Abstract
Background
In December 2019, novel coronavirus (SARS-CoV-2) pneumonia (COVID-19) was reported in Wuhan and has since rapidly spread throughout China. We aimed to clarify the characteristics and clinical significance of peripheral lymphocyte subset alteration in COVID-19.
Methods
The levels of peripheral lymphocyte subsets were measured by flow cytometry in 60 hospitalized COVID-19 patients before and after treatment, and their association with clinical characteristics and treatment efficacy was analyzed.
Results
Total lymphocytes, CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells decreased in COVID-19 patients, and severe cases had a lower level than mild cases. The subsets showed a significant association with inflammatory status in COVID-19, especially CD8+ T cells and CD4+/CD8+ ratio. After treatment, 37 patients (67%) showed clinical response, with an increase in CD8+ T cells and B cells. No significant change in any subset was detected in nonresponsive cases. In multivariate analysis, posttreatment decrease in CD8+ T cells and B cells and increase in CD4+/CD8+ ratio were indicated as independent predictors of poor efficacy.
Conclusions
Peripheral lymphocyte subset alteration was associated with clinical characteristics and treatment efficacy of COVID-19. CD8+ T cells tended to be an independent predictor for COVID-19 severity and treatment efficacy.
Keywords: COVID-19, pneumonia, lymphocyte subset
Peripheral lymphocyte subset alteration showed a clear association with the clinical characteristics of COVID-19, especially in relation to disease severity assessment and treatment efficacy prediction, which could help to indicate timely interventions to be provided by physicians.
In December 2019, several cases of pneumonia of unknown origins were reported in Wuhan, Hubei Province, China [1, 2]. Rapidly, the disease spread throughout China. By genome-wide sequencing of samples of bronchoalveolar lavage fluid, the pathogen was confirmed to be a distinct clade of the β-coronavirus associated with human severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [3]. The novel virus was officially named SARS-CoV-2, with the disease termed COVID-19 [4].
Lymphocytes and the subsets of CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells play an important role in the maintenance of immune system function. After virus infection, alteration in total lymphocyte numbers and the subsets varies with different virus types, indicating a potential association between lymphocyte subset alteration and viral pathogenic mechanisms [5]. Recent studies indicated a clear decrease in peripheral lymphocytes in COVID-19 patients but any alteration in the subsets was still unknown [6, 7]. In this study, we aimed to clarify the characteristics and clinical significance of peripheral lymphocyte subset alteration in COVID-19, which might help elucidate the pathogenesis and develop novel biomarkers and therapeutic strategies for COVID-19.
METHODS
Study Population
We enrolled 60 patients with COVID-19, which was confirmed by detecting SARS-CoV-2 RNA in throat swab samples using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer’s protocol (Shanghai BioGerm Medical Biotechnology). All the patients were initially admitted to Zhongnan Hospital of Wuhan University from 7 January to 14 February 2020. In addition, we had previously tested 245 healthy blood donors to establish interlaboratory reference ranges for various parameters. These reference values were used to provide data for the healthy controls in this study.
The study was approved by the ethics committee of Zhongnan Hospital of Wuhan University (No. 2020011). Written informed consent was obtained from patients.
Data Collection
The following information on each patient was extracted from electronic medical records: age, sex, medical history, symptoms, severity assessment on admission, laboratory findings, chest computed tomography (CT) or radiograph findings, treatment, and efficacy. On admission, severe illness was defined according to the following criteria: (1) breathing rate ≥30 times/min; (2) pulse oximeter oxygen saturation (Spo2) ≤93% at rest; and (3) ratio of partial pressure of arterial oxygen (Pao2) to fraction of inspired oxygen (Fio2) ≤300 mmHg. After 1 week of treatment, clinical response was defined according to the following criteria: (1) symptom alleviation (eg, fever, cough, chest distress, and shortness of breath); and (2) improvement in radiological abnormalities on chest CT or radiograph. Cases not meeting these criteria were classified as nonresponsive.
Flow Cytometry
Samples of EDTA anticoagulated peripheral blood (2 mL) were collected from patients with COVID-19 before initial treatment and a second sample was collected after 1 week of treatment. All samples were tested within 6 hours of being obtained. Briefly, CD3+/CD4+/CD8+ T-cell, CD19+ B-cell, and CD16+CD56+ NK-cell counts (cells/μL) were measured by multiple-color flow cytometry with human monoclonal anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-phycoerythrin (PE), anti-CD8-allophycocyanin (APC), anti-CD19-PE, anti-CD16-APC, and anti-CD56-PE antibodies (BD Multitest) according to the manufacturer’s instructions. The cells were analyzed on a BD FACS Canto II flow cytometry system (BD Biosciences).
Statistical Analysis
Categorical data were described as percentages and continuous data as median with interquartile range (IQR). A nonparametric comparative test for continuous data was used to compare variables between groups. Multivariate analysis was used to identify independent predictors of lymphocyte subsets for the treatment efficacy in COVID-19. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the ability of lymphocyte subsets in predicting treatment efficacy. Bootstrap test was used to compare 2 correlated ROC curves. All statistical analyses were performed using SPSS Statistics version 21.0 software. P < .05 was considered statistically significant.
RESULTS
Baseline Characteristics of 60 COVID-19 Patients
Sixty COVID-19 patients were included in the study (Table 1). The median age was 60 years (IQR, 38–66), and 22 patients (37%) were male. Hypertension (15%) and diabetes (10%) were the most common comorbidities. Fever (70%), cough (48%), and shortness of breath (32%) were the most common symptoms. According to CT or radiograph findings, 40 patients (67%) showed bilateral pneumonia. In blood tests, leukocytes, neutrophils, and platelets were below the normal range in 17 (32%), 11 (21%), and 11 (21%) patients, and above the normal range in 6 (11%), 7 (13%), and 1 (2%) patients, respectively. Lymphocytes were below the normal range in 38 patients (72%).
Table 1.
Clinical Characteristics of 60 Patients With COVID-19 Pneumonia
| Characteristic | Patients | Normal Range |
|---|---|---|
| Age, y, median (IQR) | 60 (38–66) | … |
| Male, No. (%) | 22 (37) | … |
| Comorbidities, No. (%) | ||
| Hypertension | 9 (15) | … |
| Diabetes | 6 (10) | … |
| Heart diseases | 1 (2) | … |
| Symptoms, No. (%) | ||
| Fever | 42 (70) | … |
| Cough | 29 (48) | … |
| Breath shortness | 19 (32) | … |
| Myalgia | 8 (13) | … |
| Bilateral lung distribution, No. (%) | 40 (67) | … |
| Severe illness on admission, No. (%) | 19 (32) | … |
| Blood count, ×109/L, median (IQR) | ||
| Leukocyte | 4.2 (3.3–5.9) | 3.5–9.5 |
| Neutrophil | 2.8 (2.2–4.5) | 1.8–6.3 |
| Lymphocyte | 0.8 (0.6–1.2) | 1.1–3.2 |
| Platelet | 186 (131–225) | 125–350 |
| Inflammatory indicators, median (IQR) | ||
| ESR, mm/h | 24 (11–41) | 0–15 |
| C-reactive protein, mg/L | 26 (9–67) | 0–10 |
| Interleukin-6, pg/mL | 13 (6–29) | 0–7 |
| Treatment, No. (%) | ||
| Oxygen inhalation | 28 (47) | … |
| Corticosteroid | 27 (45) | … |
| Antiviral treatment | 41 (68) | … |
| Arbidol | 22 (37) | … |
| Darunavir and cobicistat | 14 (23) | … |
| Lopinavir and ritonavir | 10 (17) | … |
| Remdesivir | 9 (15) | … |
| Ribavirin | 6 (10) | … |
| Interferon inhalation | 19 (32) | … |
| Immune enhancer | 23 (38) | … |
| Thymalfasin | 19 (32) | … |
| Immunoglobulin | 6 (10) | … |
Abbreviations: COVID-19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; IQR, interquartile range.
Peripheral Lymphocyte Subset Alteration in COVID-19
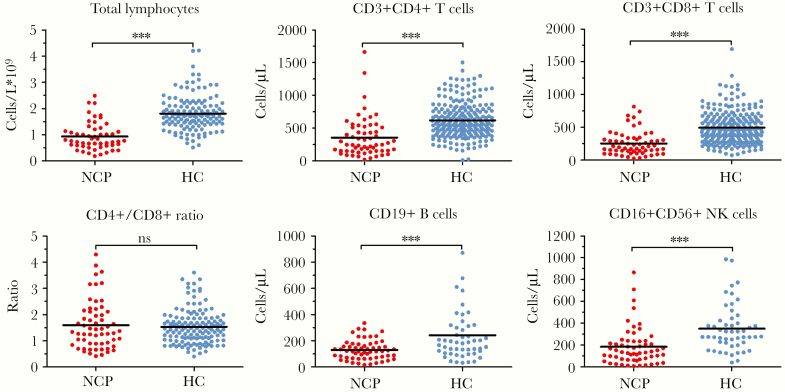
We initially analyzed the levels of lymphocyte subsets by flow cytometry in whole blood. Compared to healthy controls, COVID-19 patients had a significantly lower total lymphocytes (P < .0001), CD4+ T cells (P < .0001), CD8+ T cells (P < .0001), B cells (P = .0003), and NK cells (P < .0001) (Figure 1). No significant difference was observed in CD4+/CD8+ ratio (P = .603).
Figure 1.
Comparison of peripheral lymphocyte subsets between COVID-19 pneumonia (CP) patients and healthy controls (HC). ***, P < .001; NS, not significant.
Lymphocyte Subset Levels and COVID-19 Severity
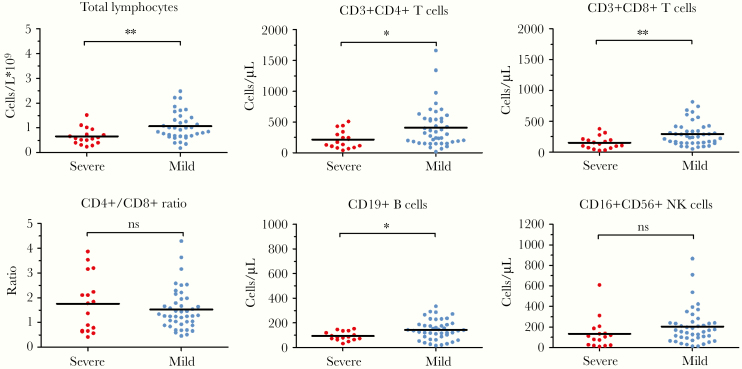
Nineteen patients (32%) were categorized as experiencing serious illness on admission. Compared to patients with mild illness, severe cases had significantly lower total lymphocytes (P = .0007), CD4+ T cells (P = .024), CD8+ T cells (P = .005), and B cells (P = .018) (Figure 2). No significant difference was observed in CD4+/CD8+ ratio (P = .392) and NK cells (P = .177).
Figure 2.
Peripheral lymphocyte subset levels and disease severity in COVID-19 pneumonia. *, P < .05; **; NS, not significant.
Lymphocyte Subset Levels and Inflammatory Status
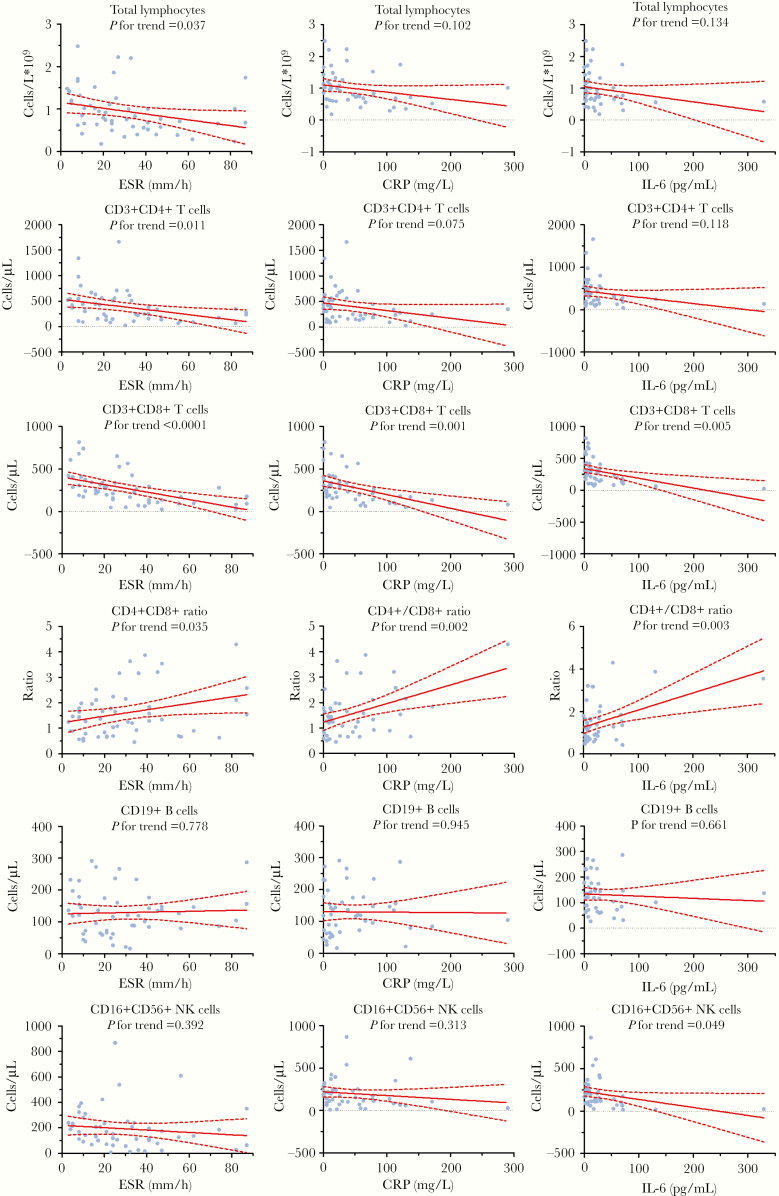
Inflammatory indicators erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and interleukin-6 (IL-6) were abnormal in 36 (71%), 34 (72%), and 30 (70%) patients on admission. Total lymphocytes and CD4+ T cells were negatively correlated with ESR (P = .037 and P = .011, respectively). CD8+ T cells were negatively correlated with ESR (P < .0001), CRP (P = .001), and IL-6 (P = .005) (Figure 3). CD4+/CD8+ ratio was positively correlated with ESR (P = .035), CRP (P = .002), and IL-6 (P = .003). B cells showed no significant correlation with ESR (P = .778), CRP (P = .945), or IL-6 (P = .661). NK cells were negatively correlated with IL-6 (P = .049).
Figure 3.
Correlation analysis of peripheral lymphocyte subset levels and inflammatory indicator levels in COVID-19 pneumonia patients. Solid line: fitted curve; dashed line: 95% confidence interval (CI) of the fitted curve. Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; IL-6, interleukin-6.
Posttreatment Lymphocyte Subset Alteration and Clinical Efficacy
After hospitalization, 28 patients (47%) were treated with oxygen inhalation, 27 (45%) with intravenous corticosteroid, 41 (68%) with antiviral treatment, and 23 (38%) with immune enhancer. The most common antiviral treatment was Arbidol administration (37%) and interferon inhalation (32%), and 50% of patients received more than 1 antiviral regimen. In immune enhancing treatment, 32% and 10% of patients received thymalfasin and immunoglobulin, respectively.
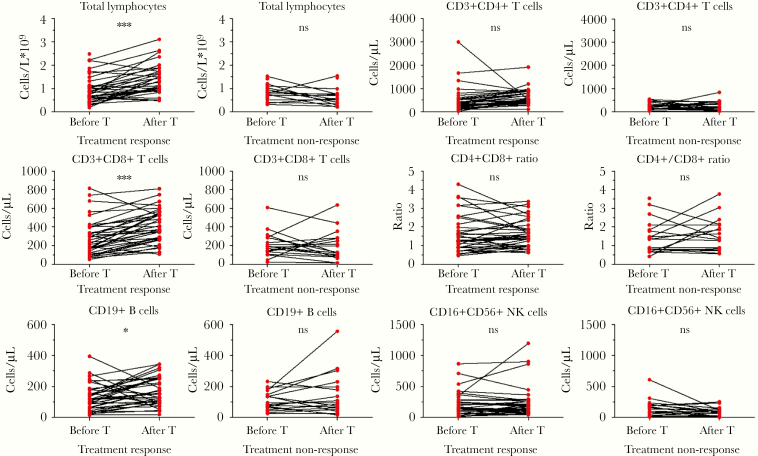
After 1 week of treatment, 37 patients (67%) reached clinical response, and 18 (33%) had not reached clinical response. In responsive patients, total lymphocytes (P < .0001), CD8+ T cells (P < .0001), and B cells (P = .026) increased significantly after treatment, and no significant change was detected in CD4+ T cells, CD4+/CD8+ ratio, and NK cells (P > .05) (Figure 4). In nonresponsive patients, no significant change was detected in any lymphocyte subsets (P > .05). Comparatively, corticosteroid treatment increased total lymphocytes significantly, while antiviral treatment increased total lymphocytes, CD4+ T cells, CD8+ T cells, and B cells significantly (data not shown). Immune enhancer had no obvious improvement in any subsets (data not shown).
Figure 4.
Peripheral lymphocyte subset alteration in clinically responsive and nonresponsive patients with COVID-19 pneumonia before (before T) and after 1 week of treatment (after T). *, P < .05; ***, P < .001; NS, not significant.
In multivariate analysis, posttreatment decrease in CD8+ T cells (P = .011) and B cells (P = .010) and increase in CD4+/CD8+ ratio (P = .032) indicated a poor efficacy when considering the factors of age, sex, disease severity on admission, oxygen inhalation, antiviral treatment, and use of corticosteroid and immune enhancer (Table 2).
Table 2.
Multivariate Analysis of Posttreatment Alteration of Peripheral Lymphocyte Subsets and Clinical Efficacy in Patients With COVID-19 Pneumonia
| Multivariate Analysisa | ||||
|---|---|---|---|---|
| Posttreatment Alteration | No. (%) | P Value | Odds Ratio | 95% CI |
| Total lymphocyte decrease | 17 (36) | .071 | 0.113 | .011–1.209 |
| CD3+CD4+ T-cell decrease | 16 (29) | .056 | 0.157 | .024–1.047 |
| CD3+CD8+ T-cell decrease | 16 (29) | .011 | 0.056 | .006–.516 |
| CD4+/CD8+ ratio increase | 30 (56) | .032 | 0.099 | .012–.821 |
| CD19+ B-cell decrease | 14 (25) | .010 | 0.033 | .002–.439 |
| CD16+CD56+ NK-cell decrease | 32 (59) | .190 | 0.310 | .054–1.787 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
aAdjusted by age, sex, disease severity on admission, oxygen inhalation, antiviral treatment, and use of corticosteroid and immune enhancer.
ROC Curve Analysis
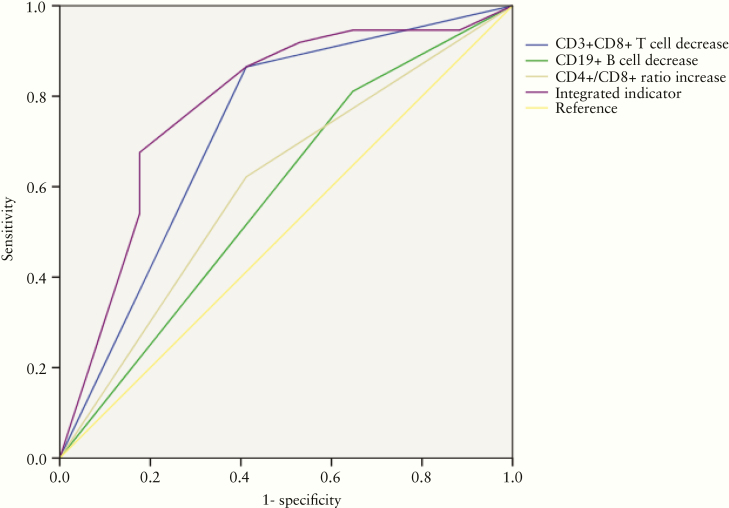
ROC curve analysis was conducted to evaluate the role of posttreatment alterations in peripheral lymphocyte subsets in predicting treatment efficacy (Figure 5). The area under the ROC curve (AUC) was 0.738 (95% confidence interval [CI], .586–.890) for CD8+ T-cell decrease, 0.605 (95% CI, .441–.769) for CD4+/CD8+ ratio increase, 0.600 (95% CI, .434–.765) for B-cell decrease, and 0.781 (95% CI, .638–.923) for the integrated indicator. Bootstrap testing indicated a higher predictive accuracy of the integrated indicator than the alteration in CD8+ T cells, B cells, CD4+/CD8+ ratio, or total lymphocytes individually (P < .05).
Figure 5.
Receiver operating characteristic (ROC) curve analysis of posttreatment alteration of peripheral lymphocyte subsets in predicting clinical efficacy in COVID-19 pneumonia.
DISCUSSION
Since December 2019, COVID-19 has been reported in Wuhan and has rapidly spread throughout China. As with MERS-Cov and SARS-Cov, SARS-CoV-2 is a member of the coronavirus family and belongs to the β-coronaviruses [8]. Infection by these coronavirus can cause sustained responses of cytokines and chemokines (namely a cytokine storm), leading to a high incidence of immune disorders and mortality [9].
Lymphocytes and their subsets play an important role in the maintenance of immune system function. As with immune diseases and other infectious disease, virus infection can also lead to dysregulation in the levels of lymphocyte subsets [10, 11]. Cellular surface molecules to CD3+, CD4+, CD8+, CD16+, CD19+, and CD56+ mark the lymphocyte T-helper cells (CD3+CD4+) and cytotoxic T cells (CD3+CD8+), B cells (CD19+), and NK cells (CD16+CD56+). These cells are involved in the humoral and cytotoxic immunity against viral infection. Thus, it is important to clarify the characteristics of lymphocyte subsets in COVID-19, which could provide novel insights to explore the immune mechanism.
In our study, lymphopenia was common in the patients with COVID-19 (72%), indicating an impairment of the immune system during the course of SARS-CoV-2 infection. In addition, decreases in CD4+ T cells, CD8+ T cells, B cells, and NK cells were also observed in the COVID-19 patients. These alterations were also found in patients with pneumonia caused by MERS-Cov and SARS-Cov [12]. In the study by Cui et al on SARS, the incidence of lymphopenia was 84%, CD4+ T cells decreased in 100% of patients, CD8+ T cells decreased in 87%, B cells decreased in 76%, and NK cells decrease in 55% [13]. In the study by Assiri et al on MERS, lymphopenia occurred in 34% of patients [14]. Lymphopenia might be caused by virus attachment or indirectly by immune injuries from inflammatory mediators. Moreover, exudation of circulating lymphocytes into inflammatory lung tissues might also lead to lymphopenia.
Among COVID-19 patients, severe cases had a lower level of total lymphocytes, CD4+ T cells, CD8+ T cells, and B cells than mild cases, which was similar to the alteration in SARS [15, 16]. CD8+ T-cell levels were negatively correlated with inflammatory indicators ESR, CRP, and IL-6, while the CD4+/CD8+ ratio was positively correlated. Total lymphocyte and CD4+ T-cell levels were negatively associated with ESR, and NK cells were negatively correlated with IL-6. These findings indicate a more obvious change in CD8+ T cells than in other lymphocyte subsets after SARS-CoV-2 infection. Thus, lymphocytes and their subsets, especially CD8+ T cells, might be a potential predictor for disease severity and clinical efficacy in COVID-19.
After 1 week of treatment, in responsive cases there was a significant increase in total lymphocytes, CD8+ T cells, and B cells, but in nonresponsive cases there was no significant change in any lymphocyte subsets. However, these findings might be confounded by therapeutic factors. First, the lympholytic effects of corticosteroid could reduce the lymphocytes directly [17]. For the patients with corticosteroid treatment, the recovery of lymphocyte population might be weakened by the lympholytic effects of corticosteroid. Thus, we conducted a multivariate analysis to evaluate the effects of these potential confounders, and identified posttreatment decrease in CD8+ T cells and B cells and increase in CD4+/CD8+ ratio as independent predictors for poor clinical efficacy, especially CD8+ T cells. Moreover, we also found that corticosteroid treatment increased total lymphocytes significantly in comparison to the patients without corticosteroid treatment. The anti-inflammatory effects of corticosteroid might have contributed to the posttreatment increase in lymphocytes, which outweighed the lympholytic effects of corticosteroid.
Importantly, CD8+ T cells have been shown to play a critical role in mediating viral clearance after acute respiratory infections of respiratory syncytial virus (RSV), influenza A virus (IAV), and human metapneumovirus [18, 19]. In animal experiments, the transfer of RSV- or IAV-immune CD8+ T cells into athymic mice significantly reduced viral titers [20, 21]. As in our results, cytotoxic immunity was involved in antiviral processes and the recovery of cytotoxic immune function (particularly CD8+ T cells) might be a reliable indicator of disease severity and recovery.
In conclusion, peripheral lymphocyte subset alteration showed a clear association with the clinical characteristics of COVID-19. CD8+ T cells tended to be an independent predictor for COVID-19 severity and treatment efficacy. These findings might help elucidate the pathogenesis and develop novel biomarkers and therapeutic strategies for COVID-19.
Notes
Financial support. This study was supported by Zhongnan Hospital of Wuhan University Program of Excellent Doctoral (Postdoctoral) Research (grant number ZNYB2019003).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mo P, Xing Y, Deng L, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China [published online ahead of print 16 March 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa270. [DOI] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak [published online ahead of print 26 February 2020]. J Autoimmun doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 2004; 189:648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print 7 February 2020]. JAMA doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malik YS, Sircar S, Bhat S, et al. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q 2020; 40:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan MH, Wong VW, Wong CK, et al. Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J Intern Med 2004; 255:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su R, Li Z, Wang Y, et al. Imbalance between Th17 and regulatory T cells in patients with systemic lupus erythematosus combined EBV/CMV viraemia [published online ahead of print 20 November 2019]. Clin Exp Rheumatol. [PubMed] [Google Scholar]
- 12. He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis 2005; 9:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui W, Fan Y, Wu W, Zhang F, Wang JY, Ni AP. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis 2003; 37:857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 2003; 326:1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peiris JS, Lai ST, Poon LL, et al. ; SARS study group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003; 361:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hollander N, Chiu YW. Relation between cortisol metabolism and its lympholytic effect in P1798 lymphosarcoma. Endocrinology 1966; 79:168–74. [DOI] [PubMed] [Google Scholar]
- 18. Wells MA, Ennis FA, Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol 1981; 126:1042–6. [PubMed] [Google Scholar]
- 19. Cannon MJ, Stott EJ, Taylor G, Askonas BA. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 1987; 62:133–8. [PMC free article] [PubMed] [Google Scholar]
- 20. Muñoz JL, McCarthy CA, Clark ME, Hall CB. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol 1991; 65:4494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol 2018; 9:678. [DOI] [PMC free article] [PubMed] [Google Scholar]