Abstract
This preliminary clinical study demonstrates the possibility of a new species of probiotic for improvement of the degeneration of knee osteoarthritis (KOA). TCI633 (Streptococcus thermophilus) is a newly founded bacterium from human breast milk, and it is able to produce hyaluronate (HA) in gastrointestinal (GI) tract. A recent study has proved that TCI633 can substantially alleviate synovial tissue inflammation and cartilage damage in the animal models, but so far it has never been applied in clinical intervention. In this study, we recruited 80 subjects and conducted 12 weeks clinical trial to validate the efficacy of TCI633 for improvement of the progression of KOA. TCI633 could improve serum collagen type II C-telopeptide (sCTX-II) and serum C-reactive protein (sCRP) by 41.58% and 39.58%, respectively, after the study. The improvement rates for sCTX-II and sCRP in TCI633 group were 54% and 57%, respectively, at 12 weeks. Compared to the results of placebo, the indistinct improvement progresses of sCTX-II and sCRP might be caused by the uneventful distribution of K/L populations between the TCI633 and placebo groups, a short term of study period, and few recruited subjects. Moreover, the results of Western Ontario and McMaster Universities (WOMAC) questionnaires show that TCI633 might retard the progression and development of KOA after the trial. In brief, this preliminary research may provide an alternative approach to the improvement of KOA by probiotics although more detailed investigations should be conducted for solid conclusions.
Keywords: Microbiology, Food microbiology, Aging, Musculoskeletal system, Streptococcus thermophilius, Probiotics, Hyaluronic acid, Knee osteoarthritis
Microbiology; Food Microbiology; Aging; Musculoskeletal System; Streptococcus thermophilius; probiotics; hyaluronic acid; Knee osteoarthritis
1. Introduction
OA, a common arthropathy, has been identified as the major cause of disability in older people around the world (Wittenauer et al., 2013). Among the populations, 80% of individuals over 65 years old tend to suffer from articular dysfunction caused by OA, especially in women (Salazar et al., 2014; Zhang and Jordan, 2010). Taking Japanese adults (40 years old) as an example, 62.4% of women and 42.0% of men were diagnosed with KOA in 2005 (Yoshimura et al., 2009). As such, OA becomes a critical challenge for worldwide healthcare systems. The onset of OA is associated with chronic cartilage degeneration followed by synovial inflammation and the damage to subchondral bone and/or osteophyte formation (Saberi Hosnijeh et al., 2016). The symptoms of OA are characterized by knee pain, stiffness, loss of function, and limitation of movement (Yu et al., 2018). Possible OA treatment drugs can be categorized into: i) structure repair drugs for reversing the damaged tissues to their original statuses, ii) symptom alleviating drugs for the alleviation of OA symptoms, which helps patients to assuage their knee pains and remain or mildly restore their intrinsic knee functions (Reginster et al., 2001). However, the structure repair drugs are not in place at present, so, more and less, symptom alleviating drugs are the predominant approach to OA management, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and symptomatic slow acting drugs for osteoarthritis (SYSADOA) (Fransen et al., 2015; Cho et al., 2019). Of these drugs, glucosamine and chondroitin are widely employed in intervening in OA progression (Ng et al., 2012). Although the exact etiology of OA is unclear, some probable theories have been proposed to elaborate on the progression (Nguyen et al., 2017). At the early stage, micro- or macro-injury somehow impairs the chondrocyte metabolism by inhibition of collagen synthesis and up-regulation of the proteolytic enzymes (i.e., matrix metalloproteinases), which result in chondrodegradation (Rose and Kooyman, 2016). Further, the up-regulation of pro-inflammatory cytokines and inflammatory enzymes (e.g., interleukin 1 (IL-1), cyclooxygenase 2 (COX-2), tumor necrosis factor alpha (TNFα)) in synovial fluid may be elicited in the presence of degraded collagen and proteoglycan fragments (Pelletier et al., 2001). The inflammatory response is possible to exasperate cartilage degradation and inflict serious damage to synovium, meniscus, periarticular ligament, and subchondral bone (Mobasheri et al., 2017). Apart from aging, the development of OA is also associated with diabetes and metabolic disorders (Mobasheri et al., 2017).
Oral probiotic supplements (e.g., Bifidobacterium, Lactobacillus species) have recently revealed a possibility for the management of OA (Parvaneh et al., 2014; Henrotin et al., 2019; So et al., 2011; Amdekar et al., 2011; Lee et al., 2018; Lei et al., 2017). In particular, Lactobacillus species are the most popular studied targets. L. casei and L. acidophilus are able to suppress the expression of OA-related inflammatory cytokines and lessen articular cartilage degradation in the animal models (So et al., 2011; Amdekar et al., 2011; Lee et al., 2018). Moreover, L. casei Shirota has demonstrated the potential to improve the symptoms of OA patients and serum C-reactive protein (CRP) levels in a clinical study (Lei et al., 2017). In a recent study, Prof. Wen group has shown that S. thermophilus (TCI633), a newly identified strain of probiotic from human breast milk, possesses the ability to ameliorate the symptoms of OA as evidenced by the improvement results of knee joint swelling, synovial tissue inflammation, and cartilage damage in the anterior cruciate ligament transection-induced rat model (Lin et al., 2020). TCI633 can successfully colonize GI tract and generate HA, and the produced HA is possibly absorbed by large intestine into the circulation system (Lin et al., 2020; Lin et al., 2015; Lin, 2017). Although the underlying mechanism for TCI633 is elusive, we assume that the benefits of TCI633 for improvement in knee OA might be due to: i) suppression of the degradation of proteoglycan and collagen in the extracellular matrix; ii) reestablishment of normal chondrocyte metabolism; iii) obstruction of chondrodegradation and apoptosis of chondrocytes; and, iv) downgrade of inflammatory responses (Kimura et al., 2016).
The goal of this research is to explore the clinical efficacy of TCI633 for alleviation of the symptoms of OA. Radiographic examination and the Western Ontario and McMaster Universities (WOMAC) questionnaire were used to assess the joint space narrowing (JSN) for Kellgren-Lawrence (K/L) grading and the symptoms of OA. Meanwhile, sCTX-II analysis was also introduced into the study given that sCTX-II has been considered a vital biomarker for the degradation of type II collagen (the most abundant constituent of collagen in cartilage) (Amiel et al., 2003).
2. Materials and methods
2.1. Ethics approval
This clinical research was approved by the ethics committee of the National Taiwan University Hospital, and the study was registered with the ClinicalTrials.gov (NCT04267432). All subjects recruited in this trial returned the written consent forms.
2.2. Study design and selection criteria
80 subjects were recruited from the outpatient clinic of the Department of Orthopedic Surgery at National Taiwan University Hospital, Taiwan. Inclusion criteria were primary knee osteoarthritis of the medial tibiofemoral compartment and a K/L grade of 1, 2, or 3 suggested by the clinical judgment of the orthopedist of National Taiwan University Hospital. Exclusion criteria were history or active presence of rheumatic diseases, traumatic knee lesions, mental disorders, and abnormal results in hematological, cardiovascular, hepatic, renal, metabolic, or other major organs’ functions. All participants could not take any medication and supplement during the study.
The study assignment was adopted a randomized (in a 2:2 ratio) and double-blind study. Subjects were informed to take four TCI633 capsules (5 × 108 bacteria per capsule; 400 mg; TCI Co., Ltd.) once daily before meal in the morning for 12 weeks. We collected the K/L grade, WOMAC, sCTX-II, and sCRP information of the subjects at the baseline and weeks 4, 8, and 12.
2.3. Treatment efficacy measures
K/L grades from 0 to 4 represent none, doubtful, mild, moderate, and severe, respectively (Reginster et al., 2001). The joint structure for the determination of K/L grade was decided by the space changes of the medial tibiofemoral compartment. Subjects remained in standing and weight bearing postures, and their knees were fully extended and the patella was placed in the central position. Subsequently, anteroposterior view radiographs for both knees were obtained.
Knee OA symptoms were assessed by the WOMAC questionnaire. The questions in the questionnaire were divided into i) joint pain (five questions); ii) joint stiffness (two questions); and, iii) physical functions of joints (seventeen questions). We modified the originally simple scoring system by numbers 0–4 with visual analogue scale (VAS) for each question. Subjects made marks on the 100 mm long lines according to their personal sensation. In the end, the total line lengths were analyzed and recorded. Low to high WOMAC scores represent slight to severe OA symptoms (the worst score in total was 2400 mm).
2.4. Serum CTX-II measurement
Fasting blood samples were collected and underwent a certain sample preparation to acquire sera, and the processed sera were stored at -20 °C before testing. The sCTX-II was analyzed by a competitive ELISA kit (Cloud-Clone Corp.).
2.5. Serum C-reactive protein (CRP) measurement
The detection principle of sCRP here was based on highly sensitive Near Infrared Particle Immunoassay rate methodology, and sCRP level was determined by the turbidimetric method. All the operation steps were following the protocol of Beckman Coulter SYNCHRON LX/DxC High Sensitivity Cardiac C-Reactive Protein.
2.6. Statistical analysis
Student t-test was used to compare the experimental results, with p < 0.05 regarded significant difference.
3. Results
3.1. Patients
80 subjects were enrolled in this trial and randomly assigned to TCI633 group or placebo group. The drop-out rates for placebo group and TCI633 group were 9 of 39 (23.1%) and 4 of 41 (9.8%), respectively. The withdrawal reasons in placebo group were due to personal reasons, abnormal hematological indices, severe lumbago, and no return visit, not caused by the concern of adverse effects. Average ages and weights of the subjects in both groups were similar (Table 1). The relatively low female to male ratio was observed in placebo group given that it might be the collateral damage of the high drop-out rate. The distribution of the recruited subjects in TCI633 group was evenly dispersed from K/L 1 to K/L 3, whereas most recruited subjects in placebo group were classified at K/L 1 (20 of 30, 66.7%). After 12 weeks treatment, some subjects in TCI633 group were improved as indicated by K/L 1: 32.4%, K/L 2: 40.6%, K/L 3: 27.0% in comparison with K/L 1: 32.4%, K/L 2: 32.4%, K/L 35.2% at the baseline. On the other hand, in placebo group, the distribution of K/L grades at 12 weeks was similar with those at the baseline.
Table 1.
Demographic and clinical OA characteristics of all subjects.
| Placebo | TCI633 | |
|---|---|---|
| Subject number (persons) | 30 | 37 |
| Age (years) | 60.8 (12.2) | 65.1 (9.3) |
| Female | 22 (73.3%) | 31 (83.8%) |
| Male | 8 (26.4%) | 6 (16.2%) |
| Body weight (kg) |
64 (11.1) |
62.7 (11.0) |
| Kellgren and Lawrence grading | ||
| Grade 1: baseline/12 weeks | 20 (66.7%)/19 (63.4%) | 12 (32.4%)/12 (32.4%) |
| Grade 2: baseline/12 weeks | 4 (13.3%)/4 (13.3%) | 12 (32.4%)/15 (40.6%) |
| Grade 3: baseline/12 weeks | 6 (20.0%)/6 (20.0%) | 13 (35.2%)/10 (27.0%) |
| Grade 4: baseline/12 weeks | N/A/1 (3.3%) | N/A |
Value indicates mean value (S.D./population percentage). Kellgren and Lawrence (K/L) grading represents: K/L 1: doubtful, K/L 2: mild, K/L 3: moderate, K/L 4: severe.
3.2. Biomarker analysis
Table 2 shows the results of sCTX-II and sCRP levels for the recruited subjects at 0, 4, 8, and 12 weeks. We leveraged the sCTX-II and sCRP values of the baseline as the standards to the normalization of sCTX-II and sCRP measurement results at 4, 8, and 12 weeks. The changes of sCTX-II in TCI633 group were from -18.40% (week 4) to -41.58% (week 12), which got much improvement progress in comparison with placebo group (from -12.24% (week 4) to -28.60% (week 12)). The significant improvement effect of sCTX-II, corresponding to the baseline result, appeared on the 4 weeks after the use of TCI633 capsules and continued over the trial. The improvement rates of sCTX-II at 4, 8, and 12 week in TCI633 group were 41%, 43%, and 54%, respectively; the improvement rates of sCTX-II at 4, 8, and 12 week in placebo group were 47%, 43%, and 53%, respectively. On the other hand, the changes of sCRP in TCI633 and placebo groups were at the similar level around -40% over the study. The improvement effect for sCRP was shown significant difference from the baseline in TCI633 and placebo groups. The improvement rates of sCRP at 4, 8, and 12 week in TCI633 group were 62%, 65%, and 57%, respectively; the improvement rates of sCRP at 4, 8, and 12 week in placebo group were 37%, 37%, and 43%, respectively. Moreover, we were curious about the improvement effect on the mild and moderate participants. We further analyzed the results of K/L grades 2, 3 (Table S1). Unfortunately, the results did not show obvious difference from the outcomes in Table 2.
Table 2.
Degree of improvement (based on KL grades 1, 2, 3) (n = 30 for placebo group, n = 37 for TCI633 group).
| Week 4 |
Week 8 |
Week 12 |
p value | |||||
|---|---|---|---|---|---|---|---|---|
| I4–I0 Difference (%) (SEM) |
Improvement rate (%) (improved/total) |
I8–I0 Difference (SE) | Improvement rate (%) (improved/total) |
I12–I0 Difference (%) (SEM) | Improvement rate (%) (improved/total) |
|||
| sCTX-II | Placebo | -12.24 ± 3.08 | 47 (14/30) | -15.37 ± 3.65 | 43% (13/30) | -28.60 ± 5.26 | 53% (16/30) | 0.002a, 0.001b, < 0.001c |
| TCI633 | -18.40 ± 4.25 | 41 (15/37) | -23.63 ± 4.90 | 43% (16/37) | -41.58 ± 5.19 | 54% (20/37) | 0.001a, < 0.001b, < 0.001c | |
| sCRP | Placebo | -33.11 ± 5.36 | 37 (11/30) | -40.74 ± 6.23 | 37% (11/30) | -43.34 ± 6.08 | 43% (13/30) | <0.001a, < 0.001b, < 0.001c |
| TCI633 | -40.59 ± 4.79 | 62 (23/37) | -39.68 ± 5.98 | 65% (24/37) | -39.58 ± 5.34 | 57% (21/37) | <0.001a, < 0.001b, < 0.001c | |
I4–I0 difference: the difference between week 0 and week 4. I8–I0 difference: the difference between week 0 and week 8. I12–I0 difference: the difference between week 0 and week 12. a: comparison between week 0 and week 4. b: comparison between week 0 and week 8. c: comparison between week 0 and week 12.
3.3. Symptom
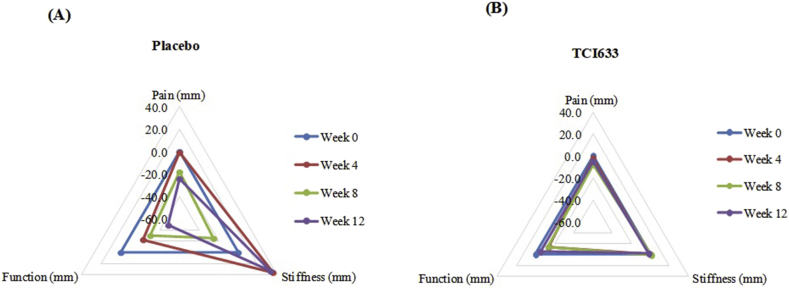
Figure 1 shows the results of WOMAC questionnaires for placebo and TCI633 groups. The values of pain, stiffness, and function at 0, 4, 8, and 12 weeks in TCI633 group displayed the similar levels no obvious difference. The values of pain and function in placebo group showed dramatic improvements after 8 weeks, but the improvement in stiffness showed fluctuated and got a negative effect.
Figure 1.
WOMAC osteoarthritis index for all subjects. (A) Placebo group. (B) TCI633 group. (Mean WOMAC score; n = 30 for placebo group, n = 37 for TCI633 group).
4. Discussion
This is the first clinical study to demonstrate a species of probiotic which can generate HA for improvement in the symptoms of OA and the prevention of knee joint degeneration. There was no any adverse effect reported from the test subjects, even better, it could help promote their bowel movements. The high drop-out rate in placebo was an uncontrolled factor because the major reasons for the withdrawal were personal inconvenience with adherence to the trial procedure/requirements. The average ages for the recruited subjects in both groups were in the appropriate study ranges (Ishijima et al., 2011). However, the variation of the female to male ratio between placebo and TCI633 groups, which may be affected by the withdrawal reason. In light of the randomized strategy, we could not assign the specific K/L grade to any group but merely obey random allocation. Unfortunately, it results in uneven subject distributions at K/L grades in placebo group. 66.7% of subjects in doubtful conditions (K/L 1) in placebo group imposed the difficulty on the fair comparison of the efficacy of symptom alleviation between placebo group and TCI633 group. Comparing the K/L results between the baseline and 12 weeks, TIC633 could improve the progression of OA in some subjects as evidenced by the portions of K/L 3 down from 35.2% to 27.0%.
The normalized levels of sCTX-II in TCI633 was improved by 41.58% after the study, the improvement progress was superior to placebo group (28.60%). The improvement rates of sCTX-II continually increased in both groups over the trial, and both groups showed a similar trend. This is, in part, because 67.7% of subjects in TCI633 group were at K/L 2 and K/L 3 and their obvious improvement progress is difficult to be observed, especially in the short period of study duration. In general, noticeable improvement effect for the symptoms of OA should take at least a half year (Lei et al., 2017). In addition, we used sCRP as an additional parameter to assess the efficacy of TCI633 although it is a controversial for OA diagnosis (Ishijima et al., 2011). Overall, the trend of sCRP in the improved population in TCI633 group was correlated with the trend of sCTX-II. Taken together, although the substantial benefits of TCI633 for improvements in OA symptoms and/or conspicuous OA treatment effect require further investigated, TCI633 does demonstrate the potential for alleviation of OA and the prevention of joint degeneration as supported by the encouraging results of the amelioration of K/L grades and the improvement rates.
According to the feedback of the subjects, patients with OA acknowledged that their keen pain, stiffness, and function remained in almost the same knee status over the trial. It implies that TCI633 can retard the progression of OA toward worse scenarios. We believe that a better treatment efficacy can be achieved by the optimization of daily dosage and long testing course. Moreover, from the perspective of the previous animal study, the improvement effect of TCI633 in human body might be attributed to the hindrance of abnormal chondrocyte metabolism and obstruction of chondrodegradation. Also, we suspected that TCI633 might be able to play some roles in gut microbiome dysbiosis and, in turn, improve the inflammatory reactions of OA (Schott et al., 2018).
Once more, this is a preliminary clinical research to unveil the possibility of TCI633 for the application of the treatment of OA. Despite the disparaging outcomes here, heavily influenced by the uneventful distribution of K/L populations, a short term of study period, and few recruited subjects, some encouraging evidences shall lead us to conduct more clinical studies for the development of an alternative SYSADOA or a novel nutraceutical supplement for prevention for the degeneration of knee joint.
5. Conclusion
This work is a first clinical research to unveil the possibility of a species of HA-generating probiotic for the symptom alleviation of OA. The unique bacteria TCI633 can generate HA in GI and possibility mitigate the chondrodegradation and apoptosis of chondrocytes through HA translocation to knee joints. The WOMAC indexes, K/L grades, and improvements of sCTX-II and sCRP indicated little positive outcomes and devised an encouraging sign for the potential for the development of an alternative SYSADOA or a novel nutraceutical supplement for prevention for the degeneration of knee joint. Admittedly, this preliminary study lacks apparently clinical advantages for the interference of the progression of OA and the improvement of the symptoms of OA; however, we open a new insight into the knee OA prevention by a new probiotic and we will undergo further investigations for more thoroughly clinical validation.
Declarations
Author contribution statement
Jia-Ling Lyu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ting-Ming Wang, Yen-Hao Chen: Performed the experiments; Analyzed and interpreted the data.
Shu-Ting Chang, Chen-Meng Kuan: Analyzed and interpreted the data; Wrote the paper.
Ming-Shiang Wu, Yung-Hao Lin, Yung-Hsiang Lin: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Amdekar S., Singh V., Singh R., Sharma P., Keshav P., Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines. J. Clin. Immunol. 2011;31:147–154. doi: 10.1007/s10875-010-9457-7. [DOI] [PubMed] [Google Scholar]
- Amiel D., Toyoguchi T., Kobayashi K., Bowden K., Amiel M.E., Healey R.M. Long-term effect of sodium hyaluronate (Hyalgan®) on osteoarthritis progression in a rabbit model. Osteoarthritis Cartilage. 2003;11:636–643. doi: 10.1016/s1063-4584(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Cho S.-K., Kim H., Park H.-R., Choi W., Choi S., Jung S.-Y., Jang E.-J., Sung Y.-K. Nonsteroidal anti-inflammatory drugs-sparing effect of symptomatic slow-acting drugs for osteoarthritis in knee osteoarthritis patients. J. Rheum. Dis. 2019;26:179–185. [Google Scholar]
- Fransen M., Agaliotis M., Nairn L., Votrubec M., Bridgett L., Su S., Jan S., March L., Edmonds J., Norton R. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 2015;74:851–858. doi: 10.1136/annrheumdis-2013-203954. [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Patrier S., Pralus A., Roche M., Nivoliez A. Protective actions of oral administration of Bifidobacterium longum CBi0703 in spontaneous osteoarthritis in Dunkin Hartley Guinea pig model. Cartilage. 2019 doi: 10.1177/1947603519841674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima M., Watari T., Naito K., Kaneko H., Futami I., Yoshimura-Ishida K. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res. Ther. 2011;13:R22. doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Maeshima T., Kubota T., Kurihara H., Masuda Y., Nomura Y. Absorption of orally administered hyaluronan. J. Med. Food. 2016;19:1172–1179. doi: 10.1089/jmf.2016.3725. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Kwon J.Y., Jhun J., Jung K., Park S.H., Yang C.W. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol. Lett. 2018;203:6–14. doi: 10.1016/j.imlet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Lei M., Guo C., Wang D., Zhang C., Hua L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef. Microbes. 2017;8:697–703. doi: 10.3920/BM2016.0207. [DOI] [PubMed] [Google Scholar]
- Lin Y.-H. 2017. Use of Streptococcus Thermophilis TCI633 in Treating Arthritis. US20170340681. [Google Scholar]
- Lin Y.-H., Su H.-L., Yu C.-H. 2015. Method of Enhancing Hyaluronic Acid Secretion Using Probiotic Strain. US20150290257. [Google Scholar]
- Lin Y.-Y., Chen N.-F., Yang S.-N., Jean Y.-H., Kuo H.-M., Chen P.-C., Feng C.W., Liu Y.-W., Lai Y.-C., Wen Z.-H. Effects of Streptococcus thermophilus (TCI633) on ACLT-induced early osteoarthritis in rat. Exp. Ther. Med. 2020 doi: 10.3892/etm.2021.9653. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A., Bay-Jensen A.C., van Spil W.E., Larkin J., Levesque M.C. Osteoarthritis year in review 2016: biomarkers (biochemical markers) Osteoarthritis Cartilage. 2017;25:199–208. doi: 10.1016/j.joca.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Ng N.T.M., Heesch K.C., Brown W.J. Strategies for managing osteoarthritis. Int. J. Behav. Med. 2012;19:298–307. doi: 10.1007/s12529-011-9168-3. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Sharma A.R., Chakraborty C., Saibaba B., Ahn M.E., Lee S.S. Review of prospects of biological fluid biomarkers in osteoarthritis. Int. J. Mol. Sci. 2017;18:601. doi: 10.3390/ijms18030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaneh K., Jamaluddin R., Karimi G., Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal. 2014;2014:595962. doi: 10.1155/2014/595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.P., Martel-Pelletier J., Abramson S.B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Deroisy R., Rovati L.C., Lee R.L., Lejeune E., Bruyere O., Giacovelli G., Henrotin Y., Dacre J.E., Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, lacebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- Rose B.J., Kooyman D.L. A Tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis. Markers. 2016;2016:4895050. doi: 10.1155/2016/4895050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi Hosnijeh F., Siebuhr A.S., Uitterlinden A.G., Oei E.H., Hofman A., Karsdal M.A., Bierma-Zeinstra S.M., Bay-Jensen A.C., van Meurs J.B. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Res. Ther. 2016;18:81. doi: 10.1186/s13075-016-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J., Bello L., Chávez M., Añez R., Rojas J., Bermúdez V. Glucosamine for osteoarthritis: biological effects, clinical efficacy, and safety on glucose metabolism. Arthritis. 2014;2014:432463. doi: 10.1155/2014/432463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott E.M., Farnsworth C.W., Grier A., Lillis J.A., Soniwala S., Dadourian G.H., Bell R.D., Doolittle M.L., Villani D.A., Awad H. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. 2018;3:e95997. doi: 10.1172/jci.insight.95997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So J.S., Song M.K., Kwon H.K., Lee C.G., Chae C.S., Sahoo A., Jash A., Lee S.H., Park Z.Y., Im S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011;88:358–366. doi: 10.1016/j.lfs.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Wittenauer R., Smith L., Aden K. World Health Organization; Geneva: 2013. Background Paper, BP 6.12 Osteoarthritis. 2013. [Google Scholar]
- Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M., Saika A., Yoshida H., Suzuki T., Yamamoto S. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J. Bone. Miner. Metab. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- Yu W., Xu P., Huang G., Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp. Ther. Med. 2018;16:2119–2125. doi: 10.3892/etm.2018.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jordan J.M. Knee osteoarthritis in women. Curr. Rev. Musculoskelet. Med. 2010;26:355–369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.