Highlights
-
•
18-month-old infants differentiate between legal and illegal phonotactics.
-
•
Infants show adult-like processing mechanisms in the electrophysiological signal.
-
•
Vascular responses do not differentiate between legal and illegal phonotactics.
-
•
Methodological and brain maturational aspects explain vascular responses.
Keywords: Phonotactics, Language acquisition, N400, Vocabulary spurt, Event-related brain potentials (ERPs), Functional near-infrared spectroscopy (fNIRS)
Abstract
The present study investigated neural correlates of implicit phonotactic processing in 18-month-old children that just reached an important step in language development: the vocabulary spurt. Pseudowords, either phonotactically legal or illegal with respect to their native language, were acoustically presented to monolingually German raised infants. Neural activity was simultaneously assessed by means of electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS). The former method excellently tracks fast processing mechanisms, whereas the latter reveals brain areas recruited. Results of the present study indicate that 18-month-olds recognize the linguistic properties of their native language based on phonotactics. This manifested in an increased N400 for legal compared to illegal pseudowords in the EEG conforming to adult-like mechanisms. Unfortunately, fNIRS findings did not support this discrimination ability. Possible methodological and brain maturational reasons might explain this null finding. This study provides evidence for the advantage of a multi-methodological approach in order to get a clear picture on neural language development.
1. Introduction
Infants possess the universal ability to acquire several languages (Eimas et al., 1971; Sebastián-Gallés, 2007; Werker and Tees, 1984, 1983; Werker and Yeung, 2005) and are able to discriminate between languages by relying on prelexical cues such as prosodic and/or phonological properties of the respective language (Cutler, 1996; Jusczyk, 1999). One important phonological cue is phonotactics, which defines possible phoneme combinations in a given language (Trask, 2004). Phonotactic structures corresponding to the subject’s native language (L1) are defined as legal (e.g., /br/ is permissible at the onset of German/English words), while phonotactic regularities corresponding to languages unknown to the subject are defined as illegal (e.g., /bz/ is not allowed at the onset of German/English words). Behavioral studies showed that infants discriminate phonotactic patterns of their L1 from illegal rules around 9 months of age (Friederici and Wessels, 1993; Jusczyk et al., 1993). This indicates that linguistic capacities are subject to a perceptual narrowing in favor of linguistic aspects of the L1 during the second half of the first year of life, which enables more targeted language learning (Kuhl et al., 2008).
Infants use phonotactic information to segment a continuous speech stream into single words which subsequently allows the learning of word meaning (Friederici and Wessels, 1993; Gonzalez-Gomez, 2013; Jusczyk et al., 1993; Mattys and Jusczyk, 2001; Sebastián‐Gallés, 2007). Around 18 months, children reach an important step in their language development: the vocabulary spurt (Goldfield and Reznick, 1990; Menyuk et al., 2014). Although varying a lot at the individual level, the vocabulary spurt is defined by an accelerating production of words (at least 10 new words every 2 weeks) (Ganger and Brent, 2004). This improvement in productive abilities aids the learning of new word meanings in the child’s respective L1. Hence, the ability to discriminate legal from illegal phonotactic cues might even get more crucial in-the-midst of this important developmental step. A behavioral study (Graf Estes et al., 2011) showed that 18-month-olds listened longer to legal pseudoword-object combinations than to illegal pairs. Performance increased with vocabulary size indicating that phonotactic knowledge indeed impacts word learning at that age.
The present study aims at investigating neural correlates of phonotactic processing in 18-month-olds. Neuroscientific studies on phonotactic processing are scarce but bear the potential to reveal mechanisms beyond the pure behavioral level (Obrig et al., 2017; Rossi et al., 2011). We simultaneously assessed electrophysiological and vascular changes by means of electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS). The EEG is very suitable to measure fast dynamic electrophysiological changes whereas the fNIRS mainly assesses slower vascular responses with a better localization of topographical origins of signals. Phonotactically legal and illegal pseudowords were presented acoustically with infants listening passively to the stimuli. We aimed at investigating implicit processing of phonotactic regularities, as previous studies regarding phonotactic processing on word level in infants mainly contained attentional cues or used additional visual aids (Friedrich and Friederici, 2005; Obrig et al., 2017).
Rossi et al. (2011) conducted a combined EEG and fNIRS study with monolingually German raised adults. Pseudowords consisting of legal or illegal onset consonant clusters were acoustically presented. EEG results (i.e., event-related brain potentials (ERPs)), revealed increased N400 amplitudes for legal compared to illegal stimuli on centro-parietal electrodes. Usually, the N400 component is linked to lexico-semantic processes, with increased negativities indicating more effort to access the mental lexicon (Kutas and Federmeier, 2011). FNIRS results showed recruitment of left fronto-temporal brain areas for legal compared to illegal pseudowords. Such evidence confirms the Dynamic Dual Pathway model (Friederici and Alter, 2004) suggesting that segmental features of language like phonotactics and phonology predominantly recruit left-hemispheric networks (Gow and Nied, 2014; Vaden et al., 2011), while suprasegmental features of language like prosody mainly recruit right-hemispheric regions (Gandour et al., 2004; Perkins et al., 1996; Zatorre et al., 1992). Results indicate that adults recognize native phonotactic rules whereas illegal cues do not launch access to the mental lexicon.
Do 18-month-old infants already show adult-like processing of phonotactic cues? Previous research revealed that the infant brain does not always act in an adult-like manner. In an EEG study with adults as well as 12- and 19-month-olds (Friedrich and Friederici, 2005), pictures of real objects were simultaneously presented with legal and illegal pseudowords. Adults displayed a typical centro-parietal N400-effect with a larger negativity for legal pseudowords. 19-month-olds showed an adult-like effect, while 12-month-olds displayed a more frontally distributed negativity for legal pseudowords not reflecting an N400 but a word priming effect. This might indicate that an adult-like N400-effect to phonotactic processing could develop around 19 months of age. Nevertheless, there is evidence from studies with picture-word-association paradigms that the N400 can occur at much earlier stages of development (Friedrich and Friederici, 2017, 2011; Junge et al., 2012; Parise and Csibra, 2012). The presence of an N400 might be subject to various criteria such as study design, materials used, and even brain maturation (Junge et al., 2012). Several studies on word processing in infants from 12 months on suggest that the N400-effect might depend on the individual level of vocabulary production skills (Friedrich and Friederici, 2010, 2004; Rämä et al., 2013; Torkildsen et al., 2009, 2008).
In younger infants, instead of an N400-effect, more frontally distributed negativities referred to as N200-500 components have been reported (Friedrich and Friederici, 2005). Interestingly, the N200-500 was also found to be more pronounced in infants at risk of dyslexia (Torkildsen et al., 2007) or in infants with very low language skills (Friedrich and Friederici, 2006). Thus, the N200-500 can be seen as an immature semantic processing mechanism that requires less processing effort and suggests an initial process of encoding word forms into memory.
Apart from semantic processing, the N200-500 might also represent a more general phonological word familiarization effect with larger frontal amplitudes for familiar/learned stimuli (Friedrich and Friederici, 2017, 2011; Goyet et al., 2010; Kooijman et al., 2013, 2005; Obrig et al., 2017; Thierry et al., 2003). In 19-month-olds, the N200-500 was more pronounced for phonotactically familiar word forms compared to non-words (Friedrich and Friederici, 2005).
With respect to hemispheric lateralization, research in adults clearly suggests a dominance of left fronto-temporal brain areas during phonotactic processing (Friederici and Alter, 2004; Rossi et al., 2011). Infant studies using diverse segmental speech stimuli also often find a predominant involvement of the left hemisphere (Dehaene-Lambertz et al., 2002; Minagawa-Kawai et al., 2011). There is growing evidence however, that neural mechanisms shift from broader and diffuse (Shultz et al., 2014) - sometimes bilateral (Obrig et al., 2017; Perani et al., 2011) - to more focal and lateralized activation during development, indicating that neural mechanisms are not yet as specialized as in the adult brain (Brauer et al., 2008; Brauer and Friederici, 2007; Harris et al., 2011; Holland et al., 2001; Johnson, 2001; Szaflarski et al., 2006).
We suggest that 18-month-olds should already reflect adult-like mechanisms as they are in-the-midst of vocabulary spurt indicating rather high word comprehension and production skills at this age. Therefore, we expect to find larger N400-amplitudes for legal compared to illegal stimuli in the EEG as well as a stronger left-lateralization for legal stimuli in the fNIRS (Rossi et al., 2011).
Pseudowords used in the present study are unknown to the infants and both legal and illegal onset clusters are presented repeatedly throughout the experiment. As infants might tend to implement new word forms faster during the vocabulary spurt, we suggest that some kind of familiarization will occur (i.e., increasing N200-N500 in the second experiment half).
2. Materials and methods
2.1. Participants
In total, 26 18-month-old infants (12 female) participated in the present study. All subjects were monolingually raised with German as their L1. Some studies showed that being raised bilingually from birth alters brain development (Liu et al., 2014; Schweizer et al., 2012; Stocco and Prat, 2014; Woumans et al., 2015). All subjects entered EEG analyses. Data of one subject had to be excluded from fNIRS analyses due to technical problems during measurements.
Infants were 547.7 days (range: 517–583) old. All infants were healthy, not born prematurely (mean week of gestation: 40; range: 37–42), and had no hearing or visual impairments. Mean birth length was 50 cm (range: 46–54) and mean birth weight 3384 g (range: 2320–4125).
As handedness is not finally developed in such young infants, we assessed handedness of family members. Most parents were right-handed. 2 out of 52 parents were left-handed and 1 was ambidextrous. In all these three cases, the respective partner was right-handed. Level of education of parents was rather high in the present study (university degree: n = 39; high school: n = 7; compulsory schooling : n = 6). In order to exclude a potential genetically driven influence of familiar speech impairments, we assessed these by means of a questionnaire. Only 1 out of 52 parents had deficits in pronunciation during childhood. In order to guarantee that general development of all infants was within a normal range, retrospective interrogation of parents was carried out and confirmed a normal development based on the following data: Infants were able to sit without help at 7 months on average (range: 5–11) and to walk without help at 13 months (range: 9–18). First words were spoken at a mean age of 12 months (range: 5–18). Thus, we can assume that all 18-month-olds were at the stage of vocabulary spurt. As subjects were part of a longitudinal study assessing infants also at 24 months of age, we were able to gather some additional language production information concerning the ability to combine two words to a sentence retrospectively. Mean starting age of two-word-sentence production was 20 months (1 missing; range: 16–24).
2.2. Material
The material was selected from a stimulus pool that was already successfully used in similar neuroscientific studies with adult subjects and infants (Obrig et al., 2017; Rossi et al., 2011). Stimuli consisted of 168 monosyllabic pseudowords of a consonant-consonant-vowel-consonant (CCVC) structure without any semantic content. All onset consonant clusters (first two consonants) of the pseudowords were either phonotactically legal (e.g., /brop/) or illegal (e.g., /bzop/) with respect to German. In total, 28 onset clusters (14 legal and 14 illegal) were constructed, each used in 6 different pseudowords. This resulted in 84 legal and 84 illegal pseudowords. Phonotactically illegal onset clusters reflected phoneme sequences of the Slovak language that has proven to be very useful in this regard, since it provides a greater variability and more combination possibilities of consonants in word onsets than German (Hanulíková, 2009). All pseudowords were controlled for consonant cluster frequency and presented in a pseudorandomized manner with the following criteria: maximally 3 legal/illegal and maximally 3 high/low frequency pseudowords in succession and an equal amount of legal/illegal pseudowords per experiment half.
All stimuli were spoken by a German/Slovak bilingual female speaker in a soundproof chamber and recorded digitally with 16 bits and 44 kHz.
In order to direct the infants’ attention to the stimuli, pseudowords were spoken in infant-directed-speech (IDS) mode based on imagined interactions with infants. IDS is a standard procedure in infant research and characterized by a higher pitch and a slower, clearer speech mode (Garnica, 1977; Soderstrom, 2007).
2.3. Procedure
In the present study, neural activity was assessed simultaneously by means of electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS). The former method excellently tracks fast processing mechanisms in the millisecond range, whereas the latter provides a good spatial resolution identifying underlying brain areas. Combining both methods has proven to be extremely beneficial for investigating acoustic stimuli, as both methods are soundless, do not interfere with each other, and provide a quite natural setting. In addition, such a non-invasive approach is very suitable for conducting neuroscientific research in infants (Rossi et al., 2012).
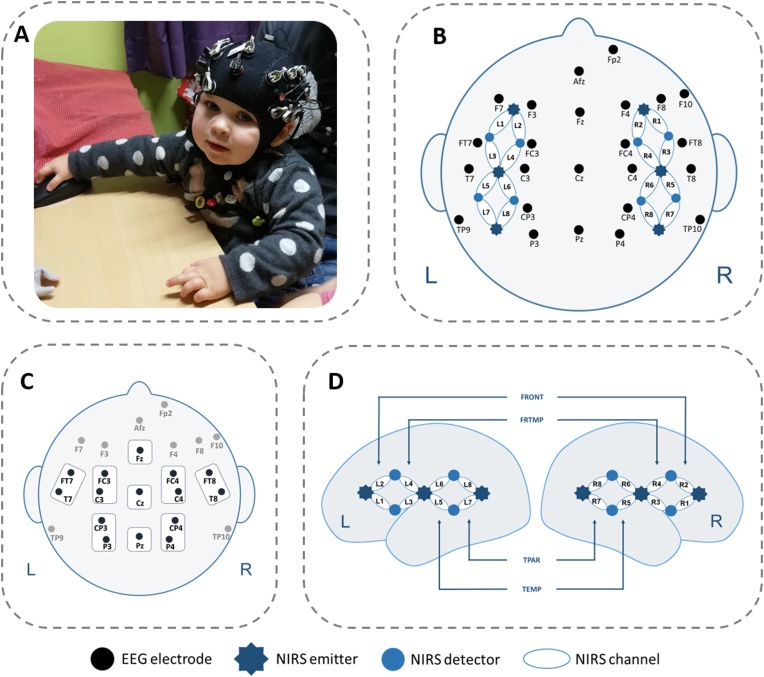
The study was approved by the local ethical committee and written informed consent was obtained from all parents prior to measurements. During the experiment, infants sat on their parent’s lap 1 m in front of a computer monitor with one experimenter aside to intervene in case of discomfort. To enable a simultaneous measurement of both methods, infants wore elastic EEG caps (EasyCap GmbH, Herrsching, Germany) in which both EEG electrodes and fNIRS optodes were integrated (cf. Fig. 1).
Fig. 1.
Simultaneous EEG-electrodes and fNIRS-channel placement. A. An 18-month-old infant wearing the measurement cap (permission to show the picture was obtained from parents). B. EEG/fNIRS configuration. C. EEG-electrode configuration including regions of interest (ROIs). D. Lateral view of fNIRS-channel arrangement. Black dots indicate EEG electrodes; stars indicate 6 fNIRS light emitters; blue dots indicate 8 fNIRS detectors; ellipses indicate fNIRS channels; L1-8 show 8 left-hemispheric fNIRS channels; R1-8 show 8 right-hemispheric fNIRS channels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
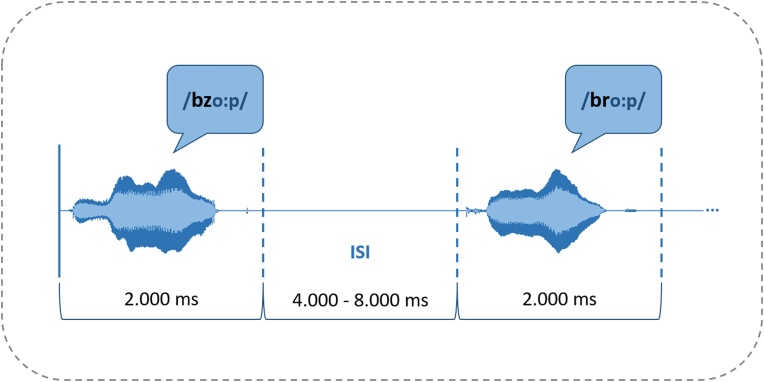
Pseudowords were acoustically presented at 70 dB via loudspeakers centered in front of the infants. As no explicit task was given, infants listened passively to the stimuli. To keep infants’ attention, a silent children’s cartoon was shown on the computer screen during experimental session. Each pseudoword was presented in a slot of 2000 ms, while inter-stimulus-intervals (ISIs) varied in length (mean = 6000 ms; range 4000−8000 ms). By introducing this variable ISI, the experimental design was adjusted to the requirements of the rather slow hemodynamic response function (hrf) measured by the fNIRS. Usually, vascular responses reach their maximum at around 5 s after stimulus onset with the activation returning to baseline after 15−20 s (Huettel and McCarthy, 2009). Therefore, variable ISIs minimize overlapping of hemodynamic responses in event-related designs (cf. Fig. 2). The experiment lasted 23 min.
Fig. 2.
Experimental design of the present study.
2.4. EEG recordings
EEG was recorded from 19 AgAgCl active electrodes (BrainProducts GmbH, Gilching, Germany) placed into an elastic EEG cap at the following positions of the 10−20 system (Jasper, 1958; Sharbrough et al., 1991): F7, F3, FT7, FC3, T7, C3, CP3, P3, F4, F8, FC4, FT8, C4, T8, CP4, P4, Fz, Pz, and Cz (Fig. 1). Vertical and horizontal electrooculogram were recorded above and next to the right eye with electrodes FP2 and F10. An electrode (TP9) at the left mastoid served as online reference. Additionally, an electrode at the right mastoid (TP10) was recorded for further re-referencing during offline analyses. The ground electrode was positioned at AFz. Electrode impedance was kept below 10 kΩ (actiCAP Control, Brain Products GmbH, Gilching, Germany). The EEG signal was measured by means of BrainVision Recorder (Brain Products GmbH, Gilching, Germany) software with a sampling frequency of 1000 Hz (amplified between 0.016−450 Hz). An anti-aliasing filter with a cut-off at 250 Hz (slope: 30 dB/oct) was applied prior to analogue-to-digital conversion.
2.5. fNIRS recordings
Vascular changes were measured by means of fNIRS. This method enables to assess concentration changes of both oxygenated [oxy-Hb] and deoxygenated hemoglobin [deoxy-Hb] in cortical brain areas by emitting light in the near-infrared spectrum on the biological tissue. Concentration changes in both hemoglobins can be calculated based on a modified Beer-Lambert law (Cope et al., 1989). The physiological basis of fNIRS is the neurovascular coupling. An increased activation in a brain region leads to several vascular and metabolic changes. Vasodilation leads to a local increase in blood volume demanding more oxygen and glucose, which in turn leads to an increase in regional cerebral blood flow and regional blood flow velocity (Logothetis and Wandell, 2004; Uludağ et al., 2004).
Consequently, the color of the blood changes. The blood flow increase overcompensates oxygen consumption and elicits a focal hyperoxygenation resulting in an increase in [oxy-Hb] as well as a decrease in [deoxy-Hb] (Fox and Raichle, 1986). [Deoxy-Hb] represents the major contribution of the BOLD signal as measured by functional magnetic resonance imaging (fMRI) (Huppert et al., 2006; Kleinschmidt et al., 1996; Obrig and Villringer, 2003; Steinbrink et al., 2006); for opposing results see (Strangman et al., 2002).
The used fNIRS system (NIRScout, NIRx Medizintechnik GmbH, Berlin) sends wavelengths at 860 and 730 nm and recorded data at 20.83 Hz. 6 light emitters and 8 light detectors were used to assess bilateral fronto-temporo-parietal brain areas. Interoptode distance was 2.5 cm. This emitter-detector configuration allowed the assessing of 8 channels per hemisphere, which were distributed over frontal (positions L1, L2, R2, and R1), fronto-temporal (positions L3, L4, R4, and R3), temporal (positions L5, L6, R6, and R5), and temporo-parietal (positions L7, L8, R8, and R7) brain regions (Fig. 1). Positioning of fNIRS light emitters and detectors was based on the standard EEG 10−20 electrode positioning system (Jasper, 1958; Sharbrough et al., 1991). Recent studies in adults (Okamoto et al., 2004) and infants (Kabdebon et al., 2014) used this electrode positioning to project to underlying anatomical structures in order to provide a better mapping of signals assessed from the scalp.
2.6. Data analyses
2.6.1. EEG data
EEG data was filtered offline with a 45 Hz low-pass Butterworth zero-phase filter (high cutoff: 45 Hz; slope: 12 dB/oct) and a 50 Hz notch filter. Data was then segmented from −200 ms to 1300 ms with 0 ms representing pseudoword onset. Overly contaminated channels were rejected manually from each segment via visual inspection. Only infants in whom per condition at least one third of all segments in at least half of all electrodes survived this procedure were included in the final analyses. Afterwards, data was re-referenced to averaged mastoids (TP9, TP10) and a pre-stimulus baseline of 200 ms was applied. Event-related brain potentials (ERPs) were extracted by averaging segments for each condition (legal first experiment half, legal second experiment half, illegal first experiment half, illegal second experiment half) and each subject. In order to select time windows for final statistical analyses, a 50-ms-analysis was performed including paired-sampled t-tests between legal and illegal items (for halves separately) in consecutive 50-ms steps between 100 and 1000 ms on each electrode. Results from this analysis and visual inspection of the grand averages revealed 500−1000 ms as the time window indicating clear differences between conditions and was selected to perform further statistical analyses.
Because topographical localization of EEG is only rough, we decided to merge lateral electrodes to 6 regions of interest (ROIs). Following lateral ROIs were defined: left anterior inferior (FT7, T7), right anterior inferior (FT8, T8), left anterior superior (FC3, C3), right anterior superior (FC4, C4), left posterior (CP3, P3), and right posterior (CP4, P4) (Fig. 1). We then performed a three-way repeated-measures ANOVA with the within-subject factors Legality (legal vs. illegal), Half (first vs. second experiment half), and Region (6 lateral ROIs as well as 3 midline electrodes Fz, Cz, and Pz) for the time window of 500−1000 ms. Significance level was set at p ≤ .050 and adjusted according to Bonferroni. Whenever Mauchly’s test of sphericity became significant, Greenhouse-Geisser correction (Greenhouse and Geisser, 1959) was applied.
2.6.2. fNIRS data
To analyze concentration changes (mmol/l) of [oxy-Hb] and [deoxy-Hb], the collected reflected light was transformed by means of the modified Beer Lambert function (Cope et al., 1989). Artifacts (mainly arising from head movements, leading to fast spikes and shifts from baseline values) of each participant were screened manually and removed by a linear spline interpolation approach which has been frequently used in adult and infant research (e.g., Grossmann et al., 2010; Obrig et al., 2017) and preferred compared to methods rejecting artifact-contaminated segments (Brigadoi et al., 2014; Di Lorenzo et al., 2019) as it allows keeping a large amount of data (Brigadoi et al., 2014; Scholkmann et al., 2010). Even though a manual identification of artifacts might be considered quite subjective as it could differ based on the person inspecting the data, we tried to minimize this drawback (1) by using experienced researchers performing artifact identification and (2) by comparing both raw signals and z-score data for artifact identification. Afterwards, marked segments were padded by interpolation. A 0.3 Hz low-pass filter (Butterworth, third order) was used to attenuate high-frequency artifacts mainly arising from heartbeat.
We applied a general-linear-model (GLM) instead of averaging data as (1) it can better describe the complex hemodynamic response and (2) results are better comparable to existing fMRI data where GLM usage is standard procedure. A GLM further allows better handling of partial overlap of hrfs due to the relatively short stimulation and ISIs integrated in an event-related design (de Roever et al., 2018; Issard and Gervain, 2018). For the GLM, data were correlated with a predictor generated by convolving the boxcar function of the stimulus design including 4 different conditions (legal first experiment half, legal second experiment half, illegal first experiment half, illegal second experiment half) with the canonical hrf (Boynton et al., 2012; Huettel and McCarthy, 2009). During this modelling, a stimulation period of 2 s (i.e., on-condition) and a resting period (i.e., off-condition; silence) resulting from ISIs was assumed and a high-pass filter of 20 s to remove drifts and slow fluctuations was applied (for similar procedures please see Altvater-Mackensen and Grossmann, 2018, 2016; Koch et al., 2006; Obrig et al., 2017; Telkemeyer et al., 2009). GLM using a canonical hrf (peak at 5 s and further 15 s for return to baseline) provides Beta-values which were used for statistical analyses.
Both a decrease in [deoxy-Hb] and an increase in [oxy-Hb] reflect increased activations (Lloyd-Fox et al., 2010; Obrig and Villringer, 2003). We performed a three-way repeated-measures ANOVA with the within-subject factors Legality (legal vs. illegal), Half (first vs. second experiment half), and Channel (16 channels) for [oxy-Hb] and [deoxy-Hb], separately. We did not opt to build ROIs for fNIRS data as we wanted to keep the more accurate topographical resolution of the fNIRS methodology as fine-grained as possible.
Significance level was set at p ≤ .050 and adjusted according to Bonferroni. Whenever Mauchly’s test of sphericity became significant Greenhouse-Geisser correction (Greenhouse and Geisser, 1959) was applied.
3. Results
3.1. EEG results
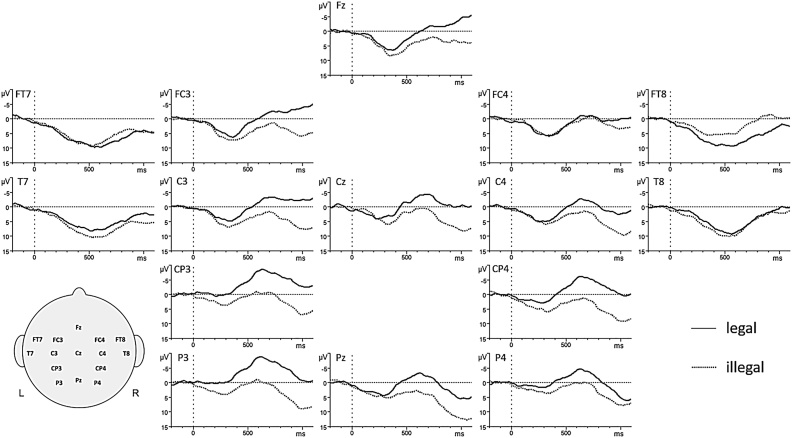
A three-way ANOVA (Legality x Half x Region) revealed a significant main effect for Legality [F(1,23) = 4.611, p = .043]. This effect was reflected by a larger negativity for legal (M=-.16; SD=9.37) compared to illegal (M=4.00; SD=11.56) pseudowords (cf. Fig. 3).
Fig. 3.
ERP results on all electrodes for legal compared to illegal pseudowords, merged across experiment halves. Negative polarity is plotted upwards.
3.2. fNIRS results
Three-way ANOVAs (Legality x Half x Channel) neither for [oxy-Hb] nor for [deoxy-Hb] revealed any significant main effect or interaction (please refer to Supplementary Fig. S1 and Table S1).
4. Discussion
The present study investigated implicit processing of phonotactic regularities in 18-month-olds by applying two methods simultaneously to gain electrophysiological and vascular results. Phonotactically legal and illegal pseudowords were presented acoustically to infants raised with German as their L1. Since subjects listened passively to the stimuli, our results provide first-time evidence that phonotactic cues induce lexical processing mechanisms in infants even when no specific task or additional visual aids are given.
Event-related brain potentials (ERPs) revealed a larger negativity for phonotactically legal than illegal pseudowords. The topography of this finding extended from frontal to centro-parietal areas, reflecting a mixture of a centro-parietal N400 component similar to that found in adult studies (Friedrich and Friederici, 2005; Rossi et al., 2011), but also a more frontally distributed N200-500 component. As an increased N400 usually indicates more effort to access the mental lexicon (Kutas and Federmeier, 2011), authors interpret this effect as a successful access to the mental lexicon for regularities known from the L1. In this sense, illegal rules not belonging to the subjects’ L1 do not launch lexical access and thus show a reduced N400 (Rossi et al., 2011). ERP studies with 9-, 10-, and 12-month-olds repeating phonotactically legal words (Goyet et al., 2010; Junge et al., 2012; Kooijman et al., 2005) showed an N200-500 effect. With the familiarization level rising, also amplitudes increased in negativity in fronto-temporal and fronto-central brain regions. Therefore, EEG results of the present study indicate that 18-month-olds recognize the specific regularities of their L1 as familiar rules, while illegal cues seem to be irrelevant at a prelexical level and are therefore discarded. Such a processing strategy is defined as perceptual narrowing to the L1 (Kuhl et al., 2008) and reflects a very beneficial ability supporting rapid word learning during vocabulary spurt by directing the infants’ attention to speech samples relevant for their target language. In adults, N400s are normally found between 300 and 600 ms after stimulus onset. In our case, they were found at later processing stages (500−1000 ms). Thus, on an electrophysiological level, children of the present study already used mature processing strategies to induce lexical search mechanisms that were very similar but not yet identical to adult mechanisms.
Until now, adult-like neural mechanisms during phonotactic processing reflected in a lexical N400 were only found in 19- but not in 12-month-olds, indicating that phonotactic processing strategies seem to become more mature in this period of time (Friedrich and Friederici, 2005). However, previous studies did not examine implicit processing of acoustically presented phonotactic cues, but used additional visual aids integrated into learning paradigms. Recognizing rules based on previous knowledge of native phonotactics simply through passive listening without any visual or semantic cues is a challenging task. Thus, it is intriguing that adult-like phonotactic mechanisms are elicited implicitly already at 18 months of age.
FNIRS findings unfortunately did not reveal any significant main effect or interaction, neither for [oxy-Hb] nor for [deoxy-Hb]. Several reasons may account for this null effect depending on the respective approach (developmental competence, methodological aspects, and brain maturation).
One explanation which first comes to mind is a potential insensitivity of 18-month-olds with respect to phonotactic regularities. A similar insensitivity reflected by null effects was found in a previous fNIRS study on phonemic variations based on vowel duration in 10–12-month-old infants (Minagawa-Kawai et al., 2007). Authors interpreted this transient insensitivity as a neural reorganization process. Such an interpretation might also apply to the findings in our study assuming that 18-month-olds are in the midst of the vocabulary spurt and thus more focused on word production than perception. However, our EEG results very well show phonotactic differentiation processes suggesting that electrophysiological and vascular signals are not linearly related (Issard and Gervain, 2018) and that other factors might underlie our null effect revealed by the fNIRS.
One fNIRS study specifically investigating the differentiation of native and non-native phonotactic and vowel duration contrasts in a change detection paradigm in infants from 3 to 14 months, also revealed a null effect with respect to both contrasts (Minagawa-Kawai et al., 2013). In this study, first a baseline block including only native pseudowords was presented, followed by the target block alternating native and non-native (phonotactic or vowel duration) pseudowords. The authors discuss several methodological issues having potentially caused the null effect. The usage of two different contrast types might have complicated processing. An additional investigation in adult participants (Minagawa-Kawai et al., 2013) in fact revealed increased activations when only one contrast was used. In our study, we indeed used only one contrast but we presented a great variety of different consonant clusters for both legal and illegal phonotactic rules. Such a material introduces an increased stimulus complexity, which was reported to negatively affect vascular signals leading to reversed or null effects (Benavides-Varela et al., 2012; Issard and Gervain, 2018). Our motivation to provide a greater stimulus variability was to optimize ecological validity of the material set. Even though such an interpretation is plausible, it does not seem to account for our null effect as the EEG results clearly show a differentiation ability between legal and illegal phonotactic rules. Further, the stimulus duration was discussed as a crucial factor for producing reliable hemodynamic responses (Minagawa-Kawai et al., 2013). In particular, a stimulation duration of at least 15 s proofed to elicit stronger activations in adults compared to the 9 s stimulation adopted in the infant study. Longer stimulus durations allow for a better estimation of baseline levels and beta-values when using a GLM model (Cristia et al., 2013). In our study, stimulation duration was very short (around 2 s) and can thus have negatively impacted hemodynamic responses. Also, the duration of the inter-stimulus-interval (ISI) has been shown to affect vascular response. ISIs longer than 15 s with a jittered interval have been found to be optimal for fNIRS studies in order to prevent an overlap of hemodynamic responses (Aarabi et al., 2017). In our study, ISI was jittered but much shorter (6 s on average, range 4−8 s) than proposed. Thus, also this factor might have been a driving force for our null effect. It should be, however, noted that longer stimulation and ISI periods prolong the experiment which leads to more attrition rates due to an increased number of movement artifacts especially in infants as well as to increased habituation effects (Cristia et al., 2013). Furthermore, the selection of the study design is a crucial factor impacting signal strength. FNIRS as well as fMRI designs can basically be subdivided into block and event-related designs. Pure block designs present stimuli of the same condition fast, repeatedly, and in succession in one block allowing systematic overlap of hemodynamic responses and introduce rest periods without stimulation in between. Such a design works well for fNIRS, but bears the risk of habituation effects because of many repetitions of the same stimulus category. As a consequence, neural response suppressions were found (Issard and Gervain, 2018). Event-related designs prevent from habituation, but necessitate long ISIs between randomly presented stimuli, in order to minimize hemodynamic response overlap. This in turn prolongs experimental time which is again a problem in restless infants. As previously mentioned, a jittering of ISIs is one preferred method to reduce experimental time, but they have to be of sufficient length (Aarabi et al., 2017). In general, however, event-related designs are better suitable for assessing fast electrophysiological signals than slow vascular responses. Such an observation is supported by our findings leading to clear phonotactic discrimination in the EEG, but not in the fNIRS. An optimization of the simultaneous EEG-fNIRS design would be the introduction of alternating mini-blocks in which short blocks of stimuli of one condition are alternated with resting periods and further short blocks of another condition. Future studies are necessary in regard investigating phonotactic processing in infants using such an alternating mini-block design. Apart from that, the choice of analyses methods of fNIRS can further impact the result pattern. Deconvolution methods such as the application of a general linear model with several assumptions about the hemodynamic response function (hrf) were found to outperform conventional averaging (Aarabi et al., 2017), especially when small numbers of trials, short stimuli, overlapping hrfs, and a low signal-to-noise ratio are present (de Roever et al., 2018; Issard and Gervain, 2018). Even though a GLM was applied in the present study, the previously mentioned factors though seem to predominantly account for the null effect.
Another explanation for the null effect in our 18-month-old infants refers to brain maturation. Biological processes such as neurovascular coupling, brain metabolic demands, angiogenesis, synaptogenesis, and synaptic pruning develop at a different pace during infant and childhood development (de Roever et al., 2018; Harris et al., 2011; Schmithorst et al., 2015; Vasung et al., 2019). Particularly relevant for eliciting altered vascular responses in fNIRS studies in infants are an immature brain vasculature, often found to lead to inverted hemodynamic responses (Issard and Gervain, 2018) as well as an increased metabolic rate in infants compared to adults (Vasung et al., 2019). Furthermore, the maturation of the brain network is subject to different developmental rates. Even though major brain regions are at work from birth on or even prenatally, the brain size in infants aged 2–4 weeks is 36 % of the adult volume and has grown to 83 % of the adult volume by the second birthday (Knickmeyer et al., 2008). Compared to this grey matter development, the small-world brain network organization including hubs (i.e., dense connection within the whole network) and modules (i.e., dense links between nodes but less connections with the rest of the network) develops at an even slower pace throughout childhood (Cao et al., 2017; Yin et al., 2019). In particular, association fibers develop during early childhood (Huang et al., 2006) and myelination processes start around 2.5 months, but end in adolescence (Luders et al., 2010; Qiu et al., 2015). Especially relevant for our study in 18-month-old infants is the finding that long-range connections, among others responsible for signal transmission between hemispheres, particularly develop between 1 and 2 years of life (Cao et al., 2017; Yin et al., 2019). Mature long-range connections improve small-world organization and global efficiency (Yin et al., 2019). At the age of 2 years, bilateral superior medial frontal regions relevant for higher-order cognitive functions are indeed present as hubs but functional hub distribution is still immature (Gao et al., 2011). These regions and connections are important for lexical processes. Thus, it seems plausible to assume that this immature brain connectivity might be at least partially responsible for our null effects found in the fNIRS. Further support for this interpretation comes from the individual variability in functional connectivity, which has been found to be quite stable first at birth, to even decrease until 12 months of age, but to increase afterwards during the second year of life (Gao et al., 2014). A greater variability between individual subjects is often a cause of differential activation patterns dissolving at the group level and increasing the risk for null effects.
Overall, results of the present study indicate that 18-month-olds recognize the linguistic properties of their native language based on phonotactics, which in turn supports rapid word learning during the vocabulary spurt. Neural responses already reflect adult-like mechanisms in the EEG. Legal phonotactic cues initiated increased responses indexed by an adult-like N400 component. However, EEG findings are unfortunately not supported by the fNIRS. Such a divergence indicates that both methods assess differential neural signals which sometimes conform but sometimes do not. Nevertheless, this study emphasizes the benefit of a multi-methodological approach in order to provide detailed insights into neural language processes. Having adopted fNIRS as a single method might have led to an incorrect conclusion about the sensitivity of 18-month-olds to differentiate legal from illegal phonotactic rules. A combination of EEG and fNIRS therefore bears the potential to provide profound insight in neural processing strategies. Methodological issues such as longer stimulation and inter-stimulus-interval durations as well as the adoption of an alternating mini-block design should be considered in future research. Further, an immature vascular response at 18 months of age might additionally be a driving force behind the null effect shown by fNIRS. Future studies need to elaborate more on methodological fundamentals to improve simultaneous EEG-fNIRS designs as well as on maturational brain changes from infancy to early childhood.
Author contributions
S.R. conceived the experiment, S.S. and S.R. performed the experiment, S.S. and S.R. analyzed the data, S.S. prepared the figures, S.S. and S.R. wrote the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
We would like to thank all parents for participating in our study with their infants and Bettina Johst for helping in programming the experiment. We further thank Alicia Kratky, Nicola König, Lukas Aretz, Miriam Ebersberg, Klaus Hueber, Claudia Hofmann, Julia Gamper, Michél Hartmann, David Böhm, Lisa Parigger and Carolin Huter for their help during EEG/fNIRS measurements.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100784.
Contributor Information
Sarah Steber, Email: sarah.steber@tirol-kliniken.at.
Sonja Rossi, Email: sonja.rossi@i-med.ac.at.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aarabi A., Osharina V., Wallois F. Effect of confounding variables on hemodynamic response function estimation using averaging and deconvolution analysis: an event-related NIRS study. NeuroImage. 2017;155:25–49. doi: 10.1016/j.neuroimage.2017.04.048. [DOI] [PubMed] [Google Scholar]
- Altvater-Mackensen N., Grossmann T. The role of left inferior frontal cortex during audiovisual speech perception in infants. NeuroImage. 2016;133:14–20. doi: 10.1016/j.neuroimage.2016.02.061. [DOI] [PubMed] [Google Scholar]
- Altvater-Mackensen N., Grossmann T. Modality-independent recruitment of inferior frontal cortex during speech processing in human infants. Dev. Cogn. Neurosci. 2018;34:130–138. doi: 10.1016/j.dcn.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Varela S., Hochmann J.-R., Macagno F., Nespor M., Mehler J. Newborn’s brain activity signals the origin of word memories. PNAS. 2012;109:17908–17913. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton G.M., Engel S.A., Heeger D.J. Linear systems analysis of the fMRI signal. NeuroImage. 2012;20:975–984. doi: 10.1016/j.neuroimage.2012.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brauer J., Neumann J., Friederici A.D. Temporal dynamics of perisylvian activation during language processing in children and adults. NeuroImage. 2008;41:1484–1492. doi: 10.1016/j.neuroimage.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi S., Ceccherini L., Cutini S., Scarpa F., Scatturin P., Selb J., Gagnon L., Boas D.A., Cooper R.J. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Huang H., He Y. Developmental connectomics from infancy through early childhood. Trends Neurosci. 2017;40:494–506. doi: 10.1016/j.tins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M., Delpy D.T., Wray S., Wyatt J.S., Reynolds E.O.R. A CCD spectrophotometer to quantitate the concentration of chromophores in living tissue utilising the absorption peak of water at 975 nm. In: Rakusan K., Biro G.P., Goldstick T.K., Turek Z., editors. Oxygen Transport to Tissue XI. Springer US; Boston, MA: 1989. pp. 33–40. [DOI] [PubMed] [Google Scholar]
- Cristia A., Dupoux E., Hakuno Y., Lloyd-Fox S., Schuetze M., Kivits J., Bergvelt T., van Gelder M., Filippin L., Charron S., Minagawa-Kawai Y. An online database of infant functional near InfraRed spectroscopy studies: a community-augmented systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A. Prosody and the word boundary problem. In: Morgan J.L., Demuth K., editors. Signal to Syntax: Bootstrapping From Speech To Grammar in Early Acquisition. Psychology Press; 1996. pp. 87–99. [Google Scholar]
- de Roever I., Bale G., Mitra S., Meek J., Robertson N.J., Tachtsidis I. Investigation of the pattern of the hemodynamic response as measured by functional near-infrared spectroscopy (fNIRS) studies in newborns, less than a month old: a systematic review. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo R., Pirazzoli L., Blasi A., Bulgarelli C., Hakuno Y., Minagawa Y., Brigadoi S. Recommendations for motion correction of infant fNIRS data applicable to multiple data sets and acquisition systems. NeuroImage. 2019;200:511–527. doi: 10.1016/j.neuroimage.2019.06.056. [DOI] [PubMed] [Google Scholar]
- Eimas P.D., Siqueland E.R., Jusczyk P., Vigorito J. Speech perception in infants. Science. 1971;171:303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- Fox P.T., Raichle M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. PNAS. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D., Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Wessels J.M.I. Phonotactic knowledge of word boundaries and its use in infant speech perception. Percept. Psychophys. 1993;54:287–295. doi: 10.3758/BF03205263. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. N400-like semantic incongruity effect in 19-Month-Olds: processing known words in picture contexts. J. Cogn. Neurosci. 2004;16:1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. Lexical priming and semantic integration reflected in the event-related potential of 14-month-olds. Neuroreport. 2005;16:653–656. doi: 10.1097/00001756-200504250-00028. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. Early N400 development and later language acquisition. Psychophysiology. 2006;43:1–12. doi: 10.1111/j.1469-8986.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. Maturing brain mechanisms and developing behavioral language skills. Brain Lang. 2010;114:66–71. doi: 10.1016/j.bandl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. Word learning in 6-month-olds: fast encoding–weak retention. J. Cogn. Neurosci. 2011;23:3228–3240. doi: 10.1162/jocn_a_00002. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. The origins of word learning: brain responses of 3-month-olds indicate their rapid association of objects and words. Dev. Sci. 2017;20:e12357. doi: 10.1111/desc.12357. [DOI] [PubMed] [Google Scholar]
- Gandour J., Tong Y., Wong D., Talavage T., Dzemidzic M., Xu Y., Li X., Lowe M. Hemispheric roles in the perception of speech prosody. NeuroImage. 2004;23:344–357. doi: 10.1016/j.neuroimage.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ganger J., Brent M.R. Reexamining the vocabulary spurt. Dev. Psychol. 2004;40:621–632. doi: 10.1037/0012-1649.40.4.621. [DOI] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Elton A., Zhu H., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J. Neurosci. 2014;34:11288–11296. doi: 10.1523/JNEUROSCI.5072-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica O.K. Some prosodic and paralinguistic features of speech to young children. In: Snow C.E., Ferguson C.A., editors. Talking to Children. Cambridge University Press; 1977. pp. 63–88. [Google Scholar]
- Goldfield B.A., Reznick J.S. Early lexical acquisition: rate, content, and the vocabulary spurt*. J. Child Lang. 1990;17:171–183. doi: 10.1017/S0305000900013167. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gomez Nazzi. Effects of prior phonotactic knowledge on infant word segmentation: the case of nonadjacent dependencies. J. Speech Lang. Hear. Res. 2013;56:840–849. doi: 10.1044/1092-4388(2012/12-0138). [DOI] [PubMed] [Google Scholar]
- Gow D., Nied A.C. Rules from words: a dynamic neural basis for a lawful linguistic process. PLoS One. 2014;9:e86212. doi: 10.1371/journal.pone.0086212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyet L., de Schonen S., Nazzi T. Words and syllables in fluent speech segmentation by French-learning infants: an ERP study. Brain Res. 2010;1332:75–89. doi: 10.1016/j.brainres.2010.03.047. [DOI] [PubMed] [Google Scholar]
- Graf Estes K.G., Edwards J., Saffran J.R. Phonotactic constraints on infant word learning. Infancy. 2011;16:180–197. doi: 10.1111/j.1532-7078.2010.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse S.W., Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. doi: 10.1007/BF02289823. [DOI] [Google Scholar]
- Grossmann T., Oberecker R., Koch S.P., Friederici A.D. The developmental origins of voice processing in the human brain. Neuron. 2010;65:852–858. doi: 10.1016/j.neuron.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanulíková A. Syllabification and its role in lexical segmentation of German and Slovak. In: Fuchs S., Loevenbruck H., Pape D., Perrier P., editors. Some Aspects of Speech and the Brain. Peter Lang Verlag; Frankfurt am Main: 2009. pp. 331–361. [Google Scholar]
- Harris J.J., Reynell C., Attwell D. The physiology of developmental changes in BOLD functional imaging signals. Dev. Cogn. Neurosci. 2011;1:199–216. doi: 10.1016/j.dcn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Huang H., Zhang J., Wakana S., Zhang W., Ren T., Richards L.J., Yarowsky P., Donohue P., Graham E., van Zijl P.C.M., Mori S. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huettel Song, McCarthy . Sinauer Associates; Sunderland, MA: 2009. Functional Magnetic Resonance Imaging. [Google Scholar]
- Huppert T.J., Hoge R.D., Diamond S.G., Franceschini M.A., Boas D.A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage. 2006;29:368–382. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issard C., Gervain J. Variability of the hemodynamic response in infants: Influence of experimental design and stimulus complexity. Dev. Cog. Neuro. 2018;33:182–193. doi: 10.1016/j.dcn.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H.H. The ten-twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnson M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Junge C., Cutler A., Hagoort P. Electrophysiological evidence of early word learning. Neuropsychologia. 2012;50:3702–3712. doi: 10.1016/j.neuropsychologia.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Jusczyk P.W. How infants begin to extract words from speech. Trends Cogn. Sci. 1999;3:323–328. doi: 10.1016/S1364-6613(99)01363-7. [DOI] [PubMed] [Google Scholar]
- Jusczyk P.W., Friederici A.D., Wessels J.M.I., Svenkerud V.Y., Jusczyk A.M. Infants′ sensitivity to the sound patterns of native language words. J. Mem. Lang. 1993;32:402–420. doi: 10.1006/jmla.1993.1022. [DOI] [Google Scholar]
- Kabdebon C., Leroy F., Simmonet H., Perrot M., Dubois J., Dehaene-Lambertz G. Anatomical correlations of the international 10–20 sensor placement system in infants. NeuroImage. 2014;99:342–356. doi: 10.1016/j.neuroimage.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Obrig H., Requardt M., Merboldt K.-D., Dirnagl U., Villringer A., Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J. Cereb. Blood Flow Metab. 1996;16:817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.P., Steinbrink J., Villringer A., Obrig H. Synchronization between background activity and visually evoked potential is not mirrored by focal hyperoxygenation: implications for the interpretation of vascular brain imaging. J. Neurosci. 2006;26:4940–4948. doi: 10.1523/JNEUROSCI.3989-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman V., Hagoort P., Cutler A. Electrophysiological evidence for prelinguistic infants’ word recognition in continuous speech. Cogn. Brain Res. 2005;24:109–116. doi: 10.1016/j.cogbrainres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kooijman V., Junge C., Johnson E.K., Hagoort P., Cutler A. Predictive brain signals of linguistic development. Front. Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P.K., Conboy B.T., Coffey-Corina S., Padden D., Rivera-Gaxiola M., Nelson T. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e) Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363:979–1000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu. Rev. Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rossi S., Zhou H., Chen B. Electrophysiological evidence for domain-general inhibitory control during bilingual language switching. PLoS One. 2014;9:e110887. doi: 10.1371/journal.pone.0110887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Wandell B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Luders E., Thompson P.M., Toga A.W. The development of the Corpus callosum in the healthy human brain. J. Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattys S.L., Jusczyk P.W. Phonotactic cues for segmentation of fluent speech by infants. Cognition. 2001;78:91–121. doi: 10.1016/S0010-0277(00)00109-8. [DOI] [PubMed] [Google Scholar]
- Menyuk P., Liebergott J.W., Schultz M.C., Liebergott J.W., Schultz M.C. Psychology Press; 2014. Early Language Development in Full-Term and Premature Infants. [DOI] [Google Scholar]
- Minagawa-Kawai Y., Mori K., Naoi N., Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. J. Neurosci. 2007;27:315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Cristià A., Dupoux E. Cerebral lateralization and early speech acquisition: a developmental scenario. Dev. Cogn. Neurosci. 2011;1:217–232. doi: 10.1016/j.dcn.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Cristia A., Long B., Vendelin I., Hakuno Y., Dutat M., Filippin L., Cabrol D., Dupoux E. Inights on NIRS sensitivity from a cross-linguistic study on the emergence of phonological grammar. Front. Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H., Villringer A. Beyond the visible—imaging the human brain with light. J. Cereb. Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Obrig H., Mock J., Stephan F., Richter M., Vignotto M., Rossi S. Impact of associative word learning on phonotactic processing in 6-month-old infants: a combined EEG and fNIRS study. Dev. Cog. Neuro. 2017;25:185–197. doi: 10.1016/j.dcn.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Dan H., Sakamoto K., Takeo K., Shimizu K., Kohno S., Oda I., Isobe S., Suzuki T., Kohyama K., Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Parise E., Csibra G. Electrophysiological evidence for the understanding of maternal speech by 9-month-old infants. Psychol. Sci. 2012;23:728–733. doi: 10.1177/0956797612438734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C., Poloniato A., Lohmann G., Friederici A.D. Neural language networks at birth. PNAS. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J.M., Baran J.A., Gandour J. Hemispheric specialization in processing intonation contours. Aphasiology. 1996;10:343–362. doi: 10.1080/02687039608248416. [DOI] [Google Scholar]
- Qiu A., Mori S., Miller M.I. Diffusion tensor imaging for understanding brain development in early life. Annu. Rev. Psychol. 2015;66:853–876. doi: 10.1146/annurev-psych-010814-015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rämä P., Sirri L., Serres J. Development of lexical–semantic language system: N400 priming effect for spoken words in 18- and 24-month old children. Brain Lang. 2013;125:1–10. doi: 10.1016/j.bandl.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Rossi S., Jürgenson I.B., Hanulíková A., Telkemeyer S., Wartenburger I., Obrig H. Implicit processing of phonotactic cues: evidence from electrophysiological and vascular responses. J. Cogn. Neurosci. 2011;23:1752–1764. doi: 10.1162/jocn.2010.21547. [DOI] [PubMed] [Google Scholar]
- Rossi S., Telkemeyer S., Wartenburger I., Obrig H. Shedding light on words and sentences: near-infrared spectroscopy in language research. Brain Lang. 2012;121:152–163. doi: 10.1016/j.bandl.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Vannest J., Lee G., Hernandez‐Garcia L., Plante E., Rajagopal A., Holland S.K. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum. Brain Mapp. 2015;36:1–15. doi: 10.1002/hbm.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann F., Spichtig S., Muehlemann T., Wolf M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010;31:649–662. doi: 10.1088/0967-3334/31/5/004. [DOI] [PubMed] [Google Scholar]
- Schweizer T.A., Ware J., Fischer C.E., Craik F.I.M., Bialystok E. Bilingualism as a contributor to cognitive reserve: evidence from brain atrophy in Alzheimer’s disease. Cortex. 2012;48:991–996. doi: 10.1016/j.cortex.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Sebastián-Gallés N. Biased to learn language. Dev. Sci. 2007;10:713–718. doi: 10.1111/j.1467-7687.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- Sharbrough Chatrian, Lesser L.üders, Nuwer Picton. American Electroencephalographic Society guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 1991;8:200–202. [PubMed] [Google Scholar]
- Shultz S., Vouloumanos A., Bennett R.H., Pelphrey K. Neural specialization for speech in the first months of life. Dev. Sci. 2014;17:766–774. doi: 10.1111/desc.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom M. Beyond babytalk: re-evaluating the nature and content of speech input to preverbal infants. Dev. Rev. 2007;27:501–532. doi: 10.1016/j.dr.2007.06.002. [DOI] [Google Scholar]
- Steinbrink J., Villringer A., Kempf F., Haux D., Boden S., Obrig H. Illuminating the BOLD signal: combined fMRI–fNIRS studies. Magn. Reson. Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Stocco A., Prat C.S. Bilingualism trains specific brain circuits involved in flexible rule selection and application. Brain Lang. 2014;137:50–61. doi: 10.1016/j.bandl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Strangman G., Culver J.P., Thompson J.H., Boas D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage. 2002;17:719–731. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum. Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S., Rossi S., Koch S.P., Nierhaus T., Steinbrink J., Poeppel D., Obrig H., Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G., Vihman M., Roberts M. Familiar words capture the attention of 11-month-olds in less than 250 ms. NeuroReport. 2003;14:2307. doi: 10.1097/00001756-200312190-00004. [DOI] [PubMed] [Google Scholar]
- Torkildsen J., von K., Syversen G., Simonsen H.G., Moen I., Lindgren M. Brain responses to lexical-semantic priming in children at-risk for dyslexia. Brain Lang. 2007;102:243–261. doi: 10.1016/j.bandl.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Torkildsen J., von K., Svangstu J.M., Hansen H.F., Smith L., Simonsen H.G., Moen I., Lindgren M. Productive vocabulary size predicts event-related potential correlates of fast mapping in 20-month-olds. J. Cogn. Neurosci. 2008;20:1266–1282. doi: 10.1162/jocn.2008.20087. [DOI] [PubMed] [Google Scholar]
- Torkildsen J., von K., Friis Hansen H., Svangstu J.M., Smith L., Simonsen H.G., Moen I., Lindgren M. Brain dynamics of word familiarization in 20-month-olds: effects of productive vocabulary size. Brain Lang. 2009;108:73–88. doi: 10.1016/j.bandl.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Trask R.L. Routledge; 2004. A Dictionary of Phonetics and Phonology. [DOI] [Google Scholar]
- Uludağ K., Dubowitz D.J., Yoder E.J., Restom K., Liu T.T., Buxton R.B. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. NeuroImage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Vaden K.I., Piquado T., Hickok G. Sublexical properties of spoken words modulate activity in Broca’s area but not superior temporal cortex: implications for models of speech recognition. J. Cogn. Neurosci. 2011;23:2665–2674. doi: 10.1162/jocn.2011.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L., Abaci Turk E., Ferradal S.L., Sutin J., Stout J.N., Ahtam B., Lin P.-Y., Grant P.E. Exploring early human brain development with structural and physiological neuroimaging. NeuroImage. 2019;187:226–254. doi: 10.1016/j.neuroimage.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J.F., Tees R.C. Developmental changes across childhood in the perception of non-native speech sounds. Can. J. Psychol. Can. Psychol. 1983;37:278–286. doi: 10.1037/h0080725. [DOI] [PubMed] [Google Scholar]
- Werker J.F., Tees R.C. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behav. Dev. 1984;7:49–63. doi: 10.1016/S0163-6383(84)80022-3. [DOI] [Google Scholar]
- Werker J.F., Yeung H.H. Infant speech perception bootstraps word learning. Trends Cogn. Sci. 2005;9:519–527. doi: 10.1016/j.tics.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Woumans E., Santens P., Sieben A., Versijpt J., Stevens M., Duyck W. Bilingualism delays clinical manifestation of Alzheimer’s disease*. Biling. Lang. Cogn. 2015;18:568–574. doi: 10.1017/S136672891400087X. [DOI] [Google Scholar]
- Yin W., Chen M.-H., Hung S.-C., Baluyot K.R., Li T., Lin W. Brain functional development separates into three distinct time periods in the first two years of life. NeuroImage. 2019;189:715–726. doi: 10.1016/j.neuroimage.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Evans A.C., Meyer E., Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.256.5058.846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.