Highlights
-
•
MARK2 plays an important role in the chemoresistance mechanism of osteosarcoma stem cells.
-
•
Down-regulation of MARK2 in CD133+ MG-63 and MNNG/HOS cells inhibits the expression of DNA-PKcs by inhibiting the activity of the PI3K/Akt/mTOR pathway.
-
•
New clues for the osteosarcoma chemotherapy strategy.
Keywords: MARK2, osteosarcoma stem cells, PI3K/Akt/mTOR, DNA-PKcs
Abstract
Objective
This study aims to explore the role of MARK2 in chemotherapeutic resistance and potential mechanism within cisplatin resistance models of CD133+ MG-63 and MNNG/HOS cells.
Methods
CD133− and CD133+ MG-63 and MNNG/HOS cells were differentiated and obtained by MACS(Magnetic bead sorting). Cell activity was determined by CCK-8 assay. siRNA was employed to down regulate the Microtubule Affinity Regulated Kinase 2 (MARK2) expression. Immunofluorescence detection and RT-qPCR were used to measure the expressions of MARK2 and DNA-PKcs at both protein and mRNA levels. Western blot was applied to test the levels of MARK2, γH2AX (S139), DNA-PKcs, Phospho-PI3 Kinase p85 (Tyr458), Akt, phospho-Akt (T308) antibodies, mTOR, phospho-mTOR (Ser2448).
Results
Compared with CD133− MG-63 cells, CD133+ MG-63 cells showed significantly strong cisplatin resistance, with high levels of MARK2, DNA-PKcs and potent DNA damage repair ability (p<0.05). Down regulation of MARK2 reduced the cisplatin resistance of CD133+ MG-63 cells, with deceasing expression of DNA-PKcs (p<0.05). PI3K/Akt/mTOR pathway was potentially activated in CD133+ MG-63 cells, and involved in the cisplatin resistance of MG-63 cells. The similar results were observed in CD133+ MNNG/HOS cells. The reduction of MARK2 retarded the activity of PI3K/Akt/mTOR pathway and further impeded the cisplatin resistance in CD133+ MG-63 and MNNG/HOS cell.
Conclusion
Our data suggested that MARK2 was related to cisplatin resistance in CD133+ MG-63 and MNNG/HOS cells. The decrease of MARK2 restricted the cisplatin resistance of CD133+ MG-63 and MNNG/HOS cells by down regulating the expression of DNA dependent protein kinase catalytic subunit (DNA-PKcs) and inhibiting activity of PI3K/Akt/mTOR signaling pathway, which provides new clues for the osteosarcoma chemotherapy strategy.
1. Introduction
Osteosarcoma represents a type of primary cancerous tumor in a bone and is considered to derive from mesenchymal stem cells [1]. The introduction of chemotherapy, along with corresponding surgical resection, has remarkably increased the survival rate by 60–70% [2]. Nevertheless, chemotherapy resistance frequently contributes to the failure in the treatment of osteosarcoma and causes recurrent, metastatic, or unresectable osteosarcomas, which limits the improvement of efficacy of current therapy. The reverses of chemotherapy resistance are therefore of great significance in further anti-cancer strategy [3]. Cluster of differentiation 133 (CD133), the antigen of which as a pentaspan membrane glycoprotein, is recognized as a stem cell marker for normal and cancerous tissues. CD133+ cells within osteosarcoma cell lines were identified with many features of cancer stem cells [4]. Notably, our previous study revealed that CD133+ osteosarcoma stem cells presented significant cisplatin resistance, while the down-regulation of DNA-PKcs expression could affect the reduction of chemoresistance [5]. However, the upstream target of this mechanism remains elusive.
Microtubule affinity regulated kinase 2 (MARK2) has been implicated in the regulation of a multitude of cellular processes. Previous evidence showed that loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity [6]. MARK2 is also involved in the modulation of neurodegeneration, by turning on phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) at Thr-313, a mutation site in Parkinson disease, for instance [7]. It has been found that MARK2 plays an important role in DNA damage repair and cell cycle activation [8]. Recent study illustrated that the attenuation of DDP (cisplatin) resistance in lung cancer was associated with down regulation of MARK2 and p-Akt [9]. The exact function of MARK2 in the regulation of osteosarcoma is still to be determined. In this study, we aim to explore the role of MARK2 in chemotherapeutic resistance and potential mechanism within an in vitro cisplatin resistance model of CD133+ MG-63 and MNNG/HOS cells.
2. Materials and methods
2.1. Cell culture
Human osteosarcoma MG-63 and MNNG/HOS cell line were both purchased from ATCC (Manassas, VA, USA). The cells were cultured with Dulbecco's minimal essential medium (DMEM), containing 10% fetal calf serum (both from Gibco, Grand Island, NY, USA) and streptomycin (Solarbio Co., Ltd., Beijing, China). The cell density was adjusted at about 1 × 105/cm2, cultured at 37 °C, 5%CO2, and the medium was changed on the following day.
2.2. CD133+ MG-63 and MNNG/HOS cells obtained by MACS (magnetic bead sorting)
According to the manufacturer's instructions, CD133 microprobe kit (Miltenyi Biotec, Auburn, CA, USA) was used to for cell sorting. The MG-63 and MNNG/HOS cells were collected and counted, and the cell suspensions were prepared by adding the cell sorting solution (60 μL separator per 107 cells). 20 μL FcR blocking buffer and 20 μL CD133 magnetic activated cells were added into each 107 cells successively. After incubation at 4 °C for 15 min, the cells were washed and resuspended. LS magnetic activated cell sorting column and magnetic activated cell separator were applied (Miltenyi Biotechnology, Bergisch Gladbach, North Rhine-Westphalia, Germany). Magnetic labeled CD133+ MG-63 and MNNG/HOS cells and unlabeled CD133- MG-63 and MNNG/HOS cells were collected. In order to ensure the purity of CD133 cells, the column was re-sorted. After sorting, the cells were maintained by serum-free suspension cell culture, including 1% N2 additive (Gibco, Waltham, MA, USA), 1% methylcellulose (Gibco, Waltham, MA, USA), 20 ng/ml. Fresh epidermal growth factor (EGF) (PeproTech, London, UK), 20 ng/ml (basic fibroblasts growth factor) was added to the ultra-low adsorption culture plate. EGF and bFGF were supplemented every other day.
2.3. Cell activity
The selected cells were inoculated in 96 well plates with a density of 5000 cells /well and cultured in cell incubator. After the cells were adhered, CDDP (cisplatin) complexes were added to the plate at the different concentrations of 0 μM, 5 μM, 10 μM, 15 μM, 20 μM, 30 μM, 40 μM, 50 μM (CDDP, Qilu Pharmaceutical Co., Ltd., Jinan, Shandong, China). After the treatment of cisplatin for 24 h, 10 μL CCK-8 reagent was added (BestBio, Shanghai, China), and cells were further incubated at 37 °C for 2 h. The absorbance of the holes at 450 nm wavelength was determined by means of light absorption enzyme labeling instrument (EMax Plus, Molecular Devices, Sunnyvale, CA, USA). At the same time, blank control (CCK 8 reagent, without cells) and control group (CCK 8 reagent without drug treatment, with cells) were set up and the experiment was repeated at least three times. Cell activity (%) = (OD value of cisplatin-OD value of blank group) / (OD value of control group-OD value of blank group) × 100%. Half inhibitory concentration (IC50) was calculated by GraphPad Prism 5 software (La Jolla, CA, USA).
2.4. RNA interference
The transfection of MARK2 siRNA (GAGGCACUUUAGAGCAAAUTT) was carried out according to the following method: the CD133+ MG-63 or MNNG/HOS cells were inoculated in a serum-free medium in a 6 well plate. Serum-free media included DMEM medium, 20 ng/mL EGF (PeproTech, London, UK), 20 ng/ml bFGF (PeproTech, London, UK) and 1% N2 additive (Gibco, Waltham, MA, USA). Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for cell transfection with siMARK2 or control siRNA (GenePharma Co., Ltd., Shanghai, China). After the transfection for 48 or 72 h, qRT-PCR or Western-blot was performed to detect the gene or protein expressions in collected cells respectively.
2.5. AKT inhibitor treatment of cells
AKT inhibitor MK-2206 2HCl solution (5 mM) was added for the final concentration of 10 μM (Selleck, Houston, TX, USA) in the cell medium. The cells were collected after treatment for 24 h. qRT-PCR and Western-blot experiments were then performed.
2.6. Immunofluorescence
The cells were seeded on the cell-climbing slide in a 24 well plate. After the cells were adhered, the culture medium was removed. PBS was used for cell washing and cells were then fixed with 4% paraformaldehyde for 15 min. The immobilized cells were permeated in 0.3% Triton X-100 for 10 min. 10% normal goat serum was added to the cell-climbing slide for 30 min at room temperature. After cells were absorbed by seal solution, 200 μL of an anti-solution prepared by PBS was added, and incubated overnight at 4 °C in humid environment. The first antibody involved in this study included rabbit polyclonal anti-human MARK2 antibody (ab133178, 1:100) and mouse monoclonal anti-human DNA-PKcs antibody (ab1832, 1:100) (Abcam, Cambridge, MA, USA). Cells were rewarmed at 37 °C for 45 min, and then the second antibody labeled with fluorescent substances, including Alexa Fluor 488 goat anti-rabbit (zf-0511, 1:200) and Alexa Fluor 594 goat anti-rat (zf-0513, 1:200) (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) antibodies, was added for incubation at room temperature for 1 h. The nuclear staining was carried out with 4-diamino-2-phenylindole (6-Diamidino-2-phenylindole) and incubated at room temperature for 5 min in the dark. The slide was inverted onto the slide with anti-fluorescence quenching agent. The slide was observed under inverted fluorescence microscope (BX52; Olympus Corp., Tokyo, Japan) and the images were collected.
2.7. Quantitative real-time polymerase chain reaction (qRT-PCR)
TRIzol lysis buffer (Toyobo, Osaka, Japan) was used to extract total RNA according to the manufacturer's instructions. The first-strand complementary DNA (cDNA) was then synthesized by using ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). In brief, the total RNA of 1 μg was added into the volume of 10 μL, which contained 2 μL 5× RT buffer, 0.5 μL RT Enzyme Mix, 0.5 μL Primer Mix. Reverse transcription was carried out in the thermal cycler (TGradient 96, Biometra GmbH, Gttingen, Germany). The temperature cycle program was under the condition of 37 °C, 15 min, and 98 °C enzyme inactivation, 5 min. After the reaction, the cDNA was preserved at −20 °C. Using cDNA as the template, SYBR Green Realtime PCR Master Mix kit (Toyobo, Osaka, Japan) was used for PCR amplification. The reaction solution (20 μL) of PCR consisted of 2 μL cDNA template, 6.4 μL distilled water, 10 μL 2× SYBR Green Realtime PCR Master Mix, 0.8 μL forward primer (10 μm) and 0.8 μL reverse primer (10 μm). (MARK2: forward 5′-AACTGAACTCCTCCAGCCTCCAG-3′, reverse 5′-GCCACTAGCGTACTCCATGACAAG-3′. DNA-PKcs:forward 5′-ACAGAGATCCAGAAAGTGAGACA-3′, reverse 5′-AGCAACCGGTCCAAGGTATT-3′). The amplification condition was as follows: pre-denaturation 95 °C, 30 s, 40 cycles of 95 °C, 5 s, 55 °C, 10 s, 72 °C, 15 s. GAPDH was used as the internal reference. Relative gene expression was semiquantitative analyzed by 2−△△Ct method. 2−△△Ct = gene copy number in test group/gene copy number in control. Experiments were carried out in triplicates.
2.8. Western-blot analysis
The cells were collected and the total protein was treated by Radio Immunoprecipitation Assay (RIPA) (Beyotime, Shanghai, China). The protein concentration was determined by the enhanced bicinchoninic acid (BCA) protein detection kit (Beyotime, Shanghai, China). The protein sample of the same quality was underwent sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 80 V. The protein on the gel was transferred to the polyvinylidene fluoride (PVDF) membrane with the electric current of 200 mA. The PVDF membrane was sealed with 5% skim milk for 1.5 h at room temperature, and further washed three times with TBST (Tris-buffered saline and Tween-20). The PVDF membrane was incubated overnight at 4 °C with primary antibody. Rabbit monoclonal anti-human MARK2 antibody (ab133724, 1:1000), rabbit polyclonal anti-human γH2AX (S139) antibody (ab2893, 1:1000), rabbit polyclonal anti-human DNA-PKcs antibodies (ab230, 1:2000) were bought from Abcam (Cambridge, MA, USA). Rabbit polyclonal anti-human PI3 Kinase p85 Antibody (4292S, 1:1000), rabbit polyclonal anti-human Phospho-PI3 Kinase p85 (Tyr458) antibody (4228S, 1:1000), rabbit polyclonal anti-human Akt antibody (9272S, 1:1000). Rabbit polyclonal anti-human phospho-Akt (T308) antibody (sc-16646-R, 1:100) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal anti-human mTOR antibody (2972S, 1:1000), and rabbit monoclonal anti-human phospho-mTOR (Ser2448) antibody (2791S, 1:1000) were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-human β-actin antibody (TA-90, 1:500) was acquired from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China). The secondary antibody (goat anti-rabbit secondary antibody (ZB-2301, 1: 5000) or goat anti-mouse secondary antibody (ZB-2305, 1: 5000) (both from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)) conjugated with horseradish peroxidase was used for 1 h incubation at room temperature. After the membrane was washed for three times with PBST, chemiluminescence detection reagent (EMD Millipore, Billerica, MA, USA) was used to develop and fix.
2.9. Statistical analysis
Statistical Product and Service Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing. Each experiment was carried out three times. Continuous data are presented as means ± standard deviation (SD), and were analyzed by using one-way ANOVA, with the Tukey's post hoc test. P<0.05 suggested that the difference was statistically significant.
3. Results
3.1. CD133+ MG-63 and MNNG/HOS cells are more resistant to CDDP than CD133− MG-63 and MNNG/HOS cells with high expression of MARK2
After immunomagnetic bead sorting, CD133+ cells and CD133− MG-63 and MNNG/HOS cells were collected and treated with different concentrations of CDDP for 24 h, respectively. All results obtained in CD133+ MG-63 and MNNG/HOS cells were similar. Cell viability was determined by CCK-8 and the results showed that CD133+ MG-63 and MNNG/HOS cells were strongly resistant to CDDP compared to CD133− MG-63 and MNNG/HOS cells (Fig. 1A). The IC50 values of CD133+ MG-63 and MNNG/HOS cells were significantly higher than that of CD133− MG-63 and MNNG/HOS cells (P<0.05) (Fig. 1B). Both the mRNA and protein levels of MARK2 in CD133+ MG-63 and MNNG/HOS cells were dramatically elevated compared with those in CD133− MG-63 and MNNG/HOS cells (Fig. 1C and D).
Fig. 1.
The resistant ability to CDDP and expression of MARK2 in CD133+ and CD133− MG63 and MNNG/HOS cells. (A and B) CD133+ MG-63 and MNNG/HOS cells were more resistant to CDDP than CD133− MG-63 and MNNG/HOS cells with higher IC50 value. The expression of MARK2 mRNA (C) and protein (D) was higher in CD133+ MG-63 and MNNG/HOS cells than that in CD133− MG-63and MNNG/HOS cells. *P<0.05, vs CD133− group.
3.2. Down-regulation of MARK2 expression reduces the tolerance of CD133+ MG-63 and MNNG/HOS cells to CDDP
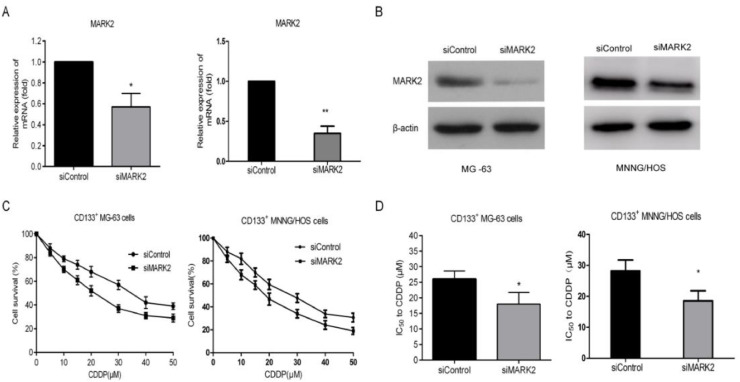
Since the CD133+ MG-63 and MNNG/HOS cells showed strong resistance to CDDP and the expression of MARK2 was increased, we next investigated whether MARK2 can play a role in CDDP resistance of CD133+ MG-63 and MNNG/HOS cells. Specific siRNA targeting MARK2 gene was employed. After transfection, the level of MARK2 was obviously down regulated in CD133+ MG-63 and MNNG/HOS cells (Fig. 2A and B). The reduction of MARK2 impeded the CDDP resistance of CD133+ MG-63 and MNNG/HOS cells with the decrease of IC50 value (Fig. 2C and D).
Fig. 2.
The effect of MARK2 on the resistance of CD133+ MG63 and MNNG/HOS cells to CDDP. The expression of MARK2 mRNA (A) and protein (B) in CD133+ MG-63 and MNNG/HOS cells was inhibited after the treatment of siMARK2. (C and D) The inhibition of MARK2 reduced the resistance of CD133+ MG-63 and MNNG/HOS cells to CDDP with deciling IC50 value. *P<0.05, vs siControl group.
3.3. CD133+ MG-63 and MNNG/HOS cells present potent DNA damage repair ability after CDDP treatment
After immunomagnetic beads sorting, CD133+ and CD133− MG-63 and MNNG/HOS cells were treated with CDDP (10 μM) for 24 h, and the double-strand break DSB marker γH2AX (S139) and DNA damage repair marker DNA-PKcs were then detected. Of note, the expression of γH2AX (S139) in CD133+ MG-63 and MNNG/HOS cells was evidently lower than that of CD133− MG-63 and MNNG/HOS cells (Fig. 3A), whereas the expression of DNA-PKcs was apparently higher than that of CD133− MG-63 and MNNG/HOS cells (Fig. 3A and B), indicating that the CDDP resistance of CD133+ MG-63 and MNNG/HOS cells is implicated to DNA damage repair.
Fig. 3.
DNA damage repair ability of MG-63 and MNNG/HOS cells. (A) The level of double-strand break DSB marker γH2AX (S139) was lower in CDDP-treated CD133+ MG-63 and MNNG/HOS cells than that in CDDP-treated CD133− MG-63 and MNNG/HOS cells with higher expression of DNA damage repair marker DNA-PKcs by western blotting. (B) Immunofluorescence test indicated the level of DNA-PKcs in CDDP-treated CD133+ MG-63 and MNNG/HOS cells was higher than that in CDDP-treated CD133− MG-63 and MNNG/HOS cells. (C) The suppression of MARK2 increased theγH2AX (S139) level while reduced DNA-PKcs expression by Western blotting. (D) Immunofluorescence test showed that downregulation of MARK2 reduced DNA-PKcs expression.
3.4. The decrease of MARK2 expression impairs the DNA damage repair ability of CD133+ MG-63 and MNNG/HOS cells
After 10 μm CDDP treatment, the expressions of γH2AX (s139) and DNA-PKcs were detected within the transfection of siRNA targeting MARK2 or scramble siRNA. We found that the down regulation of MARK2 considerably increased γH2AX (s139) level but reduced DNA-PKcs expression (Fig. 3C and D). The results suggested that the decline of MARK2 level restricted the DNA damage repair ability of CD133+ MG-63 and MNNG/HOS cells against CDDP.
3.5. PI3K/Akt/mTOR pathway was involved in the expression of DNA-PKcs in CD133+ MG-63 and MNNG/HOS cells
In order to determine the involvement of PI3K/Akt/mTOR pathway in regulation of DNA damage repair ability of MG-63 and MNNG/HOS cells, the levels of p-PI3K/p85 (Tyr458), P-Akt (T308) and P-mTOR (Ser2448) in CD133+ and CD133− MG-63 and MNNG/HOS cells were examined. As a result, it was found that the expressions of p-Akt (T308), p-PI3K/p85 (Tyr458) and p-mTOR (Ser2448) of CD133+ MG-63 and MNNG/HOS cells were higher than those of CD133− MG-63 and MNNG/HOS cells (Fig. 4A). The results indicated that the PI3K/Akt/mTOR pathway was excessively activated in CD133+ MG-63 and MNNG/HOS cells. Notably, after treatment with 10 μM Akt inhibitor MK-2206 2HCl, the expression levels of p-mTOR (Ser2448) and DNA-PKcs were evidently down-regulated (Fig. 4A). The inhibition of MK-2206 2HCl reduced the resistance of CD133+ MG-63 and MNNG/HOS cells to CDDP with deciling IC50 value. *P<0.05, vs Control group, confirm the role of the PI3K/AKT/mTOR pathway in resistance to CDDP (Fig. 4C). These results manifested that the PI3K/Akt/mTOR pathway is further induced in involved in MG-63 and MNNG/HOS cells with strong DNA damage repair ability, and the deactivation of PI3K/Akt/mTOR signaling can reduce the level of DNA-PKcs.
Fig. 4.
PI3K/Akt/mTOR pathway in the regulation of DNA damage repair ability of MG63 and MNNG/HOS cells. (A) The expressions of p-Akt (T308), p-PI3K/p85 (Tyr458), p-mTOR (Ser2448) and DNA-PKcs of CD133+ MG63 and MNNG/HOS cells were higher than those of CD133− MG63 and MNNG/HOS cells whereas the Akt inhibitor MK-2206 2HCl reduced the levels of p-mTOR (Ser2448) and DNA-PKcs of CD133+ MG63 and MNNG/HOS cells. (B) The decrease of MARK2 inhibited the expressions of p-Akt (T308), p-PI3K/p85 (Tyr458), p-mTOR (Ser2448) and DNA-PKcs of CD133+ MG63 and MNNG/HOS cells. (C) The inhibition of MK-2206 2HCl reduced the resistance of CD133+ MG-63 and MNNG/HOS cells to CDDP with deciling IC50 value. *P<0.05, vs Control group.
3.6. Down-regulation of MARK2 reduced DNA-PKcs level through decreasing the activity of PI3K/Akt/mTOR pathway in CD133+ MG-63 and MNNG/HOS cells
Similar to the effect of Akt inhibitor MK-2206 2HCl on the DNA-PKcs level, we found that, after the transfection of siMARK2, the expressions of p-PI3K/p85 (Tyr458), p-Akt (T308), p-mTOR (Ser2448) and DNA-PKcs were potentially inhibited in CD133+ MG-63 and MNNG/HOS cells (Fig. 4B). These results indicated that down-regulation of MARK2 limited the DNA damage repair ability of CD133+ MG-63 and MNNG/HOS cells through deactivating PI3K/Akt/mTOR pathway.
4. Discussion
Osteosarcoma stem cells (CSCs) were firstly cultured by Gibbs using serum-free culture techniques [10]. Later, it has been found that a variety of drug-resistant proteins are expressed on osteosarcoma stem cells, which contribute to high drug resistance [11]. In addition, most of the cancer stem cells are in a resting or dormant phase, making them insensitive to chemotherapeutic drugs that mainly act on the cell cycle. CD133+ MG-63 and MNNG/HOS osteosarcoma cells are known as osteosarcoma stem cells. Our previous study found that CD133+ MG-63 and MNNG/HOS osteosarcoma stem cells presented stronger CDDP resistance than CD133− MG-63 and MNNG/HOS cells [5]. Therefore, this study selected CD133+ MG-63 and MNNG/HOS cells as a drug resistance model.
The DNA damage repair ability of Tumor cell is an important mechanism of chemotherapy resistance. The activity of DNA-dependent protein kinase (DNA-PK) and the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) are the main indicators for DNA damage repair. Phosphorylation of the Thr2609 site in DNA-PKcs is a necessary process for non-homologous end joining (NHEJ) to repair the DNA double strands cleavage pathway. A number of studies have found that DNA-PK activity and DNA-PKcs expression are elevated in gliomas, cervical cancer, non-small cell lung cancer, and B-cell chronic lymphocytic leukemia [5, 13]. Antisense nucleic acids, small interfering RNAs or inhibitors of this enzyme can increase the chemo-sensitivity of tumor cells [14]. Our previous study indicated that CD133+ osteosarcoma tumor cells exhibited the characteristics of cancer stem cells, with high expression of DNA-PKcs, and showed potent chemotherapy resistance, tumor formation and malignant biological behavior of tumors. The data demonstrated that high expression of DNA-PKcs was associated with poor prognosis of osteosarcoma. The positive rate of DNA-PKcs in patients with recurrence and metastasis was significantly higher than that in patients without tumor survival. In this scenario, DNA-PKcs can be used as one of the prognostic factors affecting tumor-free survival [12]. In vitro cell experiments also implicated that DNA-PKcs was elevated in CD271+ osteosarcoma cells [11]. Inhibition of DNA-PKcs activity can reduce DNA repair capacity, cause cycle arrest and increase apoptosis rate, and enhance chemotherapy sensitivity of bone flesh tumor cells [5, 12]. Therefore, it is of great significance to investigate the down-regulation of the upstream mechanism of DNA-PKcs.
Microtubule affinity-regulating kinase 2 (MARK2) belongs to a kind of serine/threonine protein kinase that is a key component of microtubule-associated protein phosphorylation, and is involved in cell cycle regulatory proteins, type II histones, etc. The study found that MARK2 plays an important role in neural differentiation, neurodegeneration, cell polarization, intracellular transport and cell migration through animal gene knockout experiments [15, 16]. There are four kinds of MARK genes in the human body: MARK1, MARK2, MARK3, and MARK4. MARK3 is overexpressed in hepatoma cells and is associated with intranuclear aggregation of β-catenin. However, MARK2 is closely related to the malignant biological behavior and drug resistance of tumors. It is overexpressed in CDDP-resistant cervical cancer cell lines. Through gene chip analysis, MARK2 gene expression in CDDP-resistant Hela cell line is about 4 times than that of non-CDDP-resistant Hela cell line, and qPCR results also demonstrated high expression of the MARK2 gene in the CDDP-resistant Hela cell line at the mRNA level. In addition, the high expression of MARK2 was not found highly expressed in the vincristine-resistant or doxorubicin-resistant Hela cell line, indicating that MARK2 plays a specific role in CDDP resistance in cervical cancer. After MARK2 gene silencing, the sensitivity of cells to CDDP was increased, whereas the sensitivity to vincristine and paclitaxel was changed significantly [17]. It has also been found that MARK2 expression is closely related to CDDP resistance in non-small cell lung cancer [8]. Overexpression of MARK2 is closely related to DNA hypomethylation and replication, indicating that the mechanism of drug resistance is related to DNA damage repair. Experiments with both continuous cell lines and primary cells have shown an important role for MARK2 in cell cycle activation and DNA damage repair. After down-regulation of MARK2 expression, DNA damage was aggravated in the lung cancer cells, and S-phase arrest occurred in the cell cycle. This study suggests that MARK2 can increase cell DNA damage repair by regulating cell cycle. In addition, after the down regulation of MARK2 gene, lung cancer cell activity and non-dependent attachment growth were inhibited, indicating that MARK2 exerted a critical function in lung cancer cell activity and non-dependent attachment growth. Our result showed that MARK2 was highly expressed in osteosarcoma CD133+ MG-63 and MNNG/HOS cell line. As the treatment with gradient CDDP, the expression of MARK2 and DNA-PKcs was significantly increased, suggesting that MARK2 may have a close relationship in DNA damage repair after osteosarcoma chemotherapy.
The PI3K/AKT/mTOR signaling pathway is an imperative mediator of cell growth, survival and movement. The dysregulation of the PI3K/AKT/mTOR pathway is associated with chemoresistance in a variety of tumors [18], [19], [20]. Studies have shown that the activity of PI3K/AKT/mTOR is increased in breast cancer stem cells, and the tumorigenic ability of breast cancer stem cells in vitro and in vivo is decreased after inhibition of PI3K/AKT/mTOR [21, 22]. In our previous study, after using small molecule compound (MDA19) to effectively inhibit the activity of PI3K/AKT/mTOR in tumor cells in vitro, the downstream factors Cyclin D1, P70S6K and p-P70S6K and upstream factor VEGF were down-regulated, leading to the alleviation of epithelial-mesenchymal transition (EMT) inhibition, cell proliferation, invasion and metastasis. It implied that PI3K/AKT/mTOR pathway activity may be related to chemotherapy resistance of osteosarcoma cells [14]. mTOR serves as a key point in the regulation of the PI3K/AKT pathway, and its overactivation plays an important role in tumor CDDP resistance. The activation state of mTOR (p-mTOR) is highly expressed in lung adenocarcinoma, large cell carcinoma and squamous cell carcinoma. The expression of mTOR has become the main indicator for chemotherapy evaluation and prognosis of non-small cell lung cancer. The study found that in tumor-resistant strains, over-expressed AKT mediated the protein of tuberous sclerosis complex 2 (TSC2), disintegrated TSC2 and TSC1, thereby terminating the two small G proteins (Ras homolog enriched in brain, Rheb) inhibition, which in turn activated mTOR. PI3K/AKT/mTOR pathway participates in the DNA damage response of tumor cells such as myeloma, suggesting that its mechanism of action in tumor resistance may be related to the repair of DNA damage [23, 24]. Previously, it was mentioned that MARK2 was also involved in DNA damage repair in tumor resistance mechanisms. This study found that down-regulation of MARK2 expression can inhibit the activity of PI3K/AKT/mTOR pathway. Meanwhile, the expression of DNA-PKcs was down-regulated by the inhibition of PI3K/AKT/mTOR pathway, suggesting that down-regulation of MARK2 can inhibit DNA-PKcs expression via suppressing PI3K/AKT/mTOR pathway activity, thus improving the sensitivity of CD133+ MG-63 and MNNG/HOS cells to CDDP. However, the in vivo study is still required to validate the effect of MARK2 in the chemoresistance strategy.
5. Conclusions
In conclusion, our data demonstrate that MARK2 plays an important role in the chemoresistance mechanism of osteosarcoma stem cells. Down-regulation of MARK2 in CD133+ MG-63 and MNNG/HOS cells inhibits the expression of DNA-PKcs by inhibiting the activity of the PI3K/Akt/mTOR pathway. Our result provides theoretical basis for the improvement of osteosarcoma chemoresistance.
Declaration of Competing Interest
None.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81672655).
References
- 1.Jo V.Y., Fletcher C.D. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 2.Longhi A., Errani C., De Paolis M. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat. Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Kawai A., Yonemori K., Takahashi S. Systemic therapy for soft tissue sarcoma: proposals for the optimal use of pazopanib, trabectedin, and eribulin. Adv. Ther. 2017;34:1556–1571. doi: 10.1007/s12325-017-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirino V., Desiderio V., d'Aquino R. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS One. 2008;3:e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K., Li X., Tian J. Downregulation of DNA-PKcs suppresses P-gp expression via inhibition of the Akt/Nf-kappaB pathway in CD133-positive osteosarcoma MG-63 cells. Oncol. Rep. 2016;36:1973–1980. doi: 10.3892/or.2016.4991. [DOI] [PubMed] [Google Scholar]
- 6.Hurov J.B., Huang M., White L.S. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5680–5685. doi: 10.1073/pnas.0701179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matenia D., Hempp C., Timm T. Microtubule affinity-regulating kinase 2 (MARK2) turns on phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) at THR-313, a mutation site in Parkinson disease: effects on mitochondrial transport. J. Biol. Chem. 2012;287:8174–8186. doi: 10.1074/jbc.M111.262287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubaux R., Thu K.L., Vucic E.A. Microtubule affinity-regulating kinase 2 is associated with DNA damage response and cisplatin resistance in non-small cell lung cancer. Int. J. Cancer. 2015;137:2072–2082. doi: 10.1002/ijc.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Mei J., Tan Y. Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 2017;50:93–100. doi: 10.3892/ijo.2016.3768. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Zhong X.Y., Li Z.Y. CD133 expression in osteosarcoma and derivation of CD133(+) cells. Mol. Med. Rep. 2013;7:577–584. doi: 10.3892/mmr.2012.1231. [DOI] [PubMed] [Google Scholar]
- 11.Tian J., Li X., Si M. CD271+ osteosarcoma cells display stem-like properties. PLoS One. 2014;9:e98549. doi: 10.1371/journal.pone.0098549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Tian J., Bo Q. Targeting DNA-PKcs increased anticancer drug sensitivity by suppressing DNA damage repair in osteosarcoma cell line MG-63 and MNNG/HOS. Tumour Biol. 2015;36:9365–9372. doi: 10.1007/s13277-015-3642-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z., Xia Q., Cui J. Reversion of P-glycoprotein-mediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol. Rep. 2014;31:2720–2726. doi: 10.3892/or.2014.3154. [DOI] [PubMed] [Google Scholar]
- 14.Liu B., Xu L., Dai E.N. Anti-tumoral potential of MDA19 in human osteosarcoma via suppressing PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181501. BSR20181501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drewes G., Ebneth A., Preuss U. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 16.Matenia D., Mandelkow E.M. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem. Sci. 2009;34:332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z.Z., Lu H.P., Chao C.C. Identification and functional analysis of genes which confer resistance to cisplatin in tumor cells. Biochem. Pharmacol. 2010;80:262–276. doi: 10.1016/j.bcp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Polivka J., Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014;142:164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X., Fraser M., Moll U.M. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 21.Murohashi M., Hinohara K., Kuroda M. Gene set enrichment analysis provides insight into novel signalling pathways in breast cancer stem cells. Br. J. Cancer. 2010;102:206–212. doi: 10.1038/sj.bjc.6605468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Zhou N., Wang J. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018;196:56–62. doi: 10.1016/j.lfs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Demel H.R., Feuerecker B., Piontek G. Effects of topoisomerase inhibitors that induce DNA damage response on glucose metabolism and PI3K/Akt/mTOR signaling in multiple myeloma cells. Am. J. Cancer Res. 2015;5:1649–1664. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Cong L., He J. Photothermal treatment with EGFRmAb-AuNPs induces apoptosis in hypopharyngeal carcinoma cells via PI3K/AKT/mTOR and DNA damage response pathways. Acta Biochim. Biophys. Sin. 2018;50:567–578. doi: 10.1093/abbs/gmy046. [DOI] [PubMed] [Google Scholar]