Abstract
Background
Lungs from patients with coronavirus disease 2019 (COVID-19) have shown typical signs of acute respiratory distress syndrome (ARDS), formation of hyaline membrane mainly composed of fibrin and ‘ground-glass’ opacity. Previously, we showed plasminogen itself is a key regulator in fibrin degradation, wound healing and infection.
Aim
We aimed to investigate whether plasminogen can improve lung lesions and hypoxemia of COVID-19.
Design
Thirteen clinically moderate, severe or critical COVID-19 patients were treated with atomization inhalation of freeze-dried plasminogen.
Methods
Levels of their lung lesions, oxygen saturation and heart rates were compared before and after treatment by computed tomography scanning images and patient monitor.
Results
After plasminogen inhalation, conditions of lung lesions in five clinically moderate patients have quickly improved, shown as the decreased range and density of ‘ground glass’ opacity. Improvements of oxygen saturation were observed in six clinically severe patients. In the two patients with critical conditions, the oxygen levels have significantly increased from 79–82% to 91% just about 1 h after the first inhalation. In 8 of 13 patients, the heart rates had slowed down. For the five clinically moderate patients, the difference is even statistically significant. Furthermore, a general relief of chest tightness was observed.
Conclusion
Whereas it is reported that plasminogen is dramatically increased in adults with ARDS, this study suggests that additional plasminogen may be effective and efficient in treating lung lesions and hypoxemia during COVID-19 infections. Although further studies are needed, this study highlights a possible hope of efficiently combating this rapid epidemic emergency.
Introduction
Since December 2019, a sudden outbreak of infectious pneumonia (coronavirus disease 2019 [COVID-19]) caused by novel coronavirus, SARS-CoV-2, occurred in Wuhan, Hubei Province, China and is quickly spreading national wide and also to other parts of the world.1 As of 4 March 2020, the virus has been found in 59 countries with 80 710 confirmed cases in China, including 3045 fatalities.2 Due to rapid transmission dynamics and multiple transmission ways, COVID-19 has caused panic in the world.
The clinical features of COVID-19 are very similar to that of severe acute respiratory syndrome-like coronavirus (SARS) outbreak in 2003.3,4 In the early phases of the disease, acute respiratory infection symptoms occur. Although in most cases the disease is acute and can be resolved by itself, in severe or critical cases, patients may suffer and even die from acute respiratory distress syndrome (ARDS), acute respiratory failure and other serious complications, mainly due to massive diffuse alveolar damage and early hyaline membrane formation.4,5
As a predominant and early pathological feature, formed hyaline membranes consist of fibrin matrix incorporating the whole spectrum of serum proteins and cellular debris,6–8 which forms a barrier to inhibit gas exchange and pulmonary surfactant resulting in ARDS. Moreover, in hyaline membrane disease of premature infants, fibrin deposition in alveoli and alveolar ducts is a crucial factor that may result in death.9,10
The plasminogen activator (PA) system is a broad-spectrum protease system where the active component, plasmin, is formed from its precursor, plasminogen and is important in degrading main components of the extracellular matrix including fibrin. Plasminogen is classically regarded as inert and excessive, and the regulation of the PA system is by the activators and inhibitors of the system.11 However, we have shown before that plasminogen itself is a key regulator in many pathological processes including fibrinolysis, wound healing and infection.12–15
Previous studies have demonstrated different results on whether plasminogen is sufficient in ARDS. Premature infants with hyaline membrane disease and ARDS have been reported to have low levels of circulating plasminogen and PA system,9,16–18 but increased levels of the PA inhibitor.16 Thus, intravenous supplementation of plasminogen was effective in lowering the occurrence of premature infant ARDS and death.9,17,18 However, other studies later on showed that in the bronchoalveolar lavage of the normal adult patients with ARDS, dramatically increased and sufficient amounts of plasminogen, reduced levels of urokinase-type PA and markedly enhanced levels of PA inhibitor-1 and α2-antiplasmin were found.19,20
Hence, although hyaline membrane disease in premature infants and COVID-19 pneumonia mainly in normal adults have completely distinct aetiologies, inspired by our previous studies, we hypothesize that additional plasminogen supplementation to these COVID-19 patients may still benefit them. As the first attempt, in this study, we investigated the therapeutic effects of plasminogen by atomization inhalation on COVID-19. The results suggest that using plasminogen by atomization inhalation is an effective and efficient way to combat this rapid epidemic emergency.
Materials and methods
Patient involvement
The 13 study subjects were 30–78 years old females and males diagnosed as COVID-19 with clinically moderate, severe or critical clinical severities, in accordance with the National Health Commission guidelines.21 The clinical study was performed during 10th to 20th of February 2020, as a joint research between Talengen Institute of Life Sciences and Beijing Chang’an Chinese and Western Integrated Medicine Hospital and executed at Xiaogan Hospital and Suixian Hongshan Hospital. Ethics Committee of Beijing Chang’an Chinese and Western Integrated Medicine Hospital approved the study and the study was performed according to the Declaration of Helsinki. Subjects gave their informed consent before included in the study. Written informed consent was waived by the Ethics Committee for emerging infectious diseases.
Administration of plasminogen
Sterile, freeze-dried plasminogen of 5 mg per vial from human plasma fraction III was produced at facilities with GMP compliance and provided by Talengen Institute of Life Sciences. The safety of the virus inactivation process was confirmed by an independent third party testing organization. Atomization inhalation of 10 mg plasminogen dissolved in 2-ml sterile water was given twice daily for severe and critical COVID-19 subjects, and once daily for moderate COVID-19 subjects. Treatment profile is shown in Table 1.
Table 1.
Treatment profiles of plasminogen for COVID-19 patients
| Patient ID | Gender | Age (years old) | Severity of disease | Inhalation times of plasminogen |
|---|---|---|---|---|
| 1 | Male | 58 | Moderate | 2 |
| 2 | Male | 30 | Moderate | 2 |
| 3 | Female | 49 | Moderate | 2 |
| 4 | female | 48 | Moderate | 3 |
| 5 | Male | 48 | Moderate | 2 |
| 6 | Male | 46 | Severe | 6 |
| 7 | Male | 78 | Severe | 6 |
| 8 | Male | 47 | Severe | 2 |
| 9 | Male | 65 | Severe | 2 |
| 10 | Female | 56 | Severe | 4 |
| 11 | Male | 78 | Severe | 1 |
| 12 | Male | 48 | Critical | 5 |
| 13 | Male | 47 | Critical | 2 |
Computed tomography scan
High-resolution computed tomography (CT) scan (NeuViz 16 Classic, Neusoft, China) of the chest was taken at Suixian Hongshan Hospital a few days before the first and after the last administration of plasminogen for the five moderate COVID-19 subjects.
Oxygen saturation and heart rate examination
Real-time oxygen saturation and heart rates were monitored by patient monitor (iPM5, Mindary, China) and the values were documented at 1 h before and few hours after atomization inhalation of plasminogen.
Statistical analysis
Statistical analysis was performed on SPSS 16.0 (IBM Corporation). Heart rate of the same severity group before and after administration of plasminogen was analyzed with two-tailed t-test, and *P < 0.05 was considered statistically significant.
Results
Plasminogen improves lung lesions of clinically moderate COVID-19 patients
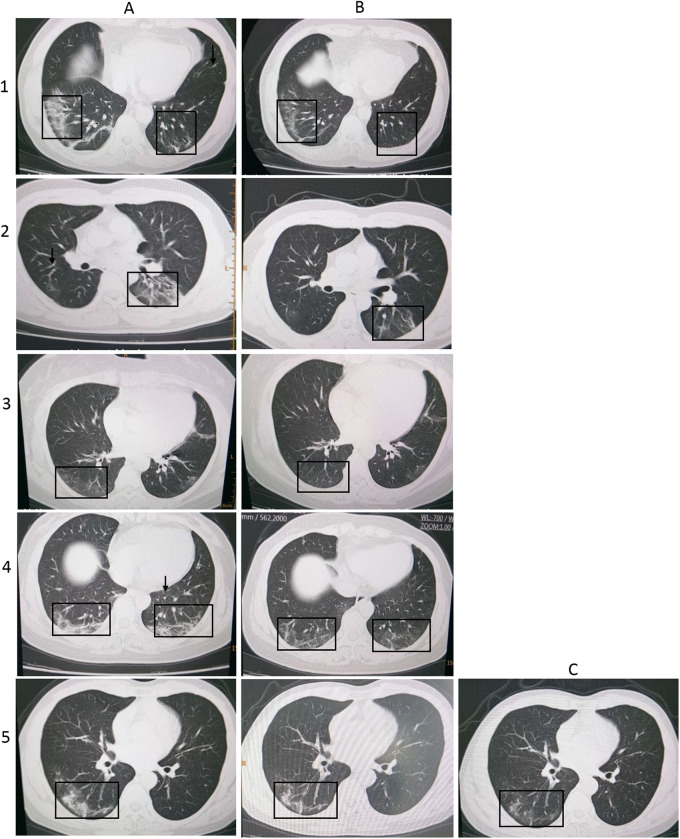
To study the effects of plasminogen on lung lesions, high-resolution CT scanning of chest was performed on COVID-19 patients. Before administration of plasminogen, the chest CT images of five moderate COVID-19 patients showed multiple patchy/punctate ‘ground-glass’ opacities in bilateral lungs with unclear margins and heterogeneous density, and also scattered punctate density in mediastinum (Figure 1 and Table 2). Despite the five patients received the daily common supportive treatments with antibiotics and traditional Chinese medicine, the density and range of ‘ground glass’ opacities in lung increased over time, suggesting a rapid deleterious progress of the disease (CT scanning examination record, not shown here). Surprisingly, just after two to three times of plasminogen inhalation, the CT results showed that the numbers, the range and the density of lung lesion-focal in all of the five patients treated have diminished or even partially disappeared. Patchy or punctate ‘ground glass’ opacities have significantly decreased or absorbed. This indicated that atomization inhalation of plasminogen rapidly improves lung lesions caused by COVID-19 infection.
Figure 1.
High-resolution CT images of moderate COVID-19 patient with individual IDs shown to the left. (A column) The chest CT images before plasminogen inhalation. (B column) The chest CT image of the corresponding patient after plasminogen inhalation. (C column) Additional chest CT image of Patient 5 taken on 5 days after B image was taken. The black arrows and boxes indicate the abnormalities.
Table 2.
High-resolution chest CT scanning record in moderate COVID-19 patients
| Patient ID | CT results before the first inhalation of plasminogen | CT results after the last inhalation of plasminogen |
|---|---|---|
| 1 | Multiple patchy/punctate ‘ground-glass’ opacities in bilateral lungs characterized with unclear margin and heterogeneous density, especially consolidation at lower lobe of right lung | Number and range of lesion focal decreased and partially disappeared |
| 2 | Multiple ‘ground-glass’ appearance in bilateral lungs characterized with unclear margin and heterogeneous density, left consolidation at lower lobe | ‘Ground-glass’ appearance in lower lobes of both lungs, flaky denser area in left lower lobe decreased and partially absorbed |
| 3 | Multiple patchy/punctate ‘ground-glass’ opacities in bilateral lungs characterized with unclear margin and heterogeneous density, mild consolidation at right lower lobe | ‘Cloud dense’ area in bilateral lungs, number and area of lesion-focal decreased and almost all absorbed |
| 4 | Multiple ‘ground-glass’ appearance in bilateral lungs characterized with unclear margin and heterogeneous density, consolidation at lower lobes of bilateral lungs | Smaller patchy/punctate ‘ground-glass’ opacities in lower lobes of both lung, mostly absorbed |
| 5 | Multiple ‘ground-glass’ appearance in bilateral lungs characterized with unclear margin and heterogeneous density, consolidation at right middle and lower lobes | Patchy/punctate ‘ground-glass’ opacities significantly decreased in right lower lobes |
Plasminogen improves oxygen saturation of clinically severe and critical COVID-19 patients
We further investigated the effects of plasminogen on oxygen saturation. Throughout the entire plasminogen administration period, the oxygen saturation levels were detected by patient monitors on clinically severe or critical COVID-19 patients with oxygen supplementation by a nasal cannula that delivered oxygen at constant concentrations of at least 80% or 100%, respectively. As shown in Table 3, although generally having good oxygen saturation levels, the oxygen saturation values of five out of six severe COVID-19 patients still increased 1–4% after plasminogen administration. Only in one patient, perhaps due to administration of plasminogen for just once, the oxygen saturation value decreased from 91% to 89%. In these two patients with critical conditions, the oxygen levels have significantly increased from 79–82% to 91% just about 1 h after the first inhalation and stabilized at around 91% thereafter (Detailed data not shown here). However, due to the critical conditions of these two patients, they were transferred to intensive care unit, so that the follow-up treatment of plasminogen was interrupted. Nevertheless, these data indicate that plasminogen generally improves the oxygen saturation levels of severe and critically severe COVID-19 patients, especially in these patients that have particularly low oxygen levels.
Table 3.
Oxygen saturation values of severe and critical COVID-19 patients
| Patient ID | Severity of disease | Oxygen saturation values before the first inhalation of plasminogen (%) | Oxygen saturation values after the last inhalation of plasminogen (%) |
|---|---|---|---|
| 6 | Severe | 93 | 97 |
| 7 | Severe | 95 | 96 |
| 8 | Severe | 93 | 95 |
| 9 | Severe | 93 | 96 |
| 10 | Severe | 91 | 95 |
| 11 | Severe | 91 | 89 |
| 12 | Critical | 79 | 91 |
| 13 | Critical | 82 | 91 |
Plasminogen improves heart rates of COVID-19 patients
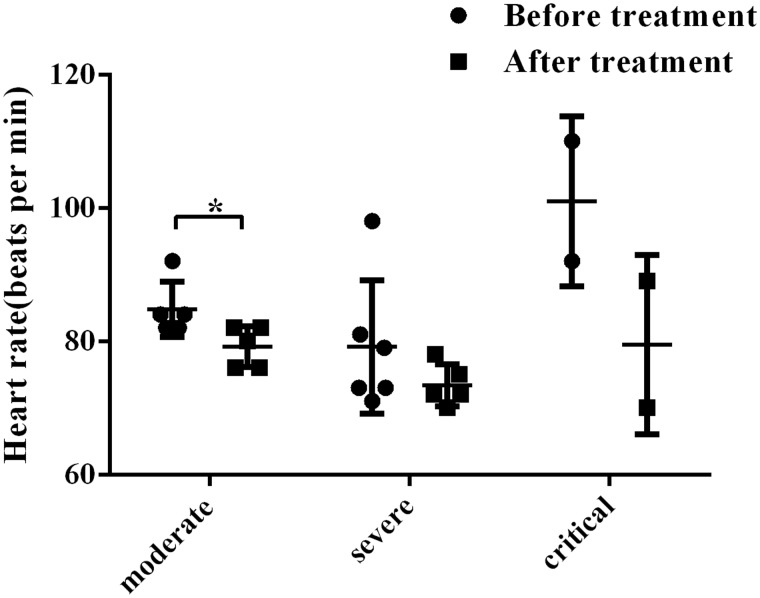
All of the COVID-19 patients also underwent heart rate examination during the study. The results showed that whereas before inhalation of plasminogen, these patients generally showed higher heart rates. After treatment, in 8 out of 13 COVID-19 patients, the heart rates had slowed down as much as 26 beats per min, 2 increased and 3 no changes. Statistical analysis showed that despite patient samples are sparse, the average heart rates after treatment in general have decreased in all of the three groups and for the moderate group, the statistics reached significance (P < 0.05) (in Table 4 and Figure 2). These results indicate that plasminogen can generally slow down the high beating rates and alleviate the burden of hearts of COVID-19 patients.
Table 4.
Heart rate of COVID-19 patients
| Patient ID | Severity of disease | Heart rate before the first inhalation of plasminogen (beats per min) | Heart rate after the last inhalation of plasminogen (beats per min) |
|---|---|---|---|
| 1 | Moderate | 92 | 76 |
| 2 | Moderate | 84-104 | 80 |
| 3 | Moderate | 82 | 82 |
| 4 | Moderate | 82 | 82 |
| 5 | Moderate | 84 | 76 |
| 6 | Severe | 98 | 72 |
| 7 | Severe | 81 | 70 |
| 8 | Severe | 73 | 75 |
| 9 | Severe | 73 | 73-75 |
| 10 | Severe | 79 | 78 |
| 11 | Severe | 71 | 72 |
| 12 | Critical | 92 | 70 |
| 13 | Critical | 110 | 89 |
Figure 2.
Heart rates of clinically moderate, severe and critical COVID-19 patients before (●) and after (■) inhalation treatment of plasminogen. All values are presented as means ± SD. *P < 0.05.
Discussion
As of 4 March 2020, COVID-19 epidemic has quickly become a global health-care emergency that affects more than 95 902 people in 59 countries.22 In this study, by atomization inhalation of plasminogen for just a few times, lung lesions and oxygen saturation levels of COVID-19 patients have got significantly improved, suggesting plasminogen may be used as an effective and efficient way to treat the complications of the COVID-19 infection.
Examinations on postmortem biopsies from one patient died from severe infection with SARS-CoV-2 show that the major pathological characteristics of SARS-CoV-2 pneumonia are diffuse alveolar damage with cellular fibromyxoid exudates and hyaline membrane formation.5 It is known that fibrin, the classic substrate for the PA system, is the main component of inflammatory exudation, and fibrin accumulation in the lung is a hallmark of alveolar hyaline membrane formation resulting in acute lung injury and ARDS, which may further lead to pulmonary fibrosis and death.9
Traditionally, as an inactive pre-enzyme, it is well known and almost a doctrine that plasminogen is excessive, inert and does not have any functional roles due to plasminogen circulates in the blood at a steadily concentration about 0.2 mg/ml.23
However, we have shown that although plasminogen levels are significantly increased in the wounded area of diabetic mice, systemic supplementation of plasminogen results in an even further local increase and faster wound healing, suggesting that plasminogen itself has active functional roles during wound healing.12 Previous studies showed that adults with ARDS have dramatically increased and sufficient amounts of plasminogen, reduced levels of urokinase-type PA and markedly enhanced levels of PA inhibitor-1 and α2-antiplasmin.19–20 Therefore, it is reasonable, similar to that during diabetic wound healing, we speculated that addition of plasminogen can also improve the lung lesions in COVID-19 patients, at least by promoting the degradation of alveolar fibrin.
Meanwhile, our CT results precisely demonstrated that administration with plasminogen indeed decreases the ‘ground glass’ opacity and lesion focal in bilateral lungs of moderate COVID-19 patients. Bilateral lung involvement with ‘ground glass’ opacity is the most common finding from CT images of the COVID-19 patient chests.24 ‘Ground glass’ opacity shown on CT image of viral pneumonia typically represents the filling of the alveolar spaces with macrophages, fibrin, hyaline membranes, proliferating and reactive pneumocytes and hemorrhage.25 Interestingly, it is known that the PA system regulates many of the above components found in the filling of the alveolar space. Thus, these findings suggest that supplementation of plasminogen, such as through atomization inhalation, can be an effective way to remove the ‘ground glass’ opacity, and therefore to restore respiratory function during viral pneumonia in general.
Inflammatory exudation and hyaline membranes form a barrier to gas exchange and inhibit pulmonary surfactant, which is essential for proper pulmonary expansion,26,27 leading to acute respiratory distress and tissue hypoxia. The oxygen saturation results in our study demonstrated that plasminogen does improve hypoxemia of severe and critically severe COVID-19 patients. Of interest, in 1970s, two double-blind randomized clinical studies have indicated that plasminogen improves the clinical conditions and decreases the death rate caused by the hyaline membrane disease, although the studies were performed on premature newborns that were reported to have low circulating plasminogen.9,17 In our study, all of the patients self-described the relief of chest tightness and smoother breathing soon after each administration of plasminogen.
Several studies on hospitalized patients have shown that hypertension, diabetes, cardiovascular diseases are the common comorbidities and lung, kidney, liver and heart are the organs easily affected by the COVID-19 infection.28,29 Earlier studies by us have shown that plasminogen can be used in treating these diseases and diseased organs.30–35 Thus, together with the regulation mechanism of the PA system, we predict that, intravenous supplementation of plasminogen, with or without inhalation of plasminogen, should have more comprehensive and effective therapeutic effects, although additional cautions need to note, due to the complicated nature of COVID-19 infection.
As it is the first and a quick study with limited resources, therefore, a major drawback of the study is the lack of a proper control untreated group. However, since the conditions of these COVID-19 patients are quickly worsening as described in the results section, and by our clinicians’ judgments, it would be difficult for these patients to recover by themselves or by conventional treatment methods during the treatment period. Thus, one could generally regard the conditions before plasminogen treatment are these patients’ own controls, so-called single arm study. Above all, the key findings of rapid clinical improvements by just plasminogen inhalation are stunning. This means that plasminogen inhalation is an efficient and effective method to relieve the COVID-19 patients from the hyaline membrane formation and ARDS. As ARDS is the key event that can quickly worsen the COVID-19 lung conditions from flu-like discomfort to multi-organ failures and even death. We speculate that, if this treatment method can be used more extensively at a larger scale, especially to patients with clinical conditions quickly turning from moderate to severe or critical, we might significantly lower the death rate and the severity of this terrible epidemic emergency. Notwithstanding, before the above conclusions can be firmly made, randomized controlled trials and additional examination parameters and methods are needed to determine the efficacy and optimize the safety profile of plasminogen for treatment of patients with SARS-CoV-2 infection, such work is currently being carried on by our team.
Acknowledgements
We wish to acknowledge all other staff involved in the study from but not limited to the institutions of the listed authors for their assistance.
Conflict of interest. J.L. owns Talengen Institute of Life Sciences. The institute has applied patents on the use of plasminogen in relation to COVID-19 and is developing natural and recombinant plasminogen as a drug candidate to treat COVID-19. Other authors have declared no competing interests.
References
- 1.World Health Organization. Pneumonia of Unknown Cause-China. 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (19 February 2020, date last accessed).
- 2.Real-Time Big Data Report on New Coronavirus Pneumonia. 2020. https://voice.baidu.com/act/newpneumonia/newpneumonia/? from=osari_pc_3 (4 March 2020, date last accessed).
- 3. Hui DSC, Wong PC, Wang C.. SARS: clinical features and diagnosis. Respirology 2003; 8:S20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;420–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gitlin D, Craig JM.. The nature of the hyaline membrane in asphyxia of the newborn. Pediatrics 1956; 17:64–71. [PubMed] [Google Scholar]
- 7. Van Breemen VL, Neustein HB, Bruns PD.. Pulmonary hyaline membranes studied with the electron microscope. Am J Pathol 1956; 33:769–89. [PMC free article] [PubMed] [Google Scholar]
- 8. Duran-Jorda F, Holzel A, Patterson WH.. A histochemical study of pulmonary hyaline membrane. Arch Dis Child 1956; 31:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ambrus CM, Weintraub DH, Choi TS, Eisenberg B, Henry PS, Norman GC, et al. Plasminogen in the prevention of hyaline membrane disease. Res Commun Chem Pathol Pharmacol 1973; 6:341–4. [PubMed] [Google Scholar]
- 10. Günther A, Ruppert C, Schmidt R, Markart P, Grimminger F, Walmrath D, et al. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res 2001; 2:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saksela O, Rifkin DB.. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol 1988; 4:93–126. [DOI] [PubMed] [Google Scholar]
- 12. Shen Y, Guo Y, Mikus P, Sulniute R, Wilczynska M, Ny T, et al. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood 2012; 119:5879–87. [DOI] [PubMed] [Google Scholar]
- 13. Guo Y, Li J, Hagström E, Tor NY.. Beneficial and detrimental effects of plasmin(ogen) during infection and sepsis in mice. PLoS One 2011; 6:e24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Li J, Hagström E, Tor NY.. Protective effects of plasmin(ogen) in Staphylococcus aureus-induced arthritis. Arthritis Rheum 2008; 58:764–72. [DOI] [PubMed] [Google Scholar]
- 15. Li J. Method for preventing or treating acute thrombosis and chronic thrombosis. WIPO Patent Application, WO/2017/101866.
- 16. Lieberman J, Kellogg F.. The fibrinolytic enzyme defect of hyaline membrane disease. Calif Med 1961; 95:278–82. [PMC free article] [PubMed] [Google Scholar]
- 17. Ambrus CM, Choi TS, Cunnanan E, Eisenberg B, Staub HP, Weintraub DH, et al. Prevention of hyaline membrane disease with plasminogen: a cooperative study. JAMA 1977; 237:1837–41. [PubMed] [Google Scholar]
- 18. Ambrus CM, Choi TS, Weintraub DH, Eisenberg B, Staub HP, Courey NG, et al. Studies on the prevention of respiratory distress syndrome of infants due to hyaline membrane disease with plasminogen. Semin Thromb Hemost 2008; 2:42–51. [DOI] [PubMed] [Google Scholar]
- 19. Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 1989; 84:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Günther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med 2000; 161:454–62. [DOI] [PubMed] [Google Scholar]
- 21.General Office of National Health Commission of the People’s Republic of China. Office of State Administration of Traditional Chinese Medicine. Notice on the issuance of strategic guidelines for diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (fifth edition draft) (2020-02-05) [EB/OL].
- 22.Real-Time Big Data Report on Global New Coronavirus Pneumonia. 2020. https://news.qq.com/zt2020/page/feiyan.htm? kw=ad_sy#/global (4 March 2020, date last accessed).
- 23. Aisina RB, Mukhametova LI.. Structure and function of plasminogen/plasmin system. Russ J Bioorg Chem 2014; 40:590–605. [Google Scholar]
- 24. Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Sherief AH, Gilman MD, Healey TT, Tambouret RH, Shepard JA, Abbott GF, et al. Clear vision through the haze: a practical approach to ground-glass opacity. Curr Probl Diagn Radiol 2014; 43:140–58. [DOI] [PubMed] [Google Scholar]
- 26. Avery ME. The Lung and Its Disorders in Newborn Infants. Pediatrics September 1969; 44: 465–466. [Google Scholar]
- 27. Taylor FB Jr, Abrams ME.. Effect of surface active lipoprotein on clotting and fibrinolysis, and of fibrinogen on surface tension of surface active lipoprotein. Am J Med 1966; 40:346–50. [Google Scholar]
- 28. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J. Method for preventing and treating tissue and organ fibrosis. WIPO Patent Application, WO/2018/107698.
- 31. Li J. Method for mitigating heart disease. WIPO Patent Application, WO/2018/107707.
- 32. Li J. Novel method for preventing and treating cardiovascular disease. WIPO Patent Application, WO/2017/101871.
- 33. Li J. Method for preventing and treating pulmonary fibrosis. WIPO Patent Application, WO/2018/107697.
- 34. Li J. Novel method for treating diabetes. WIPO Patent Application, WO/2018/107702.
- 35. Li J. Method and drug for regulating blood pressure. Chinese Patent Application, CN/202010119730.9