Abstract
Mortality from coronavirus disease 2019 (COVID-19) is strongly associated with cardiovascular disease, diabetes, and hypertension. These disorders share underlying pathophysiology related to the renin-angiotensin system (RAS) that may be clinically insightful. In particular, activity of the angiotensin-converting enzyme 2 (ACE2) is dysregulated in cardiovascular disease, and this enzyme is used by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to initiate the infection. Cardiovascular disease and pharmacologic RAS inhibition both increase ACE2 levels, which may increase the virulence of SARS-CoV-2 within the lung and heart. Conversely, mechanistic evidence from related coronaviruses suggests that SARS-CoV-2 infection may downregulate ACE2, leading to toxic overaccumulation of angiotensin II that induces acute respiratory distress syndrome and fulminant myocarditis. RAS inhibition could mitigate this effect. With conflicting mechanistic evidence, we propose key clinical research priorities necessary to clarify the role of RAS inhibition in COVID-19 mortality that could be rapidly addressed by the international research community.
Keywords: COVID-19, SARS-CoV-2, angiotensin-converting enzyme 2, renin-angiotensin system, cardiovascular disease
The association between coronavirus disease 2019 (COVID-19) mortality and cardiovascular disease is likely mediated by dysregulation of the renin-angiotensin system (RAS). Pharmacoepidemiologic studies are needed to clarify whether RAS inhibition mediates this association and is helpful or harmful.
Multiple early reports of the novel coronavirus disease 2019 (COVID-19) identified a strong association between cardiovascular disease (CVD) and mortality in infected patients. In an analysis of 45 000 confirmed cases in China, the crude case fatality rate was 0.9% for patients without any documented comorbidities, whereas the case fatality rate was much higher for patients with CVD (10.5%), diabetes (7.3%), or hypertension (6.3%) [1]. This pattern threatens extensive mortality worldwide given the high prevalence of these disorders but may also be clinically insightful as these conditions share pathophysiologic mechanisms that could underlie heterogeneity in COVID-19 outcomes. It is possible that this comorbidity pattern is simply an artifact of confounding by age or underlying pulmonary disease, in which case fatality rates from COVID-19 may rise as its incidence increases in countries with older populations. However, COVID-19 does not follow the bimodal age distribution that is typically seen in viral pneumonias due to relative immunocompromise [2], and it is unclear if young children are spared from most COVID-19 symptoms due to cross-protective immunity to other coronaviruses or from additional differences in physiology related to age. Conversely, the association between CVD, CVD risk factors, and mortality from COVID-19 may be related to differential expression or function of angiotensin-converting enzyme (ACE) 2, which is the functional receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. This latter mechanism warrants urgent attention from the international research community. In this report, we highlight important known and unknown features of COVID-19 related to ACE2 and renin-angiotensin system (RAS) activity and suggest clinical research strategies to clarify the role of RAS blockade in COVID-19.
COVID-19 PATHOGENESIS
The virus responsible for COVID-19, SARS-CoV-2, is a positive-sense, single-stranded RNA virus from the genus Betacoronavirus [4]. By phylogenetic analysis, SARS-CoV-2 is closely related to several other human coronaviruses that cause a range of upper and lower respiratory infections, including SARS-CoV (from the 2003 outbreak) and Middle East respiratory syndrome coronavirus (MERS-CoV) [5, 6]. Viruses from the Coronaviridae family are characterized by surface Spike (S) proteins that associate with cellular receptors to mediate infection, the nature of which determines features related to transmissibility and pathogenesis [7].
SARS-CoV-2 infection is triggered when its S-protein binds to ACE2 [3], and several clinical observations in COVID-19 likely relate to this [8]. ACE2 is a membrane-bound peptidase that is highly expressed in the heart, lungs, kidney, and gastrointestinal tract, and it plays an important role in several cardiovascular and immune pathways (Figure 1) [9]. With SARS-CoV, it was shown that ACE2 overexpression facilitated viral entry and replication in cells that were otherwise resistant to the virus [10]. A similar pathogenesis has been identified in SARS-CoV-2, where it was recently confirmed that host-cell entry also depends on ACE2 [11]. This suggests that SARS-CoV-2 could target the same spectrum of cells targeted by SARS-CoV, which, in the lungs, was primarily localized to pneumocytes and macrophages [12, 13]. Acute respiratory distress syndrome (ARDS), which is highly prevalent in both SARS and COVID-19, is likely explained by this lung tropism. At the same time, extrapulmonary manifestations of COVID-19 may be related to the systemic distribution of ACE2 in multiple organs. ACE2 in the gastrointestinal tract may underlie early gastrointestinal symptoms such as nausea, vomiting, and diarrhea, and ACE2 in the heart may be related to cardiac damage and fulminant myocarditis that have been reported in COVID-19 [14, 15].
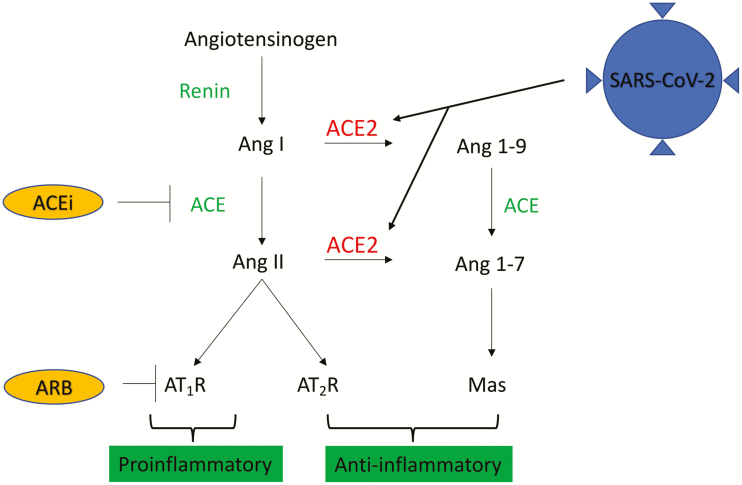
Figure 1.
SARS-CoV-2 and ACE2. ACE2 inhibits Ang II activity in the renin-angiotensin system through degradation of Ang I and Ang II into Ang 1–9 and Ang 1–7. Ang II and the AT1R have proinflammatory effects that may lead to acute lung injury or myocarditis, whereas the AT2 and Mas receptors have anti-inflammatory effects. SARS-CoV-2 uses ACE2 as its functional receptor and induces acute lung injury and myocarditis through unknown mechanisms. ACEi and ARBs inhibit the Ang II/AT1R axis, which may be anti-inflammatory; they also increase ACE2 expression, which may increase SARS-CoV-2 virulence. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ACE2, angiotensin-converting enzyme 2; Ang, angiotensin; ARB, angiotensin receptor blocker; AT, angiotensin; AT1R, AT1 receptor; AT2R, AT2 receptor; Mas, mitochondrial assembly; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
RENIN-ANGIOTENSIN SYSTEM ACTIVITY AND COVID-19
In the RAS cascade, ACE is responsible for the conversion of angiotensin (Ang) I to Ang II, and Ang II is the vasoactive peptide principally responsible for systemic vasoconstriction and stimulation of aldosterone release (Figure 1) [16]. In contrast, ACE2 counteracts the RAS cascade by providing inhibitory control over Ang II. It does this via 2 primary mechanisms. First, ACE2 directly catalyzes Ang I and Ang II, depleting their levels [17]. Second, the products of Ang I and Ang II degradation are themselves biologically active peptides that promote vasodilation, and they have additional antifibrotic, antiproliferative, and anti-inflammatory effects [17–19].
The generation of Ang II by ACE is pharmacologically inhibited by ACE inhibitors (ACEi), and angiotensin receptor blockers (ARBs) function by blocking the downstream interaction of Ang II with the AT1 receptor [16]. Conversely, while ACEi do not directly modulate ACE2, both ACEi and ARBs indirectly lead to increased ACE2 expression [20]. Additional medications, including thiazolidinediones or ibuprofen, may also increase ACE2 levels, although data on this are sparse [21]. Last, aldosterone antagonists and neprilysin inhibitors are also intimately related to RAS activation, but it is unclear whether either of these drugs have any impact on ACE2.
Three possibilities related to ACE2 expression exist that could explain an association between pre-existing CVD and COVID-19 mortality. These 3 possibilities could be synergistic or even oppose each other, although they are not mutually exclusive. At the same time, the relative degree to which any of these are at play in COVID-19 is unknown.
First, population differences in ACE2 expression or function may simultaneously predispose to the development of CVD and to the likelihood or severity of SARS-CoV-2 infection. In humans, ACE2 polymorphisms have been identified that are associated with CVD [17, 22, 23]. These same polymorphisms could impact the affinity for the S-protein of SARS-CoV-2, making infection more likely or more severe. In addition, the ACE2 gene is X-linked [24], and this could underlie some of the protective effect of female sex observed in COVID-19.
Second, either CVD itself or the pharmacologic RAS blockade used in CVD may augment ACE2 levels, increasing available substrate for SARS-CoV-2 infection within certain organs like the lung and heart. Indeed, increased ACE2 levels are seen in multiple cardiovascular conditions and related comorbidities such as diabetes and hypertension [8, 18, 25]. Paradoxically, pharmacologic RAS blockade has also been associated with increased ACE2 expression in mouse models [20]. However, it is possible that the effect of RAS blockade on COVID-19 is conditional on whether a patient has pre-existing RAS dysregulation associated with CVD, and the effect of RAS blockade may differ in the acute versus chronic setting. Thus, it is unclear whether RAS blockade would ameliorate or exacerbate the severity of COVID-19 infection in patients with CVD.
Last, SARS-CoV-2 infection may downregulate ACE2 function, possibly to a greater extent in patients with pre-existing CVD. This could lead to toxic Ang II overaccumulation, and several pathologic sequelae such as ARDS or fulminant myocarditis may follow. Based on data from the 2003 SARS-CoV, the interaction between novel coronaviruses and ACE2 function may be significant and deleterious. With SARS-CoV, animal models showed that infection with the virus downregulated ACE2 expression, generating an inflammatory response correlated with acute lung injury and impaired cardiac contractility [26–28]. If this same mechanism is present with SARS-CoV-2, this could underlie the development of ARDS, cardiac injury, and fulminant myocarditis.
RESEARCH PRIORITIES AND STUDY CONSIDERATIONS
Renin-angiotensin system activity in patients with CVD or cardiovascular comorbidities could increase the likelihood and severity of SARS-CoV-2 infection in the lung or heart and may explain heterogeneity in the clinical course of COVID-19, but in what direction? Furthermore, to what extent is this association modified by pharmacologic RAS inhibition? We lack even basic epidemiologic descriptions of medication use in COVID-19 to begin making this determination, so we can only speculate. Reflecting these disparate observations, investigators in recent weeks have variably hypothesized that both the use [29] or avoidance/withdrawal [30] of RAS blockade could lead to improved outcomes from COVID-19. Clinicians worldwide need to know if either of these suggestions has merit.
A randomized controlled trial addressing mortality reduction in COVID-19 is necessary to clarify the role of RAS blockade with the highest level of certainty. Some trials may soon be underway, yet high-quality observational data are still needed to develop and guide nuanced hypotheses (Table 1). Most important, it is unclear whether we should trial the initiation or the discontinuation of RAS blockade, and this could be rapidly informed by observational data to maximize the probability of successful therapy. Prospective identification of a community-based cohort of patients infected with SARS-CoV-2 would be ideal, and practical in an electronic era, so that mild and asymptomatic cases are represented in equal proportion with symptomatic cases. Still, it will take time and significant resources to identify a representative cohort, and answers to these questions are urgently needed. In the meantime, some data may already exist that, through international cooperation, could begin to inform these hypotheses and guide trial development.
Table 1.
Research Priorities for Coronavirus Disease 2019 and the Renin-Angiotensin System
| Unanswered Questions | Suitable Study | Key Epidemiologic Considerations |
|---|---|---|
| Are cardiovascular disease, hypertension, or diabetes independently associated with COVID-19 mortality? What subtypes of cardiovascular disease does this apply to? Are these associations mediated through acute respiratory distress syndrome, cardiomyopathy, or both? | Observational
|
Retrospective hospital cohort feasible with electronic record, greater selection bias towards null. Prospective community cohort less biased but longer time to results. Clustering may exist by country, region, or hospital. Case definition based on positive test: maximizes specificity, reduces sensitivity, will also bias to null. Covariate effects may be nonlinear (eg, age, interaction between hypertension and RAS blockade). Clear comorbidity definitions necessary to minimize misclassification bias. |
| Is RAS blockade associated with COVID-19 outcomes? Is the association positive or negative? Are these class effects? Is there any effect of neprilysin inhibition or mineralocorticoid receptor antagonism? What duration of exposure or discontinuation matters? Does this association differ for mortality, acute respiratory distress syndrome, or cardiomyopathy? | ||
| Can addition or removal of RAS blockade modify COVID-19 outcomes? Is this effect acute (within days) or chronic (over weeks to months)? Is it effective to start/stop medications once COVID-19 is already present, or must it be prophylactic? What is the relative effect of addition or withdrawal of RAS blockade in different conditions compared with COVID-19? | Randomized controlled trial
|
Direction of trials (starting or withholding RAS blockade) should be informed by existing observational data. Optimal outcomes: primary (all-cause mortality); secondary (cause-specific death, respiratory failure, fulminant myocarditis, need for intensive care). |
Abbreviations: COVID-19, coronavirus disease 2019; RAS, renin-angiotensin system.
As a first step, retrospective analysis of patients testing positive for SARS-CoV-2 in hospital-based cohorts should provide a rough estimate of the direction of effect, if not the magnitude. In admitted patients who test positive for SARS-CoV-2, we would want to compare the risk of severe disease versus mild disease associated with current use of RAS blockade at admission. Because most patients who die from COVID-19 appear to develop ARDS as they decompensate, the definition of severe disease should include death, need for intensive care and organ support such as mechanical ventilation, or ARDS. Some centers are anecdotally reporting de novo cardiomyopathy; if this is true, this too should be considered in the definition of severe disease, although the mechanism could differ, and it is unclear if cardiomyopathy associated with COVID-19 occurs in the absence of ARDS. The definition of RAS blockade exposure could also vary, given that patients may be on different drug classes (ie, ACEi or ARBs), with different doses, and with different time courses of exposure to the drug. All of these exposure parameters are important and should be considered, but a preliminary analysis with RAS blockade exposure defined at admission as a binary variable would be an important first step.
Several biases and confounders will need to be considered in observational studies in order to estimate the direct effect of RAS blockade on COVID-19 outcomes. In particular, hospital-based cohorts will exhibit selection bias for sicker patients, which is likely to bias results towards the null hypothesis (ie, no effect of RAS blockade) if patients with the least prominent risk factors are underrepresented. Significant confounding likely exists that could also obscure the direct effect of RAS blockade, notably confounding by disease indication. In addition, as a minimum set of confounders, we would purpose the following must be considered: age (measured continuously if possible, and allowing for a nonlinear relationship), tobacco exposure (ideally quantified by pack-years and distinguishing between current and former smokers), pre-existing pulmonary disease or immunocompromise, and sex.
Conclusions
Cardiovascular disease, diabetes, and hypertension are each strongly associated with mortality in COVID-19, but the mechanisms underlying this are unknown. Dysregulation of the RAS cascade is present in each of these conditions and could increase the likelihood or severity of SARS-CoV-2 infection. This suggests that pharmacologic RAS inhibition could impact COVID-19 outcomes, but the direction of effect is unclear. Mechanistic evidence suggests 2 competing hypotheses: (1) RAS blockade decreases proinflammatory activity of Ang II, decreasing the risk of ARDS, myocarditis, or mortality in COVID-19, or (2) RAS blockade increases ACE2 expression, promoting SARS-CoV-2 virulence in the lungs and heart that leads to ARDS, myocarditis, and death. Basic pharmacoepidemiologic studies with RAS blockade in COVID-19 need to be rapidly performed, which could then inform confirmatory randomized controlled trials.
These analyses will be a necessary first step to clarify the role of pharmacologic RAS blockade in COVID-19, and the role of RAS activation in COVID-19 even more broadly. Patients who require RAS inhibitors, including ACEi or ARBs, should not change their regimens without further evidence. Even if there is a causal association between RAS blockade and COVID-19 outcomes, it does not immediately follow that starting or stopping these medications will benefit patients in the short term; acute effects of these medications, and their effect on ACE2 or RAS, could be very different or even paradoxical from chronic effects. At the same time, if even a modest association exists (positive or negative) between RAS blockade and COVID-19 outcomes, these data could rapidly inform confirmatory trials of RAS blockade removal or initiation that could save countless lives in the escalating pandemic.
Notes
Disclaimer. The National Institutes of Health had no role in the design or authorship of this publication. The article contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the University of Pennsylvania, or the Massachusetts General Hospital Executive Committee on Research.
Financial support. This work was supported by the National Institutes of Health (grant numbers T32 AI007061 [to T. S. B.] and T32 AI007433 [to A. M. M.]) and the National Heart, Lung, and Blood Institute at the National Institutes of Health (grant numbers T32 HL-007891 [to T. C. H.], R00 HL-141678 [to M. O. H.], and K23-HL133843 [to J. B. C.]).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol 2020; 41:145–51. [Google Scholar]
- 2. Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Inf ect 2020. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020; 9:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol 2020. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004; 25:291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020; 525:135–40. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shieh WJ, Hsiao CH, Paddock CD, et al. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol 2005; 36:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li L-Q, Huang T, Wang Y-Q, et al. 2019 Novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol 2020. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 2017; 125:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 2016; 118:1313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anguiano L, Riera M, Pascual J, Soler MJ. Circulating ACE2 in cardiovascular and kidney diseases. Curr Med Chem 2017; 24:3231–41. [DOI] [PubMed] [Google Scholar]
- 19. Lelis D de F, de Freitas DF, Machado AS, Crespo TS, Santos SHS. Angiotensin-(1–7), adipokines and inflammation. Metabolism 2019; 95:36–45. [DOI] [PubMed] [Google Scholar]
- 20. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–10. [DOI] [PubMed] [Google Scholar]
- 21. Qiao W, Wang C, Chen B, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology 2015; 131:97–106. [DOI] [PubMed] [Google Scholar]
- 22. Pinheiro DS, Santos RS, Jardim PCBV, et al. The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: a genetic association study in Brazilian patients. PLoS One 2019; 14:e0221248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu C, Li Y, Guan T, et al. ACE2 polymorphisms associated with cardiovascular risk in Uygurs with type 2 diabetes mellitus. Cardiovasc Diabetol 2018; 17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 2010; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ortiz-Pérez JT, Riera M, Bosch X, et al. Role of circulating angiotensin converting enzyme 2 in left ventricular remodeling following myocardial infarction: a prospective controlled study. PLoS One 2013; 8:e61695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002; 417:822–8. [DOI] [PubMed] [Google Scholar]
- 28. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11:875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8::e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]