On 11 March 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak as a ‘pandemic’.1 No valid therapy for COVID-19 is actually available. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is empirically treated with antivirals, antimalarics, tocilizumab, etc.2 Production of a vaccine for COVID-19 has been attempted, although approval needs time.
We describe a possible, alternative approach for treating COVID-19. Lymphocyte count has been associated with increased disease severity risk.3 Patients who died from COVID-19 showed a significantly lower lymphocyte count than survivors, therefore this should be closely monitored.3 Repletion of lymphocytes could probably have beneficial effects on recovery.
A recent hypothesis suggests that the inhibition of the angiotensin 1 receptor (AT1R) may provide benefits to COVID-19 patients.4 This hypothesis is based on the observation that the SARS-CoV-2 virus uses angiotensin-converting enzyme 2 (ACE2) as a receptor to bind the virus to the bronchial cell membrane. The enzymes ACE and ACE2 belong to the same peptidase family but have two very different physiological functions. ACE cleaves angiotensin I to generate angiotensin II (Ang II), which binds to and activates AT1R, and thus promoting vasoconstriction. ACE2 cleaves Ang II and generates angiotensin 1-7, a powerful vasodilator acting through Mas receptors. AT1R antagonists are widely used in hypertensive patients but they increase the ACE2 cardiac expression in rats5 and the urinary concentration of ACE2.6
It has been demonstrated that the binding of virus to ACE2 leads to ACE2 down-regulation, which increases the production of Ang II but reduces angiotensin 1-7. This contributes to increased AT1-mediated pulmonary vascular permeability, thereby mediating increased lung pathology.7 Therefore, higher ACE2 expression following chronic therapy with sartans may protect COVID-19 patients from acute lung injury rather than increasing the risk for SARS-CoV-2. Two complementary mechanisms may explain such a hypothesis: sartans will continue to block excessive angiotensin-mediated AT1R activation due to the viral infection, and, in parallel, they will up-regulate ACE2, thus increasing angiotensin 1-7 production.
In such a setting, the role of neprilysin (NEP) and its inhibitor sacubitril should also be revised. Recently, Zhang et al.8 demonstrated that sacubitril/valsartan reduced the concentration of pro-inflammatory cytokines and neutrophil count, while increasing lymphocyte count more than valsartan alone or placebo.8 This finding might be related to the increase in plasma levels of atrial/brain/C-type natriuretic peptide, Ang I/II, substance P, bradykikin, and endothelin secondary to neprilisin inibition by sacubitril.8 We have recently shown that early sacubitril/valsartan administration reduces high sensitivity C-reactive protein levels and increases lymphocyte count in patients with acute heart failure.9
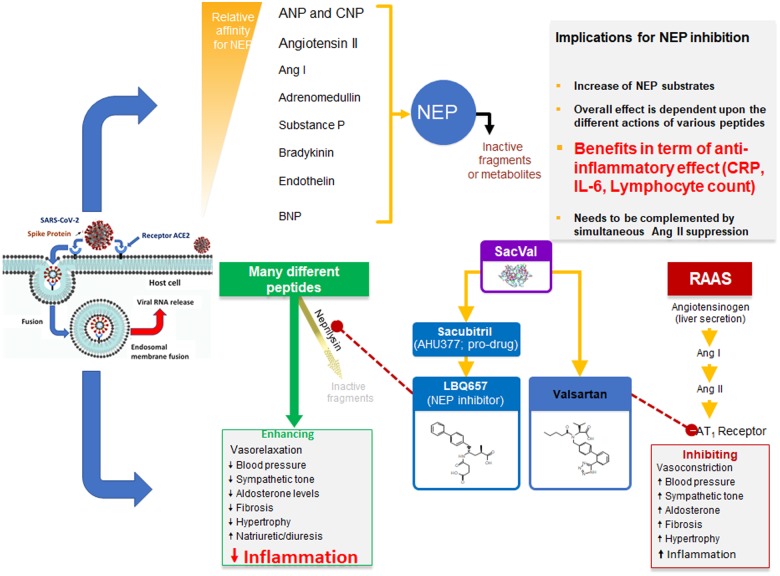
These pieces of evidence support the biological plausibility of early administration of sacubitril/valsartan in COVID-19 patients, in order to maximize the anti-inflammatory effects of sacubitril and contain the effect of Ang I on the lungs (Figure 1).
Figure 1.
Double interaction between sacubitril and NEP and between valsartan and the AT1 receptor and their consequences on the development of the inflammatory response due to COVID-19 infection. RAAS, renin–angiotensin–aldosterone system; SacVal, sacubitril/valsartan.
Conflict of interest: none declared.
References
- 1. Rothan HA, Byrareddy SN.. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham AC, Goh HP, Koh D.. Treatment of COVID-19: old tricks for new challenges. Crit Care 2020;24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y.. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020;doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Ye Y, Gong H, Wu J, Yuan J, Wang S, Yin P, Ding Z, Kang L, Jiang Q, Zhang W, Li Y, Ge J, Zou Y.. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE–AngII–AT1 and ACE2-Ang(1-7)–Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol 2016;97:180–190. [DOI] [PubMed] [Google Scholar]
- 6. Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T.. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015;28:15–21. [DOI] [PubMed] [Google Scholar]
- 7. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM.. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H, Liu G, Zhou W, Zhang W, Wang K, Zhang J.. Neprilysin inhibitor–angiotensin II receptor blocker combination therapy (sacubitril/valsartan) suppresses atherosclerotic plaque formation and inhibits inflammation in apolipoprotein E-deficient Mice. Sci Rep 2019;9:6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Acanfora D, Scicchitano P, Acanfora C, Maestri R, Goglia F, Incalzi RA, Bortone AS, Ciccone MM, Uguccioni M, Casucci G.. Early initiation of sacubitril/valsartan in patients with chronic heart failure after acute decompensation: a case series analysis. Clin Drug Investig 2020;doi: 10.1007/s40261-020-00908-4. [DOI] [PubMed] [Google Scholar]