Abstract
Since December 2019, a new coronavirus, named SARS-CoV-2, has spread globally, affecting >200 000 people worldwide with the so-called COVID-19 disease. The scientific community is actively and constantly working to identify the mechanisms involved in the diffusion of this virus and the pathogenesis of the infection, with its most frequent and severe complication, namely interstitial pneumonia. To date, SARS-CoV-2 is known to enter the host cells via the angiotensin-converting enzyme 2 protein. For this reason, the hypothesis that drugs capable of increasing the expression of this protein may have a role in the spread of the virus and in the symptomatology of affected patients has taken hold. The purpose of this Editorial is to briefly show the evidence currently available in this regard and to provide ideas for future research.
Keywords: SARS-CoV-2, Hypertension, RAS system
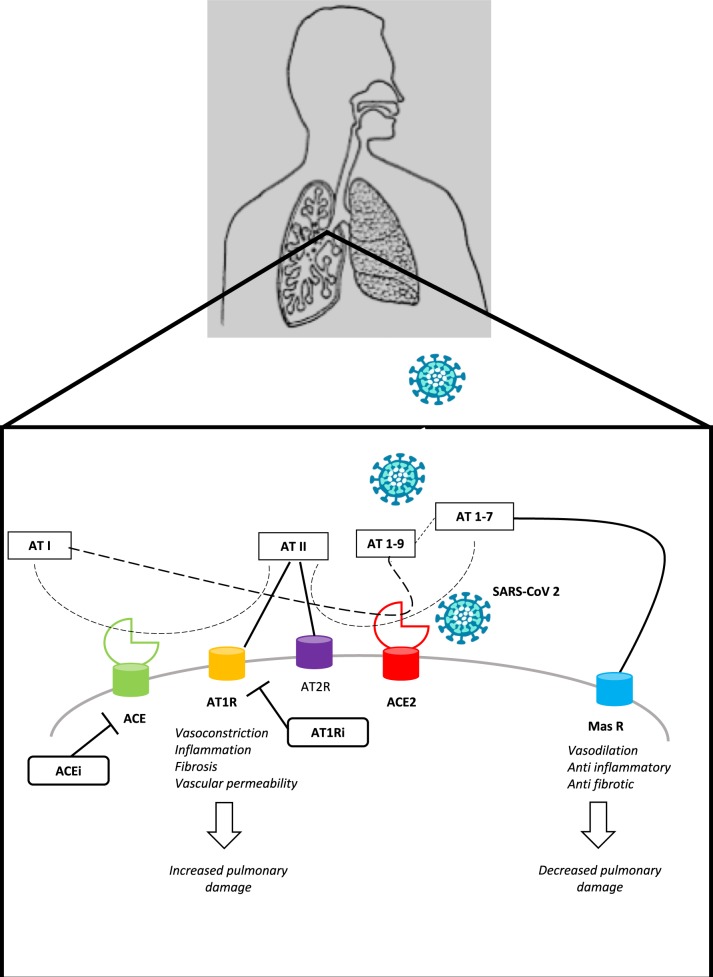
Since December 2019, starting from an initial outbreak in the Wuhan region in China, a new virus of the coronavirus family (CoVs) has spread rapidly throughout the world, being classified as ‘pandemic’ on 11 March 2020. The burden in terms of deaths of this new virus, named SARS-CoV-2, has exceeded that of the previous severe acute respiratory syndrome (SARS) epidemic during 2002. Today, >1 million people are infected worldwide, known as coronavirus disease 2019 (COVID-19) cases, most of which occur in the age group 30–80 years, with mild to moderate flu-like manifestations such as fever and cough. However, a variable percentage of people, ∼20% in China, have developed severe forms of interstitial pneumonia, up to critical conditions, with a mortality rate of >2%.1 SARS-CoV-2, which is an RNA virus, has ∼80% homology with the previous SARS-CoV.2 This evidence led to the generation of the hypothesis that the SARS-CoV-2 was able, like SARS-CoV, to use the angiotensin-converting enzyme 2 (ACE2) protein as a cell receptor through which enter cells. This has now been confirmed in different species.3 Moreover, it has been shown that the SARS-CoV-2 structure includes a distinct loop with flexible glycyl residues, leading to a receptor-binding domain with a higher affinity for ACE2 compared with SARS-CoV.4 The angiotensin-converting enzyme (ACE) and its close homologue ACE2 belong to the ACE family of dipeptidyl carboxydipeptidases, but have two opposing physiological functions. ACE cleaves angiotensin I to generate angiotensin II, which binds to the angiotensin II type 1 receptor (AT1R) to constrict blood vessels, thereby increasing blood pressure. In contrast, ACE2 inactivates angiotensin II, generates angiotensin (1-7), a heptapeptide having a potent vasodilator function via the Mas receptor,5 and might also generate angiotensin (1-9) from angiotensin I, which is then processed to become angiotensin (1-7). ACE2 is a type I membrane protein expressed in the lungs, liver, testes, heart, kidneys, and intestine.6 Previous data support an increased ACE2 expression, especially in the myocardium, in several cardiovascular diseases (CVDs) such as hypertension and heart failure (HF), as a counter-regulatory, protective mechanism.7 Similarly, preclinical evidence exists that the AT1R blockers (ARBs), commonly used as anti-hypertensive drugs, might increase ACE2 expression, especially in the heart and kidney, during both acute and chronic treatment for CVDs such as hypertension, myocardial infarction, and HF.8–10 On the contrary, evidence about the up-regulation of ACE2 expression by ACE inhibitors (ACEIs) is more conflicting.11 Mineralocorticoid receptor antagonists have also been shown to increase ACE2 levels.12 Taken together, available observations suggest that chronic AT1R blockade results in ACE2 up-regulation, even though specific data on the lungs, which would be relevant to SARS-CoV-2 infection, are lacking and the correlation between measured soluble ACE2 and membrane-bound ACE2 is not known.13
Recently, the critical importance of the renin–angiotensin system (RAS) in the pathogenesis of acute lung disease [i.e. acute respiratory distress syndrome (ARDS)] has emerged. In summary, in acute lung injury, ACE, angiotensin II, and AT1R function as lung injury-promoting factors, whereas ACE2 protects against lung injury.14 Therefore, it has been suggested that ARBs might be beneficial in acute lung injury. In this regard, previous observations on SARS-CoV might be relevant to the COVID-19 disease, which is often complicated by acute lung failure. Indeed, it has been demonstrated that the coronavirus spike protein binds to ACE2, leading to ACE2 down-regulation, which in turn results in excessive production of angiotensin II by ACE, while less ACE2 can convert it into angiotensin (1-7). This, in turn, contributes to lung injury, as angiotensin-stimulated AT1Rs lead to vasoconstriction of lung vessels, increased pulmonary vascular permeability, inflammation, and interstitial fibrosis, thereby increasing lung damage.15,16 Therefore, the higher ACE2 expression due to chronically medicating SARS-CoV-2-infected patients with ARB may protect them against acute lung injury by blocking the deleterious effect of angiotensin II, as well as by decreasing the production of angiotensin II by up-regulating ACE2, which in turn increases the production of angiotensin (1-7). This latter may mediate a beneficial effect on both lungs and heart.17 Accordingly, ACE2 has also been shown to protect against acute lung injury in several animal models of ARDS15,16 (Figure 1). Indeed, the loss of ACE2 expression in mutant mice resulted in enhanced vascular permeability, increased lung oedema, neutrophil accumulation, and worsened lung function.15 Similarly, captopril has been found to reduce lung damage by lipopolysaccharides due to an increase in ACE2 activity.18 Therefore, it has been suggested that ARBs/ACEIs might be beneficial for patients with COVID-19 suffering from pneumonia.19,20 Moreover, in the past SARS epidemic, previous findings supported the opportunity to develop ACE2 as a novel drug for ARDS in SARS, even though these similar drugs have not been released so far.14
Figure 1.
The Role of Renin Angiotensin System in lung damage. ACE, angiotensin converting enzyme; ACE2, angiotensin converting enzyme type 2; ACEi, ACE inhibitors; AT I, angiotensin I; AT II, angiotensin II; AT 1-9, angiotensin 1-9; AT 1-7, angiotensin 1-7; AT1R, angiotensin type I receptor; AT2R, angiotensin type 2 receptor; ARB, angiotensin type I receptor inhibitors.
Case series currently available have shown a variable, but relevant, percentage of people affected by hypertension (∼15–30%), diabetes, and cardiac disease among patients admitted to hospital for COVID-19 disease, and these subsets of subjects tend also to develop more frequently severe illness resulting in death.21–28 Nonetheless, it must be stated that most studies so far only enrolled small samples, without adjusting for confounding factors.24–28 Therefore, the increase in prevalence of hypertension among patients with the worst outcomes may reflect their increasing age, which has a major prognostic influence. In contrast, recent data from the Istituto Superiore di Sanità in Italy reported that the most common concurrent comorbidities observed in COVID-19 patients were hypertension (73.8%), diabetes (33.9%), ischaemic heart disease (30.1%), and atrial fibrillation (22%).29 The ongoing survey from the Centers for Disease Control and Prevention in the USA reported among 74 439 cases 10% of cases of diabetes and 9% of cases of CVDs.30
When looking at specific data on ACEI/ARB usage in retrospective studies, there is no solid evidence supporting worse outcomes.31 Despite this, a separate analysis for ACEIs and ARBs in studies with larger samples providing the necessary adjustment for comorbidities would be of great relevance. Indeed, it should be emphasized that any comorbidity of COVID-19 patients which has an indication for the use of an ACEI/ARB might also have heavily influenced their mortality and morbidity rate. Therefore, the dissection of the specific prognostic burden of each comorbidity and associated therapies may be quite hard to achieve.11 Therefore, from what we know so far, the discontinuation of an effective antihypertensive therapy with an ACEI or ARB to possibly avoid the entrance of SARS-CoV-2, as suggested by some,32 is not supported by evidence. Similar considerations have recently also been shared by authoritative international societies.33–36 Moreover, even though ACEIs and ARBs might increase ACE2 levels in the lungs, this might not be relevant to SARS-CoV-2 infection. Indeed, the intestinal epithelium has a high expression of ACE2; nonetheless, gastrointestinal symptoms are much less frequent in patients affected by COVID-19.37
Future studies should aim (i) to understand whether CVDs might influence per se the outcome of patients affected by COVID-19 disease; (ii) to determine the influence of the RAS system and active treatments on SARS-CoV-2 infection and on the development of COVID-19 disease (given the fact that in many CVDs increased levels of ACE2 play a protective role, so that its down-regulation by SARS-CoV-2 would be highly detrimental per se in this critical subset of patients due to an increase in angiotensin II); and (iii) to evaluate possible therapies for COVID-19 disease involving components of the RAS. To achieve the first goal, well-designed large case series studies and registries, which are already enrolling all over the world, would help.38–43 For the second aim, not only case series, but also autoptic exams should investigate the expression and activation of RAS components in different organs during COVID-19 infection in patients treated or not with an ACEI/ARB, also investigating ACE2 polymorphisms which could impact the affinity for the spike protein of SARS-CoV-2. Knock-in or knock-out models for RAS molecules could also be useful to understand how COVID-19 might develop interactions with the RAS. Moreover, randomized controlled trials should be initiated to show whether the modulation of RAS inhibition (starting, stopping, or continuing) would lead to better or worse outcomes in COVID-19. Lastly, experimental studies might show possible effective RAS-related therapies. In this regard, there are three active, but not yet recruiting trials, investigating the role of angiotensin (1-7) infusion, ACEIs, and losartan in patients affected by COVID-19.44–46. One randomized trial is already recruiting to assess whether a shift to a non-ARB/ACEI regimen would be beneficial or detrimental for hypertensive patients affected by COVID-19.47
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal – Cardiovascular Pharmacotherapy or of the European Society of Cardiology.
References
- 1. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W.. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Guo Y, Pan Y, Zhao ZJ.. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020;doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santos RA, Simoes E, Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T.. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 2003;100:8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S.. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1–E9. [DOI] [PubMed] [Google Scholar]
- 7. Abassi ZA, Assady S, Khoury EE, Heyman SN.. Angiotensin converting enzyme 2—an ally or a Trojan horse? Implications to SARS-CoV-2-related cardiovascular complications. Am J Physiol Heart Circ Physiol 2020;doi: 10.1152/ajpheart.00215.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM.. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004;43:970–976 . [DOI] [PubMed] [Google Scholar]
- 9. Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, Krenek P, Ochodnicky P.. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med 2015;19:1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T.. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015;28:15–21. [DOI] [PubMed] [Google Scholar]
- 11. Danser AHJ, Epstein M, Batlle D.. Renin–angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin–angiotensin system blockers. Hypertension 2020;doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karram T, Abbasi A, Keidar S, Golomb E, Hochberg I, Winaver J, Hoffman A, Abassi Z.. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol 2005;289:H1351–H1358. [DOI] [PubMed] [Google Scholar]
- 13. Patel AB, Verma A.. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 2020;doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 14. Kuba K, Imai Y, Penninger JM.. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol 2006;6:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM.. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM.. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole-Jeffrey CT, Liu M, Katovich MJ.. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol 2015;66:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Zeng Z, Li Y, Huang W, Zhou M, Zhang X, Jiang W.. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock 2015;43:395–404. [DOI] [PubMed] [Google Scholar]
- 19. Sun ML, Yang JM, Sun YP, Su GH.. [Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi.2020;43:219–222. [DOI] [PubMed] [Google Scholar]
- 20. Phadke M, Saunik S.. Rapid response: use of angiotensin receptor blockers such as telmisartan, losartsan in nCoV Wuhan corona virus infections—novel mode of treatment. Response to the emerging novel coronavirus outbreak. BMJ 2020;368:m406.32005675 [Google Scholar]
- 21. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinese Center for Disease Control and Prevention, Beijing 102206, China. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol.2020;41:145–151. [Google Scholar]
- 24. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F., Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang YChen H9, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD.. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 28. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y.. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo.pdf. (23 March 2020).
- 30.CDC COVID-19 Response Team https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6913e2H.pdf?utm_campaign=Chris+Kresser&utm_source=hs_email&utm_medium=email&utm_content=85654317&_hsenc=p2ANqtz_FbMoDa8_9_WGnsKMcaUDcVH9FiIYvjGzRDeeyW2J257DvXklRv7jUwejC46YYFsy2Ozub03dIDiaOsC6Qhd_M2ILA&_hsmi=85654317. (4 April, 2020).
- 31. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G.. Renin–angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 2020;9:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. (4 April 2020).
- 34. https://siia.it/notizie-siia/farmaci-antiipertensivi-e-rischio-di-covid-19-il-comunicato-della-siia/ (4 April 2020).
- 35. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/ (4 April 2020).
- 36.https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19
- 37. Jakovac H. COVID-19—is the ACE2 just a foe? Am J Physiol Lung Cell Mol Physiol 2020;doi: 10.1152/ajplung.00119.2020. [DOI] [PMC free article] [PubMed]
- 38. https://capacity-covid.eu (4 April 2020).
- 39. https://dcvalliance.nl/news/item/capacity-covid-registry. (4 April 2020).
- 40.https://clinicaltrials.gov/ct2/show/NCT04318418
- 41.https://clinicaltrials.gov/ct2/show/NCT04318301
- 42.https://clinicaltrials.gov/ct2/show/NCT04331574
- 43.https://clinicaltrials.gov/ct2/show/NCT04324684
- 44.https://clinicaltrials.gov/ct2/show/NTC04332666
- 45.https://clinicaltrials.gov/ct2/show/NCT04312009
- 46.https://clinicaltrials.gov/ct2/show/NCT04322786
- 47.https://clinicaltrials.gov/ct2/show/NCT04330300