Abstract
Purpose
To report clinical outcomes of Rose Bengal Photodynamic Antimicrobial Therapy (RB-PDAT) as an adjunct treatment for severe, progressive infectious keratitis.
Design
Consecutive interventional case series.
Methods
Patients with progressive infectious keratitis unresponsive to standard medical therapy underwent RB-PDAT at the Bascom Palmer Eye Institute from January 2016 through March 2018. RB-PDAT was performed by applying a solution of Rose Bengal (0.1% or 0.2% RB in BSS) to the de-epithelized cornea for 30 minutes followed by irradiation with a 6mW/cm2 custom-made green LED source for 15 minutes (5.4J/cm2).
Results
The current study included 18 patients (7 males and 11 females) ranging from 17–83 years. Acanthamoeba was the most frequent microbe (10/17; 59%), followed by Fusarium spp. (4/17; 24%), Pseudomonas aeruginosa (2/17; 12%) and Curvularia spp. (1/17; 6%) and one patient had no confirmed microbiologic diagnosis. Main clinical risk factor for keratitis included contact lens wear (79%). The average area of epithelial defect prior to first RB-PDAT was 32 ± 27mm2 and average stromal depth hyper-reflectivity measured with anterior segment OCT was 269 ± 75mm. Successful RB-PDAT (avoidance of therapeutic keratoplasty) was achieved in 72% of the cases, with an average time to clinical resolution (decreased pain and inflammation with re-epithelization and infiltrate resolution) of 46.9 ± 26.4 days after RB-PDAT. Time of follow up after RB-PDAT was 13.3 ± 5.7 months.
Conclusion
RB-PDAT can be considered as an adjunct therapy for cases of severe, progressive infectious keratitis before performing a therapeutic keratoplasty.
INTRODUCTION
Infectious keratitis is an ophthalmic emergency due to its potential rapid progression and devastating consequences if not managed promptly and effectively.1 A common sequelae of infectious keratitis is corneal stromal scarring which constitutes the fourth leading cause of blindness globally.2 More severe complications can include iris synechiae with secondary ocular hypertension and optic nerve damage, corneal perforation, endophthalmitis and hemorrhagic choroidal detachment with secondary permanent vision loss.3
The standard of care for infectious keratitis consists of frequent application of topical antimicrobials and in some cases systemic and/or periocular administration of antimicrobial agents; however, there has been a recent increase in antibiotic-resistant organisms.4 Moreover, access to compounded medications is limited in rural areas of the United States and the rest of the world. The increased resistance to standard medical treatment and limited access to treatment, has led to a rise in the clinical complications previously mentioned.5 In order to prevent corneal perforations, or further dissemination of the disease to the scleral tissue or inside the eye, a therapeutic penetrating keratoplasty (TPK) or therapeutic lamellar keratoplasty may become necessary. Unfortunately, the probability of graft failure is high after performing a corneal graft in the setting of active corneal inflammation.6,7 Due to potentially visually threatening consequences and limited treatment strategies, alternative therapies are considered in severe, progressive corneal infections.
During the last decade, corneal collagen crosslinking (CXL) procedures have been proposed as a novel treatment strategy for the management of resistant microbes and cases with progressive presumed infectious keratitis. The initial treatment was known as Photo-Activated Chromophore corneal collagen crosslinking (PACK-CXL), which consisted of riboflavin irradiated with ultraviolet A (UV-A) light.8,9 Later, Rose Bengal Photodynamic Antimicrobial Therapy (RB-PDAT) was shown in vitro to be more efficient as a treatment for fungal and methicillin-resistant Staphylococcus aureus (MRSA) keratitis.10,11 This therapy involved a photochemical process using Rose Bengal, a frequently used diagnostic dye in ophthalmology,12 excited with green light (wavelength: 500–550nm) to generate reactive oxygen species (ROS).13 Two types of ROS mechanisms have been described. Type I is associated with electron transfer reactions that form free radicals, superoxide anion, hydrogen peroxide, and hydroxyl peroxide. In type II, energy is transferred from the triplet state of the photosensitizer to the ground state of triplet molecular oxygen, which leads to the formation of toxic singlet oxygen. Singlet oxygen has higher reactivity to lipids, proteins and nucleic acids and is considered to have the greatest antimicrobial effect of all the ROS.14 In RB-PDAT, Rose Bengal is activated by the green light to stimulate the transfer of energy to nearby molecular triplet oxygen (3O2). This energy transfer results in the formation of reactive singlet oxygen (1O2) which interact with surrounding organic compounds in cells and tissues to produce a variety of effects including eradication of a wide array of bacteria, viruses, fungi and protozoa.15
Several publications have demonstrated the in vitro efficacy of Rose Bengal PDAT in inhibiting the growth of different organisms including MRSA, Fusarium solani, Aspergillus fumigatus, and Candida albicans.10,11,15,16 In vivo and ex vivo experiments have evaluated the safety profile of green light-activated Rose Bengal and have shown no significant adverse effects on keratocytes and no evidence of harm to deeper tissues such as the iris and retina.17 However, there is a paucity of information on the clinical effects and efficiency of this therapy for the treatment of infectious keratitis. The current article reports the clinical outcomes of patients diagnosed with infectious or presumed infectious keratitis unresponsive to standard medical treatment that underwent RB-PDAT as a last resort before considering a therapeutic corneal transplant.
MATERIALS AND METHODS
The current study is a retrospective chart review of all patients-with a diagnosis of presumed infectious keratitis who underwent RB-PDAT at the Bascom Palmer Eye Institute from January 2016 through March 2018. Data regarding age, sex, medical history, ophthalmic evaluation, microbiologic and histopathologic diagnosis, surgeries performed after RB-PDAT as well as imaging findings and histopathology pictures were collected. Institutional Review Board (IRB) approval from the University of Miami, which adhered to the tenets of the Declaration of Helsinki, was obtained. Written informed consent was obtained from all patients before treatment.
Patient Selection
Infections were considered resistant to treatment if they had received appropriate standard medical therapy without any clinical improvement for at least two weeks. RB-PDAT was considered in those patients with evidence of progressive disease in spite of maximal medical treatment and two faculty members of the cornea service at Bascom Palmer Eye Institute agreed the next step in treatment was surgical intervention. Exclusion criteria were age less than 12, pregnancy, and inability to remain supine for 45 minutes. There were no exclusion criteria related to morphometric ulcer characteristics (e.g. residual corneal thickness, depth and size of infiltrate).
Ophthalmic examination
All of the patients underwent a thorough ophthalmic examination prior to RB-PDAT. Risk factors for infection were evaluated (e.g. contact lens use, trauma, diabetes). The ophthalmic evaluation consisted of: measurement of best corrected visual acuity (BCVA), slit-lamp examination with and without fluorescein, slit-lamp photography, corneal pachymetry, and anterior segment optical coherence tomography (AS-OCT). During the slit-lamp examination, characteristics of infection such as location, size of the epithelial defect, and area of stromal infiltration were recorded. The slit-lamp examination also included scraping the cornea for microbiological staining, culture, and antibiotic susceptibility testing as per the Bascom Palmer Microbiology laboratory protocol.18 Infection depth was determined using the digital caliper of an optical coherence tomographer (iVue, Optovue, Freemont, CA, USA) by selecting two different images of the cornea and averaging hyperreflectivity depth at three different points of the cornea on each of these two images.
Solution preparation of Rose Bengal
Four or eight strips of Rose Bengal (RB) (Glostrips 1.3mg, Amcon, St. Louis, MO, USA) were diluted for one minute in 5mL of balanced salt solution (BSS) (Alcon Laboratories, Fort Worth, TX, USA), while shaking the container to achieve a Rose Bengal solution concentration of 0.1% or 0.2%, respectively. Concentration of Rose Bengal solution was selected by the clinician based on the severity, clinical response to prior treatments and/or in vitro data regarding the susceptibility of the microorganisms to Rose Bengal PDAT.
Rose Bengal PDAT procedure
A sterile lid speculum was placed under topical anesthesia (sterile lidocaine 1% and proparacaine 0.5%), followed by an injection of 2mL of 2% lidocaine with epinephrine 1:10,000 into the bulbar subconjunctival space at 12 o’clock and 6 o’clock with a 30-G needle. If ulcers had a small epithelial defect, the area surrounding it was debrided to obtain an 8mm de-epithelized area to increase Rose Bengal absorption. An 8mm corneal sponge (Beaver Visitec International, Waltham, MA, USA) was placed over the cornea. Three drops of 0.1% or 0.2% of Rose Bengal solution was applied to the sponge surface, followed by one drop every three minutes over the following 30 minutes to maintain saturation. The sponge was then removed and a custom-made disposable shield measuring 10mm × 15mm with a central 9mm opening was placed to protect the corneoscleral limbus from the green light irradiation. Afterwards, the anterior corneal surface was irradiated with a custom-made 6mW/cm2 green LED light source 16 for 15 minutes for a total energy density exposure of 5.4J/cm2. The anterior corneal surface was irrigated with one drop of balanced saline solution (Alcon Laboratories, Fort Worth, TX, USA) every three minutes throughout the light exposure to prevent corneal dehydration. Finally, a bandage contact lens (Airoptix AQUA, Alcon, Fort Worth, TX, USA) was placed to protect the ocular surface at the conclusion for pain management after the procedure and the ocular surface was examined at the slit-lamp. Patients were evaluated after one, three and seven days of treatment, and re-evaluated after two weeks of treatment to determine if they would benefit from another treatment. If no significant improvement was seen, but physician and doctors agreed that there was some benefit, an additional treatment was given. Patients were afterwards followed biweekly, weekly, or monthly depending on their clinical progression. Patients continued their standard medical therapy, specific to the type of microorganism, after RB-PDAT until the infection clinically resolved.
Statistical analysis
The main outcome measure was frequency of RB-PDAT success, defined as avoidance of therapeutic penetrating keratoplasty (TPK). Secondary outcome measures included number of RB-PDAT treatments, time from first RB-PDAT treatment to clinical resolution (clinical resolution defined as re-epithelization of the epithelial defect with decreased pain and inflammation and resolution of infiltrate), time from first RB-PDAT to TPK or Optical Penetrating Keratoplasty (OPK), and BCVA six months and one year after first RB-PDAT. Visual acuity progression was reported by using a value of 1/400 Snellen (logMAR = 2.6) to represent vision of counting fingers and further extrapolated hand movement, light perception, and no light perception as 2.7, 2.8, and 2.9 logMAR, respectively.19 For statistical purposes, we summarized the descriptives based on the 18 individuals treated that had available follow-up. We included information from the most severely affected eye in the patient with bilateral disease. Logistic regression analysis was performed to evaluate which factors (patient demographics, ulcer characteristics, length of standard medical treatment before presentation, BCVA at first RB-PDAT session) affected success. A paired, two-tailed t-test was additionally performed to compare BCVA before and after treatment. P value of <0.05 were considered statistically significant.
RESULTS
Study population
Between January 2016 and March 2018, 19 patients (20 eyes) underwent RB-PDAT for the indications previously outlined. Of those, follow-up was available for 18 patients (19 eyes). Of these 18 patients, 7 (39%) were male and 11 (61%) were female. One patient presented with bilateral keratitis and underwent RB-PDAT in both eyes and 17 individuals had unilateral keratitis and received treatment in one eye only. Demographics and clinical characteristics of each patient are reported in Table 1. Average age at the time of RB-PDT was 45.4 years (range 17–83 years). A microbiologic diagnosis was confirmed through culture and/or biopsy in 17 patients; no microbiologic diagnosis was determined in one patient. Of the cases identified, Acanthamoeba spp. was the most frequent microbe identified (59%, 10/17), followed by Fusarium spp. (24%, 4/17), Pseudomonas aeruginosa (12%, 2/17) and Curvularia spp. (6%, 1/17). The patient who had a negative culture, case (#6), had a clinical presentation strongly suggestive of Acanthamoeba spp., thus, even though microbiologic diagnosis was negative, she was classified and treated as an Acanthamoeba spp. infection. Patient #15 had multiple coinfections with Acanthamoeba spp., Staphylococcus spp., Candida spp. and Streptococcus spp, in the setting of a chemical burn.
Table 1.
Demographics and clinical characteristics of patients.
| N | Age | Sex | Risk Factors | Organism | Area of epithelial defect (mm2) | Area of stromal infiltration (mm2) | Depth of infection (um) | Length of SMT prior to first RB- PDAT (Weeks) | Initial BCVA | # of PDT treatments received | RB % | Successful (yes/no) | Time to clinical resolution after first RB-PDAT (Days) | Surgical intervention after RB-PDAT | Corneal graft status | BCVA last FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | M | CL | Acanthamoeba | 30 | 48.2 | 243 | 12 | HM | 2 | 0.1 | Yes | 29 | PKP | Clear | 20/30 |
| 2 | 34 | M | CL | Acanthamoeba | 12 | 16 | 197 | 64 | HM | 2 | 0.1 | Yes | 30 | PKP, CE+ IOL | Clear | 20/200 |
| 3 | 32 | F | CL | Acanthamoeba | 40 | 40 | 387 | 16 | HM | 3 | 0.1 /0.2 | Yes | 60 | Keratectomy, PKP, CE+ IOL | Clear | 20/20 |
| 4 | 17 | F | CL | Acanthamoeba | 12.7 | 12.7 | 385 | 48 | LP | 1 | 0.1 | Yes | 30 | PKP | Clear | 20/60 |
| 5 | 55 | F | CL | Acanthamoeba | 38.5 | 31.2 | 116 | 3 | HM | 2 | 0.1 | Yes | 90 | CE +IOL, DALK | Clear | 20/60 |
| 6 | 48 | F | CL | Suspicious for Acanthamoeba | 14 | 14 | No data | 4 | HM | 2 | 0.1 | Yes | 60 | DALK, CE+ IOL | Clear | 20/30 |
| 7 | 57 | F | CL | Fusarium | 4.14 | 4.14 | 320 | 4 | HM | 2 | 0.1 | Yes | 20 | CE+ IOL, PKP | Clear | 20/100 |
| 8 | 83 | F | CL | Fusarium | 26 | 3.57 | 302 | 2 | 20/2000 | 1 | 0.1 | Yes | 60 | PKP | Clear | 20/800 |
| 9 | 37 | M | CL | Pseudomonas | 81 | 81 | 314 | 4 | LP | 1 | 0.1 | Yes | 27 | PKP, CE+ IOL | Clear | 20/30 |
| 10 | 62 | F | Soil/CL | Acanthamoeba | None | 6.3 | 188 | 2 | 20/200 | 1 | 0.1 | Yes | 20 | N/A | N/A | 20/80 |
| 11 | 17 | F | CL | Acanthamoeba | 3.5 | 11.6 | 290 | 6 | 20/2000 | 2 | 0.1 | Yes | 90 | N/A | N/A | 20/80 |
| 12 | 68 | F | OCP/CL | Curvularia | None | 12 | No data | 4 | LP | 2 | 0.2 | Yes | 60 | CE+ IOL | N/A | 20/50 |
| 13 | 51 | M | CL | Pseudomonas | 81 | 81 | No data | 3 | HM | 1 | 0.1 | Yes | 30 | No data | No data | No data |
| 14 | 44 | M | CK | Acanthamoeba Candida, MRSA | 16.2 | 9 | No data | 16 | HM | 1 | 0.2 | No | Not resolved | TPK | Edema, neovascularization | HM |
| 15 | 27 | F | CL | Acanthamoeba | 36 | 36 | 229 | 8 | LP | 1 | 0.2 | No | Not resolved | TPK | Edema, Descemet folds | 20/20 |
| 16 | 40 | F | CL | Acanthamoeba | 25 | 2 | 300 | 24 | HM | 2 | 0.2 | No | Not resolved | TPK | Edema, Descemet folds | 20/80 |
| 17 | 56 | M | Soil | Fusarium | 9.6 | 81 | 252 | 2 | LP | 1 | 0.1 | No | Not resolved | TPK | Eviscerated | NLP |
| 18 | 66 | M | None | Fusarium | 81 | 36 | 243 | 4 | LP | 1 | 0.1 | No | Not resolved | TKP | Edema | HM |
M= male, F= female, CK= chemical keratitis, OCP= Ocular cicatricial pemphigoid, CL= contact lens, BCVA= best corrected visual acuity, SMT = standard medical treatment, PKP= Penetrating keratoplasty, TPK= therapeutic penetrating keratoplasty, CE+IOL= cataract extraction + intraocular lens placement, HM: hand motion, LP: Light perception
The majority of individuals reported a history of contact lens wear (79%) and 11% reported an occupation that required frequent contact with soil. Other comorbidities in our cohort included active and progressive ocular cicatricial pemphigoid (OCP) (n=1), Iridocorneal Endothelial (ICE) syndrome (n=1), a history of chemical burn (n=1).
The average area of epithelial defect prior to first treatment with RB-PDAT was 32 ± 27.3 (range: 0 – 81mm2) with an average stromal opacification area of 29.2 ± 27.5mm2 (range: 2 – 81mm2). The ulcer was central in five patients, paracentral in five, peripheral in three, and diffuse (limbus to limbus) in five. By AS-OCT, average stromal depth hyper-reflectivity was 269 ± 75μm (range: 116–387μm).
The length of standard medical treatment prior to the first RB-PDAT ranged from 2 to 64 weeks depending on the organisms (Acanthamoeba spp. = 18.5 weeks, Fusarium spp. = 3 weeks, Curvularia spp. = 4 weeks and Pseudomonas aeruginosa = 3.5 weeks) with a mean of 12.5 weeks. Nine patients received one treatment with RB-PDAT, eight patients received two treatments, and one patient underwent three treatments. Patients who received multiple treatments presented with very severe cases of infectious keratitis. Clinicians decided whether a repeat treatment was indicated if they observed minimal clinical improvement at follow-up.
RB-PDAT outcomes
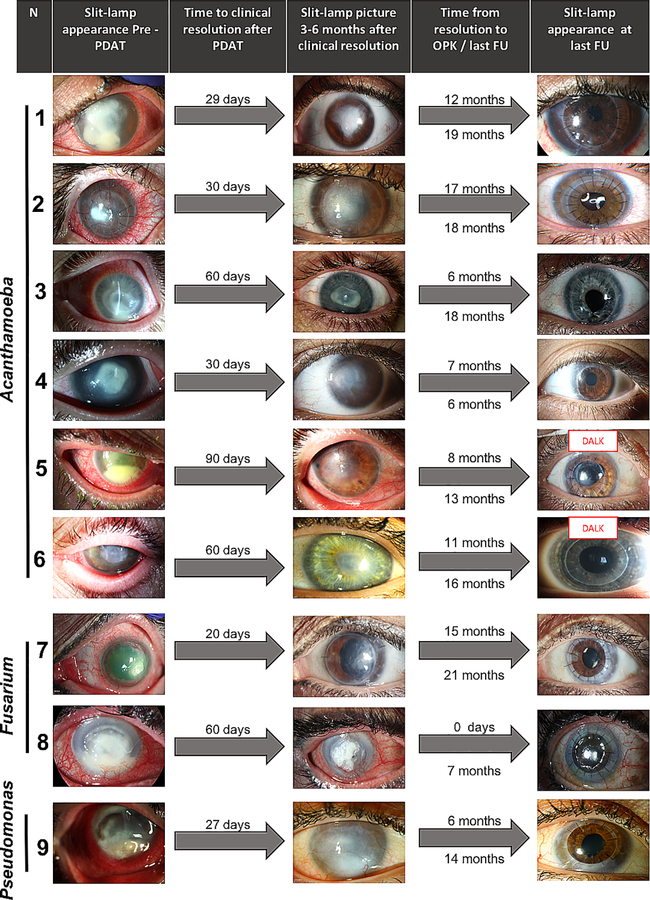
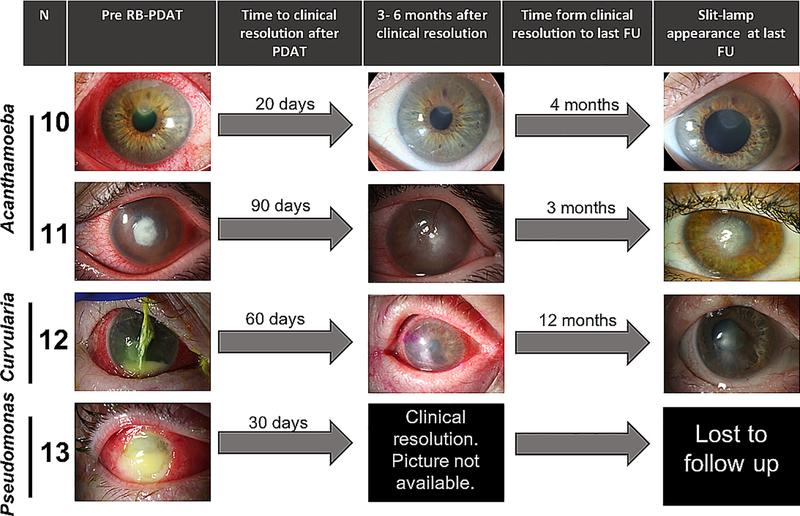
RB-PDAT was considered successful in 13 individuals, defined as control of infection without the need for a TPK. Clinical endpoints were defined as no infection with no treatment for a minimum of three months for bacterial and fungal keratitis, and six months for acanthamoeba keratitis. In these 13 individuals, time to clinical resolution after the first RB-PDT session was 46.9 ± 26.4 days. Individuals without evidence of recurrence or inflammation were given the option of undergoing optical keratoplasty at least six months after RB-PDT. Seven optical penetrating keratoplasties (PKP) and two deep anterior lamellar keratoplasties (DALK) were thus successfully performed with no recurrent infection or graft failure on mean follow-up of 6.47 ± 3.22 months (range 0.25 to 10). No organisms were identified on microbiology or pathology from the host tissue removed at the time of surgery. Figure 1 depicts the graft outcomes of these patients. Three of the remaining patients have not yet undergone any additional procedure and one was lost to follow-up. The average BCVA prior to PDAT treatment was 2.48 logMAR and improved to 1.87 logMAR after infectious resolution (p=0.046). BCVA at 6 months and 1 year after PDT was 1.83 logMAR and 0.54 logMAR in those who underwent optical corneal transplantation and 0.73 logMAR and 0.57 logMAR for those who have not yet undergone any additional intervention. Therefore, in those considered successful, BCVA improved 1.92 ± 0.8 LogMAR (p<0.01) from presentation to one-year follow-up. Clinical evolution of the patients is seen in Figure 2.
Figure 1.
Slit-lamp findings and clinical course of patients that underwent optical corneal transplantation after successful RB-PDAT.
Figure 2.
Slit-lamp findings and clinical course of patients that underwent successful RB-PDAT and have not yet required additional optical keratoplasties.
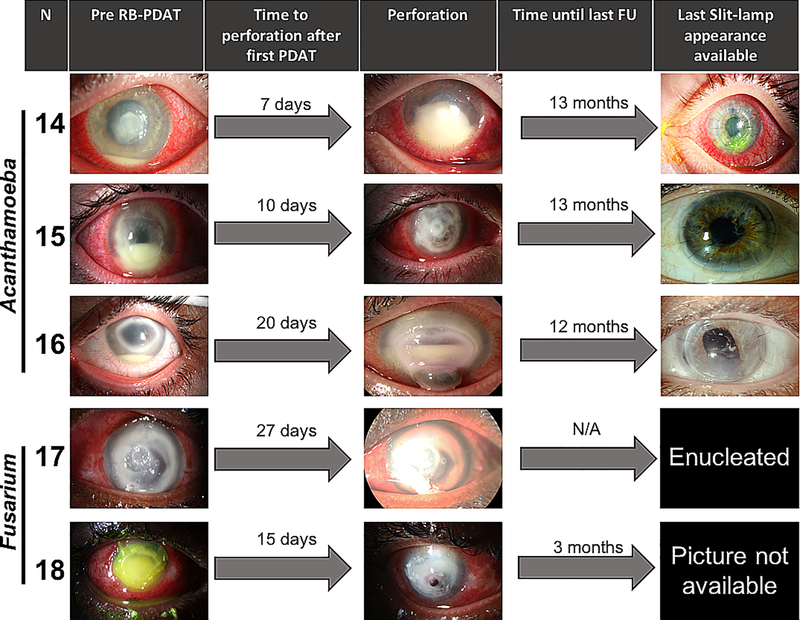
RB-PDAT was considered a failure when corneal perforation occurred after treatment with RB-PDAT. This occurred in five patients and after a mean of 15.8±8 days after the first RB-PDAT, a TPK had to be performed. Three of the five individuals were found to have microbes identified in the perforated tissue, one with Acanthamoeba spp. cysts noted the in the pathology specimen, one with Acanthamoeba spp. cyst on pathology and culture, and one with fungal elements on pathology. Two individuals had no microbes identified in the tissue suggesting microbial elimination. Outcomes after TPK were not as good as after optical corneal transplantation. While one graft remained clear after TPK, three grafts had corneal edema (n=2) and neovascularization (n=1) on mean follow-up 10.3 ± 4.86 months after TPK (range: 3–13 months). One patient underwent enucleation two days after TPK, due to intractable pain and intraocular involvement. The average BCVA prior to PDAT treatment in these patients was 2.73 logMAR and with slight improvement in vision to 1.95 and 1.78 logMAR six months and 1 year, respectively, after the procedure. BCVA improved only 0.96 ± 1.4 logMAR (p=0.19) from presentation to one-year follow-up in patients who failed RB-PDAT treatment. (Patients outcomes are portrayed in Figure 3.)
Figure 3.
Slit-lamp findings and clinical course of patients that had an unsuccessful RB-PDAT and required therapeutic keratoplasty or enucleation.
Risk factors for RB-PDT failure
Demographics (age, gender, ethnicity, race) did not predict RB-PDAT outcome (success vs. failure). Non-contact lens wearers had a higher risk of RB-PDAT failure as compared to contact lens wearers (100% vs 14%, p=0.01). Clinical characteristics of infection (e.g. depth, epithelial defect size, and area of infiltrate) and length of medical treatment before RB-PDAT also did not predicted outcomes. Additionally, there was no difference in outcomes between those patients treated with 1% Rose Bengal solution versus 2% Rose Bengal solution. Comparison of characteristics of patients and infection are summarized in Table 2.
Table 2.
Characteristics of patients and infection of successful vs. failed RB-PDAT
| Characteristics of patients and
infection of successful vs. failed RB-PDAT | |||
|---|---|---|---|
| Parameter | Successful RB-PDAT | Failed RB-PDAT | p- value |
| Age (mean ± SD) | 44.9 ± 20.3 | 46.6 ± 14.9 | 0.85 |
| Male-to-female | 4:9 | 2:3 | - |
| Average size of epithelial defect | 26.4 ± 27.8 | 33.6 ± 28.3 | 0.64 |
| Average area of stromal infiltration | 27.2 ± 27.7 | 32.8 ± 31 | 0.74 |
| Mean initial BCVA (logMAR) | 2.48 ± 0.5 | 2.73 ± 0.05 | 0.11 |
| Etiologic organisms | |||
| Acanthamoeba spp: 8 | Acanthamoeba spp: 3 | ||
| Fusarium spp: 2 | Fusarium spp: 2 | ||
| Curvularia spp: 1 | |||
| Pseudomonas aeruginosa: 2 | |||
DISCUSSION
Infectious keratitis can lead to significant ocular morbidity, greatly impacting patients’ quality of life. This sight-threatening condition can be caused by a wide range of bacteria, fungi, protozoa and/or viruses. Antimicrobial resistant strains are increasing and are associated with worse clinical presentation and visual impairment.9 Consequently, great efforts are being made to develop novel therapies to control these infections. We found that one to three sessions of RB-PDAT, in conjunction with medical therapy, eliminated infection and prevented the need for TPK in 72% of individuals with severe corneal ulcers. Even in the five individuals who failed RB-PDAT and underwent TPK, no residual infection was noted in two.
The results of the current study are supported by basic science data showing that RB-PDAT is more effective against microbes than CXL with riboflavin and UV-A light. In vitro studies of CXL with riboflavin and UV-A have demonstrated efficacy against certain bacteria including Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa, however this treatment has been shown to be ineffective against fungi including Candida spp., Fusarium spp., and Aspergillus spp.10,20,21 On the contrary, RB-PDAT has been shown to fully inhibit growth of both fungal (Candida spp., Fusarium spp., and Aspergillus spp.) and bacterial (MRSA) isolates 10,11,16 Clinically, PACK-CXL has also been used for the treatment of infectious keratitis associated with melt.9 A meta-analysis of PACK-CXL reported this treatment stabilized corneal melt in Gram-negative bacteria (13/14; 92%), Gram-positive bacteria (37/44; 84%), Acanthamoeba (5/7; 71%), and fungus (8/13; 61%).22 However, one randomized controlled trial found that the risk of perforation was higher with adjunct PACK-CXL and medical therapy as compared to medical therapy alone in cases of deep fungal keratitis.23
Current study outcomes are favorable compared to the current standard of care of TPK for resistant infections. The risk of graft failure is dramatically higher in TPK compared to OPK. Survival rates for therapeutic grafts have been reported to be 78.4, 58.3, and 37.3% at one, three, and five years.24 However, success depends on the organism involved and is higher for bacteria (~76.6%) 25 compared to fungi (~51–84%) 26–28 at one year.29 Furthermore, eradication of the infection is not ensured after TPK and ranges from 90–100% in bacterial keratitis 28,30, 69–90% in fungal keratitis 26–28 and 45–81% in Acanthamoeba.28,31–33 In our cohort, infection was clinically eradicated in 72% (13/18) and microbiologically eradicated in 83% (15/18) after RB-PDAT and the 1-year graft survival in those who later underwent OPK was 100%.
The safety of 0.1% RB-PDAT has been evaluated in vivo and ex vivo in animal models and no significant adverse effects on keratocytes, iris, or retina were observed.17 These studies along with our data demonstrating no difference between patients treated with 0.1% and 0.2% RB solution, led us to select a 0.1% RB solution as the standard for our RB-PDAT protocol. Rose Bengal is thought to interact strongly with collagen, thus limiting its penetration into healthy stroma to approximately 1000μm.34 However, considering our average depth of infection of 269 ± 75μm and our good clinical outcomes, we hypothesize that diffusion of the dye and its effects are enhanced by the stromal melting and inflammation present in keratitis. Beyond a direct antimicrobial effect, another potential consequence of photochemical crosslinking using Rose Bengal and green light is an increased resistance to collagenase digestion.35,36 Evidence to support the process of photochemical crosslinking was observed in some patients with the presence of a demarcation line.37 We therefore consider that even if antimicrobial eradication was not achieved with RB-PDAT, corneal melting and extension of the infection were halted, allowing the conventional antimicrobials administered as standard medical therapy to then eradicate infection. In the long term, RB-PDAT may also have a beneficial effect on subsequent OPK. Studies in murine models have demonstrated that CXL regresses pathological vessels and lymphatics in high risk corneal grafts38, and can thus increase survival after OPK.
As with all studies, our findings must be considered in light of the study limitations which include its retrospective nature and limited number of subjects. Additionally, due to regulatory issues, this treatment was offered only to advanced, resistant cases and these results do not apply to less severe cases or early treatment. Thus, due to the severity of the infections, no minimum corneal thickness could be determined as an inclusion criteria as corneal thickness measurements were limited by dense infiltrates, inflammation, and edema. We believe RB-PDAT outcomes were unfavorable in some patients due to inconsistent attendance of follow-up appointments and earlier treatment could have resulted in a better outcome. A controlled randomized clinical trial with a larger number of patients would be needed to compare RB-PDAT with medical treatment to medical treatment alone and determine which sub-types of infection are best treated with combined therapy. Despite these limitations, the current pilot clinical study results indicate Rose Bengal, a photosensitizing agent available to most ophthalmologists, when combined with a green LED light, resolved infection and prevented the need for TPK in a majority of individuals with sight threatening infections. This therapy may thus be considered as an adjunct therapy in severe cases of infectious keratitis in an attempt to avoid TPK and optimize future visual potential.
Supplementary Material
Acknowledgements/ Financial disclosure
a. Funding/Support:
This research was supported by the Edward D. and Janet K. Robson Foundation (Tulsa, OK), the Florida Lions Eye Bank and the Beauty of Sight Foundation (Miami, FL), Drs. K. R. Olsen and M. E. Hildebrandt, Drs. Raksha Urs and Aaron Furtado, NIH Center Grant P30EY14801, a grant from Research to Prevent Blindness to the department of ophthalmology, the Pan-American association of Ophthalmology (PAAO) and Retina Research Foundation (J. D. Martinez) and the Henri and Flore Lesieur Foundation (Chicago, IL) (J.-M. Parel)
c. Other Acknowledgments
The authors are grateful to Cornelis Rowaan, BS, Alex Gonzalez, BA, and Andres Bernal, MS, of the Ophthalmic Biophysics Center for participating to the design, development and construction of the portable irradiation source; Kenaan Mintz for chemical analyses of Rose Bengal solutions, Jennifer Phu for help with preparing the patients and coordinating treatment and Cynthia Maza, Pedro Monsalve, MD, Xiao-Yi Zhou, MD, from the Florida Lions Ocular Pathology laboratory for helping with histopathology.
Footnotes
b. Financial Disclosures
The authors have no financial disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta N, Tandon R, Gupta SK, Sreenivas V, Vashist P. Burden of corneal blindness in India. Indian J Community Med. 2013;38(4):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. [DOI] [PubMed] [Google Scholar]
- 3.Gokhale NS. Medical management approach to infectious keratitis. Indian J Ophthalmol. 2008;56(3):215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic Resistance Among Ocular Pathogens in the United States: Five-Year Results From the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015;133(12):1445–1454. [DOI] [PubMed] [Google Scholar]
- 5.Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea. 2018;37(1):84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sony P, Sharma N, Vajpayee RB, Ray M. Therapeutic keratoplasty for infectious keratitis: a review of the literature. CLAO J. 2002;28(3): 111–118. [PubMed] [Google Scholar]
- 7.Lomholt JA, Ehlers N. Graft survival and risk factors of penetrating keratoplasty for microbial keratitis. Acta Ophthalmol Scand. 1997;75(4):418–422. [DOI] [PubMed] [Google Scholar]
- 8.Tabibian D, Mazzotta C, Hafezi F. PACK-CXL: Corneal cross-linking in infectious keratitis. Eye Vis (Lond). 2016;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377–1382. [DOI] [PubMed] [Google Scholar]
- 10.Arboleda A, Miller D, Cabot F, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158(1):64–70 e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halili F, Arboleda A, Durkee H, et al. Rose Bengal- and Riboflavin-Mediated Photodynamic Therapy to Inhibit Methicillin-Resistant Staphylococcus aureus Keratitis Isolates. Am J Ophthalmol. 2016;166:194–202. [DOI] [PubMed] [Google Scholar]
- 12.Norn MS. Vital staining of the cornea and conjunctiva; with a mixture of fluorescein and rose bengal. Am J Ophthalmol. 1967;64(6):1078–1080. [DOI] [PubMed] [Google Scholar]
- 13.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Hurtado SA, Ramirez-Ramirez J, Larios-Morales AC, et al. Efficient in vitro photodynamic inactivation using repetitive light energy density on Candida albicans and Trichophyton mentagrophytes. Photodiagnosis Photodyn Ther. 2019;26:203–209. [DOI] [PubMed] [Google Scholar]
- 15.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amescua G, Arboleda A, Nikpoor N, et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea. 2017;36(9):1141–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Alt C, Webb RH, Melki S, Kochevar IE. Corneal Crosslinking With Rose Bengal and Green Light: Efficacy and Safety Evaluation. Cornea. 2016;35(9):1234–1241. [DOI] [PubMed] [Google Scholar]
- 18.Miller D, Alfonso EC. Comparative in vitro activity of levofloxacin, ofloxacin, and ciprofloxacin against ocular streptococcal isolates. Cornea. 2004;23(3):289–293. [DOI] [PubMed] [Google Scholar]
- 19.Roberts MF, Fishman GA, Roberts DK, et al. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol. 2002;86(6):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwabuchi RT, Carvalho FR, Khan YA, Hirai F, Campos MS, McDonnell PJ. Assessment of fungal viability after long-wave ultraviolet light irradiation combined with riboflavin administration. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):521–527. [DOI] [PubMed] [Google Scholar]
- 21.Martins SA, Combs JC, Noguera G, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49(8):3402–3408. [DOI] [PubMed] [Google Scholar]
- 22.Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013;3(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddaraju M, Mascarenhas J, Das MR, et al. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. Am J Ophthalmol. 2015; 160(1 ):131–134 e135. [DOI] [PubMed] [Google Scholar]
- 24.Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115(6):975–982 e971. [DOI] [PubMed] [Google Scholar]
- 25.Ti SE, Scott JA, Janardhanan P, Tan DT. Therapeutic keratoplasty for advanced suppurative keratitis. Am J Ophthalmol. 2007;143(5):755–762. [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Wang T, Xie L, et al. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology. 2010;117(5):890–896. [DOI] [PubMed] [Google Scholar]
- 27.Xie L, Zhai H, Shi W. Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea. 2007;26(2):158–162. [DOI] [PubMed] [Google Scholar]
- 28.Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. 2004; 137(4):736–743. [DOI] [PubMed] [Google Scholar]
- 29.Sharma N, Sachdev R, Jhanji V, Titiyal JS, Vajpayee RB. Therapeutic keratoplasty for microbial keratitis. Curr Opin Ophthalmol. 2010;21(4):293–300. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shehri A, Jastaneiah S, Wagoner MD. Changing trends in the clinical course and outcome of bacterial keratitis at King Khaled Eye Specialist Hospital. Int Ophthalmol. 2009;29(3):143–152. [DOI] [PubMed] [Google Scholar]
- 31.Kitzmann AS, Goins KM, Sutphin JE, Wagoner MD. Keratoplasty for treatment of Acanthamoeba keratitis. Ophthalmology. 2009;116(5):864–869. [DOI] [PubMed] [Google Scholar]
- 32.Shi W, Liu M, Gao H, Li S, Xie L. Perioperative treatment and prognostic factors for penetrating keratoplasty in Acanthamoeba keratitis unresponsive to medical treatment. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1383–1388. [DOI] [PubMed] [Google Scholar]
- 33.Kashiwabuchi RT, de Freitas D, Alvarenga LS, et al. Corneal graft survival after therapeutic keratoplasty for Acanthamoeba keratitis. Acta Ophthalmol. 2008;86(6):666–669. [DOI] [PubMed] [Google Scholar]
- 34.Verter EE, Gisel TE, Yang P, Johnson AJ, Redmond RW, Kochevar IE. Light-initiated bonding of amniotic membrane to cornea. Invest Ophthalmol Vis Sci. 2011;52(13):9470–9477. [DOI] [PubMed] [Google Scholar]
- 35.Fadlallah A, Zhu H, Arafat S, Kochevar I, Melki S, Ciolino JB. Corneal Resistance to Keratolysis After Collagen Crosslinking With Rose Bengal and Green Light. Invest Ophthalmol Vis Sci. 2016;57(15):6610–6614. [DOI] [PubMed] [Google Scholar]
- 36.Cherfan D, Verter EE, Melki S, et al. Collagen cross-linking using rose bengal and green light to increase corneal stiffness. Invest Ophthalmol Vis Sci. 2013;54(5):3426–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez JD, Naranjo A, Amescua G, et al. Human Corneal Changes After Rose Bengal Photodynamic Antimicrobial Therapy for Treatment of Fungal Keratitis. Cornea. 2018;37(10):e46–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou Y, Le VNH, Toth G, et al. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am J Transplant. 2018;18(12):2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.