Abstract
Background
Recent studies have focused on initial clinical and epidemiological characteristics of the coronavirus disease 2019 (COVID-19), which is the mainly revealing situation in Wuhan, Hubei.
Aim
This study aims to reveal more data on the epidemiological and clinical characteristics of COVID-19 patients outside of Wuhan, Zhejiang, China.
Design
This study was a retrospective case series.
Methods
Eighty-eight cases of laboratory-confirmed and three cases of clinically confirmed COVID-19 were admitted to five hospitals in Zhejiang province, China. Data were collected from 20 January 2020 to 11 February 2020.
Results and discussion
Of all 91 patients, 88 (96.70%) were laboratory-confirmed COVID-19 with throat swab samples that tested positive for SARS-Cov-2, three (3.30%) cases were clinically diagnosed. The median age of the patients was 50 (36.5–57) years, and female accounted for 59.34%. In this sample, 40 (43.96%) patients had contracted the disease from local cases, 31 (34.07%) patients had been to Wuhan/Hubei, eight (8.79%) patients had contacted with people from Wuhan, and 11 (12.09%) patients were diagnosed after having flown together in the same flight with no passenger that could later be identified as the source of infection. In particular within the city of Ningbo, 60.52% cases can be traced back to an event held in a temple. The most common symptoms were fever (71.43%), cough (60.44%) and fatigue (43.96%). The median of incubation period was 6 (interquartile range 3–8) days and the median time from the first visit to a doctor to the confirmed diagnosis was 1 (1–2) days. According to the chest computed tomography scans, 67.03% cases had bilateral pneumonia.
Conclusions
Social activity cluster, family cluster and flying alongside with persons already infected with COVID-19 were how people got infected with COVID-19 in Zhejiang.
Introduction
In early December 2019, cases of pneumonia of unknown cause were identified in Wuhan, Hubei Province of China. Bronchoalveolar lavage fluid was obtained from patients, and a novel coronavirus was identified by metagenomic analysis using next-generation sequencing in the Wuhan Institute of Virology.1 The US Centre’s for Disease Control and Prevention (CDC) named it as 2019 novel coronavirus (2019-nCoV).2 2019-nCoV shares 88% of the genetic sequence with two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21.3 It shares the same cell entry receptor (angiotensin-converting enzyme 2) with SARS-CoV.1 The 2019-CoV is listed as the seventh member of coronavirus (subgenus sarbecovirus, Orthocoronavirinae subfamily)4 and named as SARS-CoV-2.5
Coronavirus disease 2019 (COVID-19) is highly contagious and spreads rapidly through human-to-human transmissions.5,6 As of 20 February, 75 571 confirmed cases had been reported in Mainland China and 1083 confirmed cases in 24 other countries and regions. Amongst the cases in Mainland China, 2239 cases died, 11 cases of death were reported from out of Mainland China.7
There were 41 initial cases of COVID-19 that were directly or indirectly linked to the Wuhan Huanan Seafood Wholesale Market, as reported by Huang et al.8 The clinical features include fever, dry cough, dyspnoea, myalgia, fatigue, decreased leukocyte counts and computed tomography (CT) evidence of pneumonia.8 Subsequently, Chen et al.9 reported 99 cases from a single centre of Wuhan, but the severe and non-severe cases were not compared; and then Wang et al.10 published a study based on 138 hospitalized patients from Wuhan. However, all these studies are based on cases identified in Wuhan. Recently, Guan et al.11 reported 1099 cases with laboratory-confirmed SARS-CoV-2 from 552 hospitals across 31 provinces/provincial municipalities. They reported that the median age was 47.0 years, 41.90% were females, 31.30% had been to Wuhan and 71.80% had contacted people from Wuhan, and the average incubation period was 3.0 days. The most common symptoms were fever and cough.11 Together, several articles about cases from Wuhan have reported epidemiological and clinical manifestations and provided important initial background upon which we seek to furnish further in this article.8–10,12 The clinical features of COVID-19 of cases outside Wuhan are still largely unknown.
Zhejiang Province has consistently been one of the top three provinces with most cases in China and, therefore, provide a good basis to learn how COVID-19 spreads outside Wuhan. The cluster events, public transport transmissions and clinical diagnosis of this new infectious disease are of great importance to be reported as it shed light on of cases that occur outside Wuhan. Here, we report the epidemiological and clinical characteristics of COVID-19 patients from five hospitals in Zhejiang province, China.
Materials and methods
DATA sources
We performed a retrospective, multi-centre study on the epidemiological history, clinical records, laboratory results and chest radiological features of 88 laboratory-confirmed and 3 clinically diagnosed patients with COVID-19 who were diagnosed from 20 January 2020 to 11 February 2020. Final follow-up for this report lasted until 16 February 2020.
The primary method of diagnosis is to perform real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay test using throat swab specimens that were collected from upper respiratory tracts. This test is performed twice at 24-h interval. Of the 91 cases reported in here, 88 cases were tested positive for SARS-CoV-2 at least once. These laboratory confirmation assays for SARS-CoV-2 were performed at CDCs of various cities and Ningbo First Hospital following the standard protocol.8 Three further cases were reported in Ningbo cohort as clinically diagnosed COVID-19 pneumonia because of their epidemiological history, signs, symptoms and chest CT evidence according to National Health Commission of the People’s Republic of China guidance, though they tested negative for the SARS-CoV-2.13,14 The incubation period was defined as the time from the exposure to the confirmed or suspected transmission source to the onset of illness.
A team of doctors who had been treating these patients extracted the medical records of these patients and data was analysed by the working group in Ningbo. When the data were not clear or missing, the working group in Ningbo would clarify the details with the doctors in charge of treating these patients. The study has been reviewed and approved by the medical ethics committees (2020-R018). The requirement for written informed consent was waived because of the urgent need to collect clinical data and no harm could potentially be done to patients. Doctors who treated the patients collected and recorded the epidemiological characteristics by interviewing each patient on their activity history during 2 weeks before symptoms onset or admission into the hospital. All patients underwent chest CT scans. The clinical symptoms, chest CT and laboratory findings on admission were extracted from electronic medical records. Laboratory results included blood routine, blood chemistry, arterial blood gas, fibrinogen, liver and renal function, electrolytes, C-reactive protein and procalcitonin.
The diagnosed patients were divided into the severe group and mild group according to the national treatment guidelines.13,14 Questionnaires of MuLBSTA scores15 were recorded by attending physicians according to six indexes, which are multilobular infiltration, lymphopaenia, bacterial co-infection, smoking history, hypertension and age. All data were checked by two experienced physicians (G.Q. and N.Y.).
Statistical analysis
We present the summary statistics of continuous variables using the means and standard deviations or median (interquartile range, IQR); comparison across groups was performed using the Mann–Whitney U-test. Categorical variables were expressed as the counts and percentages in each category. Chi-square tests were used for categorical variables as appropriate. All analyses were analysed by IBM SPSS statistics version 26.0.
Patient and public involvement
This was a retrospective case series study; no patients and public were involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Demographic features
Of all 91 patients recruited as of 11 February, we detected 88 (96.70%) laboratory-confirmed COVID-19 pneumonia with throat swab samples that positive for SARS-CoV-2 and three (3.30%) clinically confirmed COVID-19 pneumonia since they had a definite demographic history, typical symptoms and chest CT images.
As shown in Table 1, the median age of the 91 patients was 50 years (IQR 36.5–57.0), ranged from 5 to 96 years. Amongst the patients were two young persons, including a 5 years old and a 17 years old. There were six elderly patients aged 70 or above. Adults accounted for most of the cases, with breakdown as follows: aged 18–39 (26 cases, 28.57%), aged 40–49 (16 cases, 17.58%), aged 50–59 (28 cases, 30.77%) and aged 60–69 (13 cases, 14.29%). There were 54 female patients (59.34%), and none of them were pregnant.
Table 1.
Demographics, baseline characteristics of 91 patients from Zhejiang province with COVID-19
| Age (years) | Patients (n = 91) |
|---|---|
| Medians (interquartile ranges) | 50 (36.5–57) |
| <18 | 2 (2.20) |
| 18–39 | 26 (28.57) |
| 40–49 | 16 (17.58) |
| 50–59 | 28 (30.77) |
| 60–69 | 13 (14.29) |
| ≥70 | 6 (6.59) |
| Sex | |
| Female | 54 (59.34) |
| Male | 37 (40.66) |
| Epidemiological | |
| Contact with local case | 40 (43.96) |
| Imported from Hubei | 31 (34.07) |
| Vehicle (airplane, coach, ship) | 11 (12.09) |
| Contact with Wuhan personnel | 8 (8.79) |
| Unknown original | 1 (1.10) |
| Chronic medical illness | |
| Hypertension | 15 (16.48) |
| Diabetes mellitus | 8 (8.79) |
| Cardiovascular and cerebrovascular disease | 3 (3.30) |
Values are expressed as n (%) or medians (interquartile ranges) unless stated otherwise.
As for epidemiological characteristics, 31 patients had been to Wuhan/Hubei within the past 2 weeks (G0). Local cases are defined as follows: eight patients have had contacts with persons who had been in Wuhan/Hubei within the past 2 weeks, these are the first generation of local cases (G1). Following that 40 patients contracted the disease after having had contact with the local cases, these are defined as G2 local cases. In particular, 23 of the G2 cases were all derived from an event where people collectively prayed for good luck in a temple for the new lunar year. There were 11 patients who took the same flight and we believe they were infected while flying together, with no person that could be singled out as the index patient within this group. It is impossible to establish how the remaining one patient contracted the disease. None of the patients had a history of exposure to the Huanan seafood wholesale market. No healthcare workers were found in our study. Moreover, none of the health workforce in Zhejiang was infected.
Most of the 91 patients had no underlying comorbidities, while 15 (16.48%) patients had hypertension, eight (8.79%) patients had Type 2 diabetes mellitus, among which three (3.30%) patients had both hypertension and Type 2 diabetes mellitus, and three (3.30%) patients had cardiovascular and cerebrovascular diseases.
Clinical characteristics
On admission, the most common symptoms were fever (65, 71.43%), cough (55, 60.44%) and fatigue (40, 43.96%) (Table 2). Among them, 34 (37.36%) cases have a temperature between 38.1° and 39° but none had a very high fever (body temperature >). Other symptoms included expectoration (30, 32.97%), anorexia (23, 25.27%), diarrhoea (21, 23.08%), nausea (11, 12.09%) and vomiting (6, 6.59%). In a case, her abdominal discomfort was the only symptom. Nine patients were diagnosed as severe pneumonia because of the development of pneumonia. As of 16 February, 60 (65.93%) patients were still isolated in our hospitals, and 31 (34.07%) patients had been discharged and no patients had died so far. The median of the incubation period is 6 (IQR 3–8) days, and the number of days from the first visit to a doctor till the case is confirmed is 1 (1–2).
Table 2.
Clinical features of 91 patients with COVID-19
| Patients (N = 91) | |
|---|---|
| Signs and symptoms | |
| Fever | 65 (71.43) |
| Maximal temperature | |
| <37.3 | 26 (28.57) |
| 37.3–38 | 26 (28.57) |
| 38.1–39 | 34 (37.36) |
| 39.1–41 | 3 (3.3) |
| >41.0 | 0 |
| Unknown | 2 (2.2) |
| Cough | 55 (60.44) |
| Fatigue | 40 (43.96) |
| Expectoration | 30 (32.97) |
| Anorexia | 23 (25.27) |
| Diarrhoea | 21 (23.08) |
| Chest distress | 17 (18.68) |
| Nausea | 11 (12.09) |
| Shortness of breath | 10 (10.99) |
| Dyspnoea | 3 (3.3) |
| Headache | 7 (7.69) |
| Vomiting | 6 (6.59) |
| Myalgia | 5 (5.49) |
| Back discomfort | 3 (3.3) |
| Admission to intensive care unit | 9 (9.89) |
| CT of chest | |
| CT scan | 91 |
| Lesions in bilateral lungs | 61 (67.03) |
| Lesions in unilateral lung | 25 (27.47) |
| No lesions in bilateral lungs | 5 (5.49) |
| Diagnose methods | |
| Laboratory-confirmed (real-time RT-PCR) | 88 (96.7) |
| Clinical-confirmed | 3 (3.3) |
| Incubation periods | 6 (3–8) |
| Days from visiting doctor to be confirmed | 1 (1–2) |
| Clinical outcome | |
| Remained in hospital | 60 (65.93) |
| Discharged | 31 (34.07) |
| Died | 0 |
Values are expressed as n (%) or medians (interquartile ranges) unless stated otherwise.
All 91 patients, including the 5-year-old child, underwent chest CT scans. According to their CT images, 61 (67.03%) patients demonstrated bilateral pneumonia, and 25 (27.47%) patients demonstrated unilateral pneumonia. Patchy ground-glass shadows in the lungs of these patients could be observed in the scans.
According to data of laboratory tests, half of the patients (49, 53.85%) demonstrated elevated levels of C-reactive protein, but elevated levels of procalcitonin were detected in only a minority (14, 15.39%). Data from blood routine showed that 14 (15.39%) patients had below the normal range of leucocyte and 3 (3.97%) cases with elevated levels (Table 3). Twenty-eight (30.77%) cases had lymphopaenia (<1.0 109 cells/l), while 60 (65.93%) cases were in the normal range of level. Platelet was suppressed in 10 (10.99%) cases and elevated in three cases (3.30%). Suppressed level of thrombocytocrit, elevated levels of fibrinogen and D-dimer were documented in 39 (42.86%), 22 (24.18%) and 22 (24.18%) cases, respectively.
Table 3.
Laboratory findings of COVID-19 patients on admission to hospital
| Laboratory findings | Patients (n = 91) |
|---|---|
| Blood routine | Medians (interquartile ranges), n (%) |
| Leucocytes (×10^9/l; normal range 3.5–9.5) | 4.99 (4.25–5.69) |
| Increased | 3 (32.97) |
| Decreased | 14 (15.39) |
| Neutrophils (×10^9/l; normal range 1.8–6.3) | 2.91 (2.3–3.58) |
| Increased | 3 (3.30) |
| Decreased | 10 (10.99) |
| Lymphocytes (×10^9/l; normal range 1.1–3.2) | 1.35 (0.98–1.66) |
| Increased | 3 (3.30) |
| Decreased | 28 (30.77) |
| Mononuclear leucocytes (×10^9/l; normal range 0.1–0.6) | 0.4 (0.3–0.58) |
| Increased | 18 (19.78) |
| Decreased | 0 |
| Eosinophils (×10^9/l; normal range 0.02–0.52) | 0.01 (0.01–0.05) |
| Increased | 0 |
| Decreased | 47 (51.65) |
| Basophil (×10^9/l; normal range 0.0–0.06) | 0.01 (0.01–0.02) |
| Increased | 1 (1.10) |
| Decreased | 0 |
| Red blood cell (×10^12/l; normal range 3.8–5.1) | 4.49 (4.11–4.81) |
| Increased | 0 |
| Decreased | 10 (10.99) |
| Haemoglobin (g/l; normal range 115–150) | 135 (125–145) |
| Increased | 1 (10.99) |
| Decreased | 33 (36.26) |
| Platelets (×10^9/l; normal range 125–350) | 196 (142–238) |
| Increased | 3 (3.30) |
| Decreased | 10 (10.99) |
| Thrombocytocrit (%, normal range 0.19–0.36) | 0.2 (0.15–0.24) |
| Increased | 4 (4.40) |
| Decreased | 39 (42.86) |
| Fibrinogen (g/l; normal range 2–4) | 3.4 (2.7–4.04) |
| Increased | 22 (24.18) |
| Decreased | 3 (3.30) |
| D-dimer (ng/ml; 0–243) | 300 (106–450) |
| Increased | 22 (24.18) |
| Decreased | 0 |
| Blood chemistry | |
| Alanine aminotransferase (U/l; 9–50) | 18 (13–28) |
| Increased | 7 (7.69) |
| Decreased | 6 (6.59) |
| Aspartate aminotransferase (U/L; 15–40) | 21 (17–28) |
| Increased | 9 (9.89) |
| Decreased | 9 (9.89) |
| Albumin (g/l; 40–55) | 40 (37.85–42) |
| Increased | 0 |
| Decreased | 43 (47.25) |
| Urea (mmol/L; 3.1–8.0) | 3.95 (3.3–4.49) |
| Increased | 1 (1.10) |
| Decreased | 19 (20.88) |
| Serum creatinine (umol/l; 57–97) | 66 (57.75–77) |
| Increased | 3 (3.30) |
| Decreased | 21 (23.08) |
| K+ (mmol/l, 3.5–5.3) | 4.04 (3.72–4.23) |
| Increased | 0 |
| Decreased | 7 (7.69) |
| Na+ (mmol; 137–147) | 139 (137.25–141) |
| Increased | 0 |
| Decreased | 16 (17.58) |
| Cl− (mmol/l; 99–110) | 103 (100.55–105) |
| Increased | 1 (1.10) |
| Decreased | 8 (8.79) |
| Ca2+ (2.11–2.52) | 2.16 (2.06–2.25) |
| Increased | 1 (1.10) |
| Decreased | 11 (12.09) |
| Aterial blood gas | |
| PaO2 (mmHg; 83–108) | 75.6 (53.5–90.38) |
| Increased | 6 (17.65) |
| Decreased | 18 (52.94) |
| PaCO2 (mmHg; 35–48) | 36.5 (28–41) |
| Increased | 1 (2.94) |
| Decreased | 2 (5.88) |
| Lactic acid (mmol/l; <1.6) | 1.4 (1.1–1.93) |
| Increased | 19 (55.88) |
| Decreased | 0 |
| PH (7.35–7.45) | 7.44 (7.40–7.46) |
| Increased | 10 (29.41) |
| Decreased | 0 |
| Infection-related biomarkers | |
| C-reactive protein (mg/l; 0–5) | 6.81 (1.87–15.30) |
| Increased | 49 (53.85) |
| Decreased | 0 |
| Procalcitonin (ng/ml; <0.04) | 0.02 (0–0.04) |
| Increased | 14 (15.39) |
| Decreased | 0 |
Values are expressed as medians (interquartile ranges) or n (%), where N is the number of patients. COVID-19 = 2019 novel coronavirus diseases. Data of Arterial Blood Gas were available in 34 cases.
The serum level of albumin was suppressed in 43 (47.25%) patients. ALT (7, 7.69%) and AST (9, 9.89%) were slightly elevated in the minority of cases. Suppressed calcium concentration and serum sodium concentration were less common. Some patients had an abnormality in arterial blood gas analysis, with decreased PaO2 in 18 cases, increased levels of lactic acid in 25 cases and increased PH in 14 cases.
Patients were classified by the severity of their symptoms at admission according to the national guidelines.13,14 Nine patients were diagnosed as severe pneumonia because of the development of pneumonia. Compared with those mild patients, patients diagnosed as severe pneumonia had numerous differences in laboratory results (Table 4), including higher neutrophil, lower lymphocytes count, hyponatraemia, hypocalcaemia, as well as higher levels of C-reactive protein. MuLBSTA score is used to predict mortality in viral pneumonia, amongst the nine severe pneumonia patients their MuLBSTA scores were significantly higher, indicating they had higher mortality risks.
Table 4.
Questionnaires and laboratory results compared severe and mild patients with COVID-19
| Severe (N = 9) | Mild (N = 82) | P value | |
|---|---|---|---|
| Age (years) | 66 (54–80) | 49 (35.3–56) | 0.000167 |
| MuLBSTA | 13 (11–13) | 5 (1.5–7) | <0.0001 |
| Blood routine | |||
| Leucocytes | 5.23 (4.74–6.8) | 4.97 (4.02–5.65) | 0.01078 |
| Neutrophils | 3.32 (3–5.82) | 2.8 (2.18–3.49) | 0.00041 |
| Lymphocytes | 0.9 (0.7–1.3) | 1.4 (1.05–1.75) | 0.02702 |
| Mononuclear leucocyte | 0.35 (0.3–0.7) | 0.4 (0.3–0.59) | 0.18341 |
| Eosinophils | 0.01 (0–0.01) | 0.02 (0.01–0.06) | 0.14742 |
| Basophil | 0 (0–0.01) | 0.01 (0.01–0.02) | 0.01869 |
| Red blood cell | 4.26 (3.8–4.82) | 4.49 (4.17–4.81) | 0.46518 |
| Haemoglobin | 130 (118–142) | 135 (126–147) | 0.27599 |
| Platelets | 152 (127–208) | 198 (144–248) | 0.51192 |
| Thrombocytocrit | 0.16 (0.13–0.21) | 0.21 (0.16–0.25) | 0.32815 |
| Fibrinogen | 3.8 (3.71–4.15) | 3.36 (2.64–4.08) | 0.18738 |
| D-dimer | 450 (160–485) | 300 (106–400) | 0.59192 |
| Blood chemistry | |||
| Alanine aminotransferase | 19.9 (14–26) | 18 (13–29) | 0.75424 |
| Aspartate aminotransferase | 27 (23.75–27) | 21 (17–29) | 0.89006 |
| Albumin | 38.55 (36.33–39.25) | 40.2 (38–42.4) | 0.13321 |
| Urea | 5.19 (4.66–6.14) | 3.83 (3.25–4.4) | <0.0001 |
| Serum creatinine | 81.5 (70.75–90.5) | 66 (57–76) | 0.03718 |
| K± | 3.82 (3.76–3.89) | 4.09 (3.72–4.27) | 0.35867 |
| Na± | 137.85 (135.3–139.38) | 139.6 (137.6–142) | 0.02714 |
| Cl− | 101.4 (99.28–104.5) | 103.4 (101.28–105.23) | 0.31589 |
| Ca2± | 2.01 (1.86–2.01) | 2.17 (2.07–2.25) | 0.00566 |
| Infection-related biomarkers | |||
| C-reactive protein | 30.63 (12.5–103.4) | 5.98 (1.4–11.3) | <0.0001 |
| Procalcitonin | 0 (0) | 0.03 (0–0.04) | 0.00358 |
Values are expressed as medians (interquartile ranges) or n (%), where N is the number of patients with available data. P values comparing severe and mild pneumonia patients are from χ2, Fisher’s exact test or Mann–Whitney U-test. COVID-19 = 2019 novel coronavirus diseases.
Discussion
This report, to our knowledge, is the largest case study to date of hospitalized patients with COVID-19 in Zhejiang province, which is out with of Wuhan and Hubei. As of 16 February 2020, of the 91 cases in the present study, 9 were diagnosed as severe pneumonia, 31 were discharged, 60 remain hospitalized and no patient died. Most of the infected cases were female. Thirty-one (34.07%) patients had been to Wuhan/Hubei, while 48 (52.75%) patients had not been Wuhan/Hubei, and 11 (12.09%) cases were confirmed aircraft transmission. The most common symptoms at the onset of COVID-19 were fever, cough, and fatigue. The median of incubation period was 6 (IQR 3–8) days and from the first visit to a doctor to confirmed diagnosis was only 1 (1–2) days.
Our study provided further evidence that rapid human-to-human transmission of SARS-CoV-2 outside Wuhan had occurred. None of the patient in this study had direct contact with wildlife or from Huanan Seafood Wholesale Market. While 8.79% had contact with people who had travelled from Wuhan, more than 52.75% were local Zhejiang residents with no travel history to Wuhan and Hubei. In particular, 23 cases of 38 cases of Ningbo Cohort were related to the outbreak of a temple cluster, including 11 patients had directly participated in a temple activity. Our findings have provided strong evidence that large social events should be cancelled in order to prevent infection.
Notably, there is 54 (59.34%) female cases in our study compared to 27% of the first reported study8 or 41.8% of a recent study,11 and 44% of a recently study from Zhejiang Province.16 However, it has been found that more males were infected by Middle East respiratory syndrome (MERS)-CoV and SARS-CoV.17,18 Whether it is related to endocrinology,9 social activities, or religious activities still needs further research.
Fever (71.43%), cough (60.44%) and fatigue (43.96%) are the most comment clinical characteristics in our study and is similar to the cohorts reported in the published literature.8,10,16 Furthermore, it is reported only 43.8% of COVID-19 had fever onset and 87.9% reported having had a fever during hospitalization.11 Fever is less widespread amongst those infected with COVID-19 than those with SARS-CoV (99%) and MERS-CoV (98%).19 As screening heavily on fever detection, patients that do not have fever might get suspected as being wrongly diagnosed.
Some COVID-19 cases had atypical symptoms or were asymptomatic. Furthermore, asymptomatic persons are potential sources of SARS-CoV-2 transmission.20 It appears that transmission is possible during the incubation period, and the carrier cannot be spotted.20 Recently, a study reported that they detected SARS-CoV-2 in stool samples from patients with abdominal symptoms.21 Interestingly, we had detected SARS-CoV-2 in a rectal swab of patients who were twice tested negative by throat swab specimens using RT-PCR. Therefore, it is helpful to detect atypical symptoms COVID-19 using both throat swab and rectal swab, particularly for patients who suffer symptoms with their digestive tract, like diarrhea.
Only 28 (30.77%) cases had lymphopaenia in our study, while half the patients (49, 53.85%) demonstrated elevated levels of C-reactive protein, decreased platelet in 10 cases. Most cases had normal serum levels of procalcitonin on admission (procalcitonin <0.04 ng/ml), except 14 cases had a procalcitonin level higher than 0.04 ng/ml. All patients were scanned by chest CT scan. Of the 91 patients, 61 (67.03%) had multilobe infiltration. The typical manifestation of lung CT was ground-glass opacification of bilateral multiple lobular and subsegmental areas of consolidation (Figures 1 and 2). Our study provided three cases as clinically confirmed COVID-19 pneumonia because of their epidemiological history, signs, symptoms and chest CT evidence according to guidance, though they tested negative for the SARS-CoV-2. Thus, CT could be used as an important differential method in cities where they are lacking nucleic acid kits, like Wuhan, and help to bring forward the isolation period for the infected.
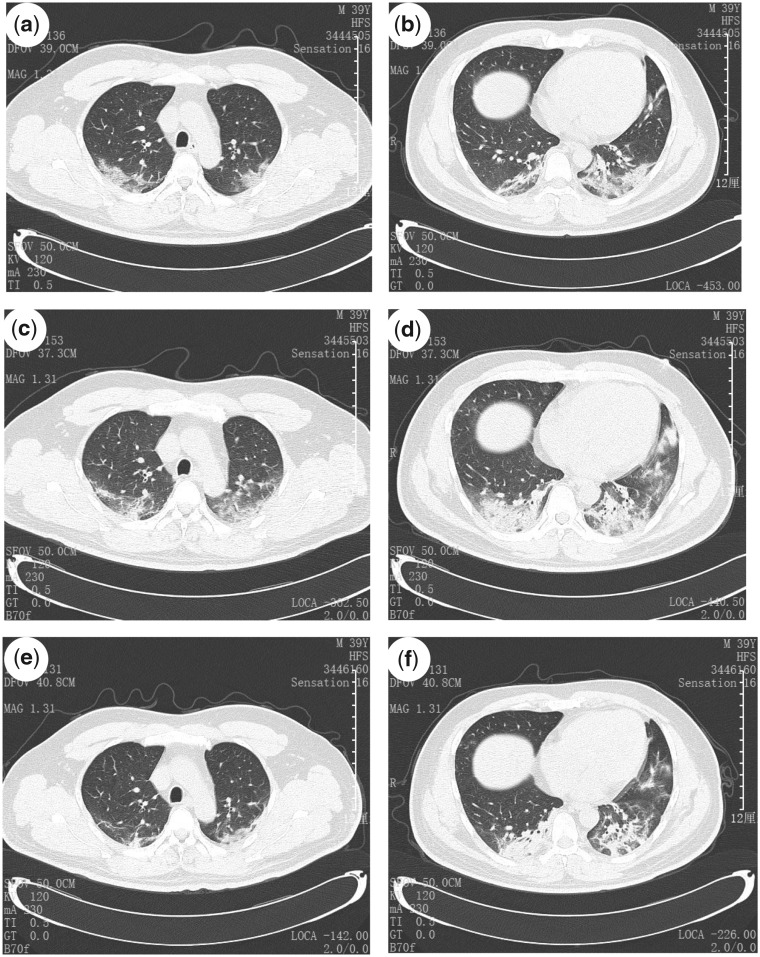
Figure 1.
Transverse chest CT from a 39-year-old man, showing ground-glass opacities of bilateral lungs near the pleura (a and b) on Day 1 from symptom onset, and increased bilateral ground-glass opacities and consolidation (c and d) on Day 5 from symptom onset, and slightly absorbed ground-glass opacities (e and f) on Day 7 from symptom onset after 7-day treatment.
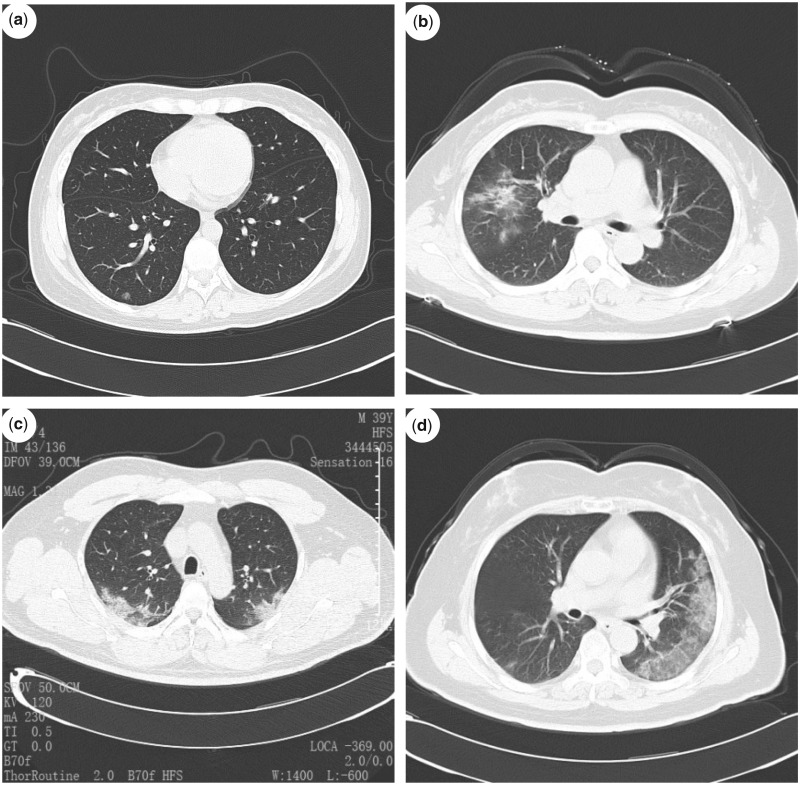
Figure 2.
(a) Normal chest CT scan from a 37-year-old man. (b) Chest CT scan from a 57-year-old woman, showing scattered opacities in middle right lobe. (c) Chest CT scan from a 39-year-old man, showing bilateral ground-glass opacities. (d) Chest CT scan from a 64-year-old woman, showing typical left pleural ground-glass opacities.
Currently, no anti-viral agents have been proven to be an effective treatment for COVID-19. It is reported that a combination of Lopinavir and Ritonavir had been applied to SARS-CoV-2 patients with substantial clinical benefit.22 As an emerging virus, all the patients received anti-viral agents in Ningbo cohort, including Kaletra (Lopinavir/Ritonavir) and Umifenovir, nine cases received methylprednisolone to treat high fever, SpO293% and hypoxaemia. The dose of methylprednisolone depends on disease severity between 1 and 2 mg/kg. However, the outcome is still unclear.
As of 16 February 2020, no death has been reported in Zhejiang province as the government authority has taken unprecedented and effective effort to reduce the risk of transmission. Early diagnosis, early isolation and early management all contributed to reducing transmission and mortality in Zhejiang. However, 60 patients are still hospitalization and their cases should be followed up in the future.
Our study has some limitations. Firstly, two-thirds of patients are still hospitalized. For those who had been released their cases are being followed up for 2 weeks, and further information can be learnt from these individuals. Secondly, we just have recruited 91 cases. Increasing the number of cases is good for observing COVID-19 outside of Wuhan/Hubei. Thirdly, we did not have many severe cases to compare the differences in epidemiological and clinical features.
Funding
This work was supported by Natural Science Foundation of Zhejiang Province (Q17H010001), Natural Science Foundation of Ningbo (2017A610246) and Shaoxing Municipal Science and Technology Plan Project.
Conflict of interest. None declared.
References
- 1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paules CI, Marston HD, Fauci AS.. Coronavirus infections—more than just the common cold. JAMA 2020; 323:707. [DOI] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Coronavirus Disease 2019 (COVID-19) Outbreak. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (18 February 2020, date last accessed).
- 6. Pongpirul WA, Pongpirul K, Ratnarathon AC, Prasithsirikul W.. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med 2020; 382:1067–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Center for Disease Control and Prevention. COVID-19 Pneumonia Epidemic Map. 2020. http://2019ncov.chinacdc.cn/2019-nCoV/index.html (18 January 2020, date last accessed). [DOI] [PMC free article] [PubMed]
- 8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. Medrxiv2020. https://www.medrxiv.org/content/10.1101/2020.02.06.20020974v1 (18 January 2020, date last accessed).
- 12. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Commission of the People’s Republic of China. The Notice of Launching Guideline on Diagnosis and Treatment of the Novel Coronavirus Pneumonia, 4th edn.http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67.shtml (27 January 2020, date last accessed). [Google Scholar]
- 14.National Health Commission of the People’s Republic of China. The Notice of Launching Guideline on Diagnosis and Treatment of the Novel Coronavirus Pneumonia, 5th edn.http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml (5 February 2020, date last accessed). [Google Scholar]
- 15. Guo L, Wei D, Wu Y, Zhou M, Zhang X, Li Q, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol 2019; 10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu X, Wu X, Jiang X, Xu K, Ying L, Ma C, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badawi A, Ryoo SG.. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016; 49:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S.. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 2017; 198:4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zumla A, Hui DS, Perlman S.. Middle East respiratory syndrome. Lancet 2015; 386:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv 2020. doi:10.1101/2020.01.30.927806.
- 22. Chu C, Cheng V, Hung I, Wong M, Chan K, Chan K, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]