):1304–1310. [Google Scholar]
- 33.Luo M., Cao Y., wang W., Chen X., Cai J., Wang L., Xiao J. Sustained-release antimicrobial gelatin film: effect of chia mucilage on physicochemical and antimicrobial properties. Food Hydrocolloids. 2019;87:783–791. [Google Scholar]
- 34.Ullah R., Nadeem M., Khalique A., Imran M., Mehmood S., Javid A., Hussain J. Nutritional and therapeutic perspectives of chia (Salvia hispanica L.): a review. J Food Sci Technol. 2016;53(4):1750–1758. doi: 10.1007/s13197-015-1967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirel M.A., Ilhan M., Suntar I., Keles H., Akkol E.K. Activity of Corylusavellana seed oil in letrozole-induced polycystic ovary syndrome model in rats. Braz J Pharmcog. 2016;26:83–88. [Google Scholar]
- 36.Gopi C., Sastry C.G., Dhanaraju M.D. Microwave-assisted synthesis, structural activity relationship and biological activity of some new quinoxaline Schiff base derivatives as highly potent spirochete bactericidal agents. Beni-Suef Univ J Basic Appl Sci. 2017;6:39–47. [Google Scholar]
- 37.Reddy S., Begum N., Mutha S., Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac J Reprod. 2016;5:116–122. [Google Scholar]
- 38.Kafali H., Iriadam M., Ozardali I., Demir N. Letrozole-induced ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35:103–108. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Shi D., Vine D.F. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012;98(1):185–193. doi: 10.1016/j.fertnstert.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Manneras L., Cajander S., Holmang A., Seleskovic Z., Lystig T., Lonn M., Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 41.Azeez O.M., Akhigbe R.E., Anibogu C.N. Oxidative status in rat kidney exposed to petroleum hydrocarbons. J Nat Sci Biol Med. 2013;4:149–154. doi: 10.4103/0976-9668.107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao M., Peng M., Zhu Z., Yan X., Wei Z., Li M. Effect of dodder total flavone on polycystic ovary syndrome rat models induced by DHEA combined HCG. Saudi J Biol Sci. 2019;26(4):821–827. doi: 10.1016/j.sjbs.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullah A., Jahan S., Razak S., Pirzada M., Ullah H., Almajwal A., Rauf N., Afsar T. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J Ovarian Res. 2017;10(62):1–8. doi: 10.1186/s13048-017-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan U., Ahmed N., Mohyud-Din S.T., Bin-Mohsin A bioconversion model for MHD flow a heat transfer over a porous wedge containing both nanoparticles and gyrostatic microorganisms. J Biol Syst. 2016;23:1–21. [Google Scholar]
- 45.Hosseini S.M.M., Mohyud-Din S.T., Ghaneai H. Variation iteration method for nonlinear age-structured population models using auxiliary parameter. J Phys Sci. 2010;65:1137–1142. [Google Scholar]
- 46.Kalgaonkar S., Almario R.U., Gurusighe D., Garamendi E.M., Buchan W., Kim K., Karakas S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386–393. doi: 10.1038/ejcn.2010.266. [DOI] [PubMed] [Google Scholar]
- 47.Salek M., Clark C.C.T., Taghizadeh M., Jafarnejad S. N-3 fatty acids as preventive and therapeutic agents in attenuating PCOS complications. Excli J. 2019;18:558–575. doi: 10.17179/excli2019-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasim-Karakas S.E., Almario R.U., Gregory L., Wong R., Todd H., Lasley B.L. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(2):615–620. doi: 10.1210/jc.2003-030666. [DOI] [PubMed] [Google Scholar]
- 49.Dennett C.C., Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr. 2015;28(2):116–120. doi: 10.2337/diaspect.28.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]