Since December 2019, the world is increasingly facing an unprecedented challenge by coronavirus disease 2019 (COVID-19) caused by a virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is structurally related to the human severe acute respiratory syndrome CoV (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV) (Zhu et al., 2020). The World Health Organization (WHO) has declared COVID-19 a pandemic and is calling for forceful action from all countries, as the number of infected patients and the number of deaths are increasing daily, causing catastrophic consequence to the daily life, economy, and society.
Like other coronaviruses, SARS-CoV-2 viruses enter the cell via endocytosis (Figure 1) into the endosomal pathway (Hong, 2005). Once in the endosomal compartments, they escape into the cytoplasm and release the positive-stranded RNA genome to trigger viral replication and assembly. Newly made viral proteins such as Spike protein and other membrane proteins enter the secretory pathway via targeting and assembling in the endoplasmic reticulum (ER). During the transport from the ER to the Golgi apparatus via a collection of tubulovesicular structures referred to as the ER–Golgi intermediate compartment (ERGIC), other viral proteins such as nucleocapsid and newly made viral RNA genome assemble onto the membrane of the ERGIC to trigger inward budding of previral particles into the lumen of the ERGIC. These previral particles mature during the transport to the trans-Golgi network (TGN) of the Golgi apparatus, from where newly assembled viral particles are released by the infected cells via transport carriers budding from the TGN and fusing with the cell surface. The endosomal compartments are dynamically linked with the TGN via various transport routes between the endosomes and the TGN. Over the years, cell biologists and virologists have identified several inhibitors to the endocytic pathway and they are traditionally used to perturb the function of the endocytic pathway, which indirectly also affects the secretory pathway (Engel et al., 2011). These inhibitors include Ammonium chloride (NH4Cl), Chloroquine, Bafilomycin A1, Concanamycin A1, and Monensin. Therefore, it is a reasonable hypothesis that inhibiting the function of the endocytic and secretory pathways will directly or indirectly suppress viral infection, replication, assembly, and/or release if the side effects are managed well.
Figure 1.
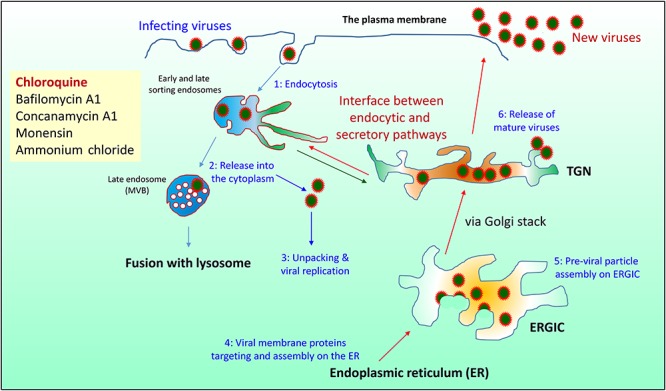
Schematic drawing of endocytic and secretory pathways in relation to the life cycle of SARS-CoV-2. SARS-CoV-2 virus enters the cell by endocytosis (step 1). The endocytic pathway consists of various early and late recycling endosomes and late endosome (also called multivesicular bodies, MVB) before maturing into the lysosome. Newly made proteins enter the secretory pathway via ER and are transported to the TGN via the ERGIC and then the Golgi stack before being transported to the cell surface via transport carriers that bud from the TGN and fuse with the plasma membrane. Some of the internalized viruses are released from the endosome into the cytoplasm (step 2) for unpacking and viral replication and assembly (step 3). Newly made viral S protein and other membrane proteins enter the ER (step 4) whereas viral RNA genome and nucleocapsid protein are assembled on the membrane of the ERGIC to trigger inward budding to form the previral particles into the lumen of the ERGIC (step 5). Matured viruses are released via transport from the TGN to the cell surface (step 6). Chloroquine directly inhibits endosomal function and likely indirectly affects the secretory pathway via perturbing the interface between the endosome and TGN, resulting in inhibition of viral endosomal release, replication, assembly, and/or release to achieve clinical benefit.
Among these inhibitors, only Chloroquine (which inhibits acidification of endosomes) is a Food and Drug Administration (FDA)-approved drug used for treating malaria infection and, therefore, it has attracted the most attention during the past few months. Chloroquine was shown by in vitro experiments to inhibit the replication of SARS-CoV and SARS-CoV-2 (Keyaerts et al., 2004; Wang et al., 2020). Therefore, it is urgent to test the effect of Chloroquine on infected patients to see its therapeutic benefit on COVID-19 in clinic. In this timely clinical study report (Huang et al., 2020), the authors have tested the clinical effects of Chloroquine on patients by monitoring viral RNA by real-time polymerase chain reaction (RT-PCR), lung function by computerized tomography (CT) scanning, and T-cell counts. Among 82 patients examined, 22 were identified to meet their enrollment criteria. These 22 patients were divided into two groups, one (n = 10) treated with Chloroquine (500 mg, oral administration, twice daily) and the other (n = 12) with Lopinavir/Ritonavir (400/100 mg, oral administration, twice daily) for 10 days, and monitored for a total of 14 days. Importantly, the Chloroquine-treated group displayed steady increases in the number of patients becoming negative for the viral RNA. By Day 13, all the Chloroquine-treated patients became negative in viral RNA test. In the Lopinavir/Ritonavir-treated group, 11 out of 12 turned negative at Day 14. CT scan imaging was used to see the lung clearance and the study revealed that lung improvement from the Chloroquine group was more than doubled compared with that of the Lopinavar/Ritonavir group, indicating that patients in the Chloroquine-treated group recover their pulmonary function more significantly than those in the group treated with Lopinavir/Ritonavir. More significantly, patients in the Chloroquine-treated group were discharged from the hospital faster than those in the Lopinavir/Ritonavir-treated group, as all 10 patients from the Chloroquine group were discharged as compared to 6 patients (50%) from the Lopinavir/Ritonavir group by Day 14. T-cell counts for the Chloroquine-treated patients did not show a significant decrease during the 10-day treatment period. Common side effects associated with Chloroquine such as vomiting, abdominal pain, nausea, diarrhea, rash or itchy, cough, and shortness of breath were observed but these were managed well by strengthening patient monitoring and strictly following the standard oral dosage of the drug. In the context of another independent report (Gautret et al., 2020), this study suggests that Chloroquine is an effective and safe treatment for the SARS-CoV-2 virus associated with the COVID-19 pandemic, and the regulatory systems in various countries should consider fast-tracking the approval of its use with strict guideline to minimize the consequence of COVID-19 pandemic. Although the molecular mechanism underlying the action of Chloroquine remains to be defined, it is envisioned that in addition to directly affecting the endosomal viral entry and release via inhibiting endosomal function, Chloroquine may potentially affect the viral assembly at the ERGIC and/or viral release from the TGN indirectly.
References
- Engel S., Heger T., Mancini R., et al. (2011). Role of endosomes in simian virus 40 entry and infection. J. Virol. 85, 4198–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hong W. (2005). SNAREs and traffic. Biochim Biophys Acta 1744, 120–144. [DOI] [PubMed] [Google Scholar]
- Huang M., Tang T., Pang P., et al. (2020). Treating COVID-19 with Chloroquine. J. Mol. Cell Biol. doi: 10.1093/jmcb/mjaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., et al. (2004). In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 323, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]


