Abstract
Objectives
To report the methods and findings of two complete autopsies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive individuals who died in Oklahoma (United States) in March 2020.
Methods
Complete postmortem examinations were performed according to standard procedures in a negative-pressure autopsy suite/isolation room using personal protective equipment, including N95 masks, eye protection, and gowns. The diagnosis of coronavirus disease 2019 (COVID-19) was confirmed by real-time reverse transcriptase polymerase chain reaction testing on postmortem swabs.
Results
A 77-year-old obese man with a history of hypertension, splenectomy, and 6 days of fever and chills died while being transported for medical care. He tested positive for SARS-CoV-2 on postmortem nasopharyngeal and lung parenchymal swabs. Autopsy revealed diffuse alveolar damage and chronic inflammation and edema in the bronchial mucosa. A 42-year-old obese man with a history of myotonic dystrophy developed abdominal pain followed by fever, shortness of breath, and cough. Postmortem nasopharyngeal swab was positive for SARS-CoV-2; lung parenchymal swabs were negative. Autopsy showed acute bronchopneumonia with evidence of aspiration. Neither autopsy revealed viral inclusions, mucus plugging in airways, eosinophils, or myocarditis.
Conclusions
SARS-CoV-2 testing can be performed at autopsy. Autopsy findings such as diffuse alveolar damage and airway inflammation reflect true virus-related pathology; other findings represent superimposed or unrelated processes.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Autopsy, Diffuse alveolar damage, Acute lung injury, Pulmonary pathology
Key Points.
This is the first report of complete autopsy findings in coronavirus disease 2019 (COVID-19) in the English language literature. A protocol detailing use of appropriate equipment is provided.
A 77-year-old man died after having fever and chills for 6 days. Postmortem nasopharyngeal swab was positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Autopsy showed diffuse alveolar damage and airway inflammation.
The second decedent was a 42-year-old obese man with muscular dystrophy. Postmortem nasopharyngeal swab was positive for SARS-CoV-2. Autopsy showed acute bronchopneumonia, with aspiration.
Case slides and more information .
Use your ASCP account information to log in. Guests use the following login information:
Username: ajcp Password: ascp20
We are currently in the midst of a global coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of the April 3, 2020, Situation Report, the World Health Organization (WHO) reported 972,303 confirmed cases of COVID-19 worldwide, with 50,321 attributed deaths, of which 4,793 deaths are in the United States.1
Coronaviruses are enveloped, nonsegmented, positive-sense single-stranded RNA viruses. Until recently, only two beta coronaviruses—severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV)—have caused significant human morbidity and mortality.2 Most patients with COVID-19 are asymptomatic or experience only mild symptoms, including fever, dry cough, and shortness of breath. However, some individuals deteriorate rapidly and develop acute respiratory distress syndrome (ARDS).3 The most common histopathologic correlate of ARDS is diffuse alveolar damage (DAD), characterized by hyaline membrane formation in the alveoli in the acute stage, and interstitial widening by edema and fibroblast proliferation in the organizing stage. DAD has a long list of potential etiologies, including infection, vaping-associated pulmonary injury, oxygen toxicity, drug toxicity, toxic inhalants or ingestants, shock, severe trauma, sepsis, irradiation, and acute exacerbations of usual interstitial pneumonia.4-9
Despite a burgeoning literature that provides insights into the clinical picture of COVID-19, very little is known about the pathologic manifestations. At the time of this writing (April 4, 2020), we are not aware of any published reports of complete autopsies on decedents with COVID-19 in the English literature. One report from China has described histopathologic findings in two patients who underwent lobectomy for lung cancer.10 After resection, these individuals developed symptoms consistent with viral infection, and were subsequently found to have COVID-19. The authors described several nonspecific histologic changes, including edema, fibrinous/proteinaceous exudates, hyperplastic pneumocytes, patchy inflammation, and multinucleated giant cells.10 Another case report, also from China, described findings in a postmortem biopsy taken from a decedent who experienced 14 days of symptoms. The biopsy showed DAD and interstitial mononuclear inflammatory infiltrates.11 Another recent report, an analysis of core biopsies obtained postmortem from four COVID-19 patients with a minimum of 15 days of symptoms, describes DAD in all biopsies, with superimposed bacterial pneumonia in one.12
The aim of this report is to share our observations on the pathology of COVID-19 based on complete autopsies in two individuals who died in Oklahoma during the COVID-19 pandemic and were found to be positive for SARS-CoV-2 by postmortem testing. We also wish to share our experience regarding the precautions and personal protective equipment (PPE) used during these autopsies.
Materials and Methods
The Oklahoma Office of the Chief Medical Examiner (OCME) is a statewide medical examiner death investigation system, serving the 77 counties of the state of Oklahoma. The office is staffed by 12 American Board of Pathology–certified forensic pathologists and 33 American Board of Medicolegal Death Investigators–certified medicolegal death investigators, in addition to a full technical and administrative support staff. The mission of the OCME is to protect the health and safety of Oklahomans through the scientific investigation of deaths as defined by state statute, via scene investigation and postmortem examination. Examples of deaths falling under the jurisdiction of the OCME include violent deaths; deaths under suspicious, unusual, or unnatural circumstances; deaths related to disease, which might constitute a threat to public health; and deaths unattended by a licensed physician. The decedents described herein were not currently being treated by a physician and exhibited symptoms suspicious for COVID-19 at the time of death. No antemortem testing for COVID-19 had been performed. Jurisdiction was therefore assumed by the OCME, citing unattended deaths and a possible threat to public health.
The decedents were transported to our facility and received full body anterior-posterior radiographs using a Lodox scanner system while remaining in a sealed body bag. Complete postmortem external and internal examinations were performed on both bodies by forensic pathologists with the assistance of a trained pathology technician. The autopsies were performed in accordance with guidelines set forth by the National Association of Medical Examiners (NAME)13 and the College of American Pathologists (CAP).14 Recently published guidelines for performing autopsies with suspected COVID-19 were followed.15,16 Examinations were performed in a state-of-the-art facility completed in 2018 with a negative-pressure autopsy suite and a separate negative-pressure isolation room. The examination tables used for autopsies were equipped with a reverse-flow air handling system Image 1. The examiners were clad in PPE, including N-95 masks, eye protection, disposable scrub caps, gowns, gloves, and rubber shoes or boots. Alternatively, the examiners were clad in a disposable body suit with shoe covers and 3M Versaflo powered air purifying respirators with M-series headgear (Image 1). The clothing worn under the PPE consisted of blue surgical scrub shirts and pants, which were removed before exiting the lab and laundered in-house. Shoes worn during autopsy remained in the laboratory. Shower rooms stocked with hygiene products and clean towels were available in the laboratory.
Image 1.
A pathology technician in complete autopsy attire. Behind her is an example of the reverse airflow tables at the Oklahoma Office of the Chief Medical Examiner autopsy stations. The main autopsy suite is equipped with 8 identical stations. Suspected coronavirus disease 2019 (COVID-19) autopsies are performed in an isolation room equipped with two identical stations.
Testing for COVID-19 was performed on nasopharyngeal swabs taken during external examination of the body. Lung tissue swabs were taken immediately after opening the body and collected in a sterile manner from an incision of the lung parenchyma. The swabs were immediately placed in transport media and sent to the State of Oklahoma Department of Health Laboratory, where real-time reverse transcriptase polymerase chain reaction (rRT-PCR) testing was performed. Nasopharyngeal swabs for a basic respiratory pathogen panel including influenza were also submitted in the same manner from both cases. In the second case, a section of lung parenchyma was collected in a sterile fashion and submitted to the State of Oklahoma Department of Health Laboratory for microbiologic cultures.
Representative sections of tissue were submitted in standard tissue cassettes and fixed in formalin. Tissue was processed, embedded in paraffin, cut onto glass slides, and stained with H&E in the usual fashion. All slides from the lungs were examined by a fellowship-trained pulmonary pathologist with 13 years of experience. Immunohistochemistry was performed using appropriate positive and negative controls by the University of Oklahoma Medical Center Laboratory in Oklahoma City. Representative sections of tissue will be retained in formalin in our storeroom for a 1-year period after completion of the cases. The blocks and slides will be retained indefinitely. Recently published guidelines provided by the Centers for Disease Control National Vital Statistics System were observed in completion of the death certificates.17
Results
The autopsy findings in both cases are summarized in Table 1.
Table 1.
Summary of Autopsy Findings
| Case 1, 77-Year-Old Man | Case 2, 42-Year-Old Man | |
|---|---|---|
| Neck | Symmetric, no trauma | Symmetric, no trauma |
| Respiratory system | Combined lung weight: 2,452 g Lungs firm and edematous diffusely Edematous right pleural adhesions No effusions Microscopic: diffuse alveolar damage, acute stage | Combined lung weight: 1,191 g Lungs mottled red/tan in upper lobes Dark red edematous lower lobes No adhesions, no effusions Microscopic: acute bronchopneumonia, focal aspiration |
| Central nervous system | Brain weight: 1,274 g No gross abnormalities | Brain weight: 1,224 g No gross abnormalities |
| Cardiovascular system | Heart weight: 402 g No adhesions, effusions, or thrombi Coronary artery disease, marked 2 vessel Microscopic: acute ischemic injury Abdominal aorta atherosclerosis | Heart weight: 372 g No adhesions, effusions, or thrombi Coronary artery disease, mild Aorta intimal fatty streaking |
| Gastrointestinal system | No mouth/tongue abnormalities No esophagus abnormalities Stomach mucosa intact Stomach contains 100 mL green/brown fluid Increased visceral adipose No bowel abnormalities No appendix present | No mouth/tongue abnormalities No esophagus abnormalities Stomach and bowel have gaseous distension Stomach mucosa intact Stomach contains 100 mL green/brown fluid Increased visceral adipose No appendix abnormalities |
| Hepatobiliary system and pancreas | Liver weight: 2,232 g Hepatic centrilobular steatosis Remote cholecystectomy Right upper quadrant adhesions No pancreas abnormalities | Liver weight: 1,683 g Hepatic cirrhosis, advanced Remote cholecystectomy No pancreas abnormalities |
| Genitourinary system | Arterionephrosclerosis Oncocytoma (3 cm) Benign prostatic hyperplasia Normal testes | Simple cortical renal cyst Grossly normal prostate Testicular atrophy |
| Endocrine system | Normal thyroid Grossly normal pituitary Grossly normal adrenals | Multinodular thyroid Grossly normal pituitary Grossly normal adrenals |
| Immunologic system | Remote splenectomy No lymphadenopathy No thymus tissue present | Splenomegaly (293 g) No lymphadenopathy No thymus tissue present |
| Musculoskeletal system | Resuscitation attempt injury Obesity Osteoarthritis Knee replacement hardware | Resuscitation attempt injury Obesity Gynecomastia Abdominal striae |
| Molecular/Microbiology | Nasopharyngeal swabs: positive for SARS-CoV-2 Lung parenchymal swabs: positive for SARS-CoV-2 Basic respiratory pathogen panel: negative | Nasopharyngeal swabs: positive for SARS-CoV-2 Lung parenchymal swabs: negative for SARS-CoV-2 Basic respiratory pathogen panel: negative Bacterial cultures: positive for nontoxigenic Escherichia coli, Proteus mirabilis, and Candida tropicalis |
Case 1
The decedent was a 77-year-old man with a history of hypertension, remote deep vein thrombosis, remote splenectomy for an unspecified genetic disease, remote pancreatitis due to cholelithiasis, and osteoarthritis status post total knee replacement in October 2019 with subsequent development of a rash over the knees and positive antinuclear antibody serology.
During his current illness, he had a 6-day history of chills and intermittent fevers but no cough. Prior to the onset of these symptoms, the decedent did not observe any known precautionary measures to prevent the disease and was exposed to numerous potential sources of infection. However, there was no history of recent travel or known exposure to sick contacts. Emergency medical services responded to a call on March 20, 2020, stating that the decedent was experiencing weakness, fever, and shortness of breath. The patient went into cardiac arrest during transport to the hospital and died shortly after arrival at the hospital.
At postmortem examination, evidence of emergency medical intervention was noted, including intubation and evidence of chest compressions (abrasions and bilateral anterolateral aligned rib fractures). The decedent was 5 ft 7 in, 208 lb, with a body mass index of 31.8 kg/m2. Postmortem radiography showed bilateral pulmonary opacities Image 2A. Internal examination revealed bilateral lungs that were heavy (right lung weight, 1,183 g; left lung weight, 1,269 g), red to maroon in color, and with an edematous parenchyma that had a diffusely firm consistency without focal lesions. The upper and lower airways were widely patent and lined by a smooth, glistening, pale cream-colored mucosa without gross abnormalities. No mucus plugging was noted.
Image 2.
Postmortem anterior-posterior chest radiographs. A, Case 1. Diffuse, dense bilateral airspace consolidations (complete “whiteout”). Multiple air bronchograms are present (arrows). The autopsy in this case showed diffuse alveolar damage. B, Case 2. Diffuse airspace opacities in both lungs, less consolidative in comparison to part A. Multiple bilateral air bronchograms are highlighted (arrows). The left lung is asymmetrically slightly more consolidated compared to the right. An endotracheal tube is shown with its tip above the level of the clavicular heads in the cervical trachea (white arrow). There is marked gastric distension with air (asterisk). The large opaque circular artifact on the right chest represents the grommet of the sealed body bag, and the small opaque circular artifacts represent buttons on clothing. Autopsy revealed acute bronchopneumonia.
Nasopharyngeal and bilateral lung parenchymal swabs for SARS-CoV-2 were reported as positive (by rRT-PCR) by the State of Oklahoma Department of Health Laboratory, and a nasopharyngeal swab for a respiratory pathogen panel including influenza was reported as negative. Results were reported in 4 days.
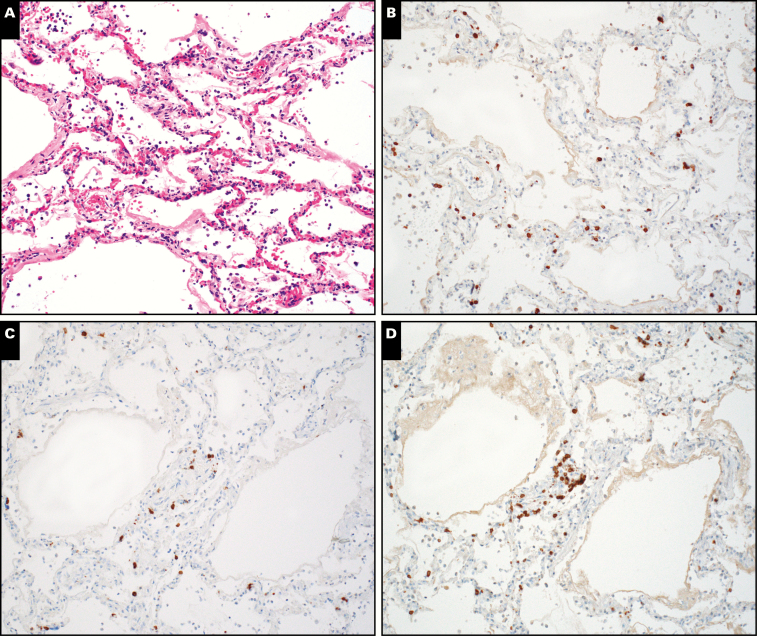
Microscopic examination of the lungs revealed DAD in the acute stage characterized by numerous hyaline membranes without evidence of interstitial organization Image 3. There was very patchy and sparse interstitial chronic inflammation composed mainly of lymphocytes. As is common in DAD, thrombi were noted within a few small pulmonary artery branches. Congestion of alveolar septal capillaries and edema fluid within the airspaces were noted focally. There was mild chronic inflammation within the bronchi and bronchioles, along with prominent mucosal edema within the bronchial mucosa (the resultant mucosal thickening is appreciable, even at low magnification in Image 3A). There was no evidence of mucus plugging within airways. No eosinophils or neutrophils were identified. Immunohistochemistry showed a sparse infiltrate of CD3-positive T-lymphocytes within the alveolar septa Image 4, with only rare CD20-positive B-lymphocytes. CD8-positive T-cells slightly outnumbered CD4-positive T-cells. CD68 highlighted a few macrophages.
Image 3.
Case 1. Microscopic findings in the lungs of a 77-year-old man who died of coronavirus disease 2019 (COVID-19). A, The airways are patent, with no evidence of mucus plugging. The upper arrow points to a patent bronchiole. The structure marked by the lower arrow is a patent bronchus. Neither airway shows evidence of mucus plugging. The pale appearance of the thickened bronchial mucosa is caused by mucosal edema. B, Diffuse alveolar damage in the acute stage. Note hyaline membranes (arrow). C, Chronic inflammation in the mucosa of an airway (arrow). The inflammatory cells are mainly lymphocytes. D, Patchy interstitial chronic inflammation. This image is taken from one of the few areas where interstitial inflammation was obvious even at low magnification. In most areas, the inflammatory infiltrate was very sparse or absent.
Image 4.
Immunohistochemistry in case 1. A, Diffuse alveolar damage with minimal, patchy chronic inflammation (H&E, x200). T-lymphocytes are highlighted by immunohistochemical stains for CD3 (B), CD4 (C), and CD8 (D).
Other findings noted at autopsy were right pleural adhesions, hypertensive heart disease with microscopic evidence of acute ischemia, coronary artery atherosclerosis with marked two-vessel disease, arterionephrosclerosis, a right renal mass (oncocytoma), evidence of remote splenectomy, marked prostatic hyperplasia, and obesity. Sections from the heart showed no evidence of myocarditis. In the final autopsy report, the cause of death was listed as COVID-19, with coronary artery disease listed under “other contributing factors.” The manner of death was listed as natural.
Case 2
A 42-year-old man with a history of myotonic muscular dystrophy presented to a community hospital on March 19, 2020, in critical condition. Reported symptoms upon admission were fever, shortness of breath, and cough. The decedent lived with two immediate family members. According to reports from the family, the decedent was disabled and used a walker to ambulate due to progressive muscle weakness caused by muscular dystrophy. His only recent exposure to a public space was eating at a local restaurant on March 13, 2020. His immediate family members had visited the grocery store and gone to work during this time. There was no history of recent travel or known exposure to sick contacts. He had a reported history of prior bowel obstructions, which resolved without surgery. Approximately 2 days prior to his death, he began experiencing abdominal pain. Computed tomography (CT) of the chest performed at the hospital shortly prior to death showed bilateral ground-glass opacities Image 5 as well as bilateral consolidations. The patient suffered a cardiac arrest soon thereafter. Overall, he survived for only for a few hours in the hospital.
Image 5.
Antemortem chest computed tomographic scan from case 2, showing bilateral ground-glass opacities. Bilateral consolidations were present elsewhere (not shown).
At postmortem examination, evidence of emergency medical intervention was present, including intubation and chest compressions. The decedent was 5 ft 10 in, 218 lb, with a body mass index of 31.3 kg/m2. Postmortem radiography showed bilateral pulmonary opacities Image 2B. The abdomen was distended by air in the stomach, small bowel, and large bowel. The lungs were heavy (right lung weight, 579 g; left lung weight, 612 g). The pulmonary parenchyma had a red/tan mottled appearance, and both lower lobes had a diffusely saturated dark red appearance.
Nasopharyngeal swabs were positive for SARS-CoV-2 by rRT-PCR. The time interval between sample collection and reporting of results was 4 days. However, bilateral lung parenchymal swabs were negative. A standard respiratory pathogen panel was also negative. Bacterial cultures (aerobic/anaerobic) of the lung tissue grew nontoxigenic Escherichia coli, Candida tropicalis, and Proteus mirabilis.
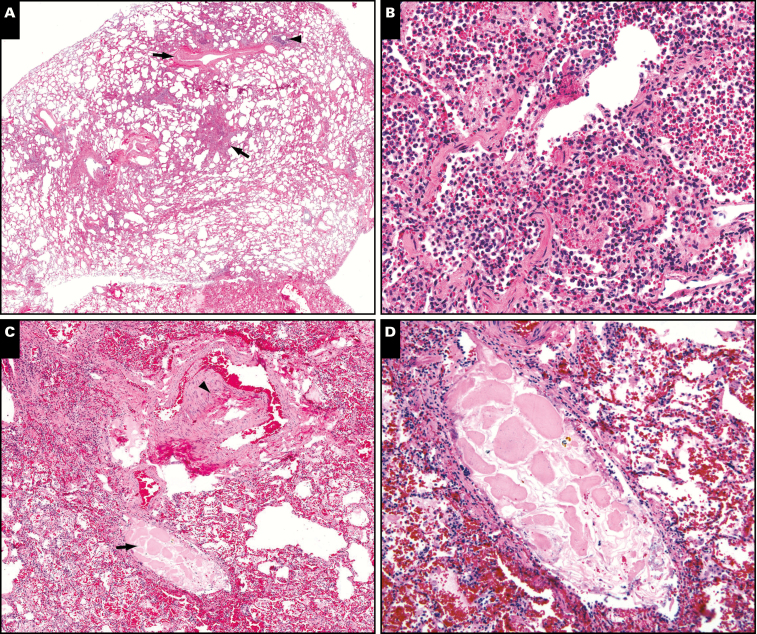
Microscopic examination of the lungs revealed foci of acute bronchopneumonia along with rare aspirated food particles Image 6. The process was characterized by the filling of peribronchiolar airspaces by neutrophils and histiocytes. There was no evidence of DAD, mucus plugging within airways, or eosinophils. Focally, aspirated foreign material, including bacteria, squamous cells, and vegetable matter, was noted within airways. Immunohistochemistry showed similar findings similar to case 1. CD68 highlighted numerous macrophages within the areas of bronchopneumonia.
Image 6.
Pathologic findings in the lungs in case 2. A, Low magnification view of a small pulmonary artery branch (upper arrow) and its partner bronchiole (arrowhead). The area indicated by the lower arrow is shown at higher magnification in B. B, The airspaces are filled by a mix of neutrophils and histiocytes (acute bronchopneumonia). C, Low magnification view of another area. The arrowhead points to a small pulmonary artery. The arrow indicates its partner airway, which contains a foreign particle. The particle is shown at higher magnification in D. D, The foreign particle is a piece of aspirated vegetable matter.
Other autopsy findings included liver cirrhosis with gynecomastia and testicular atrophy, mild coronary artery atherosclerosis in a 372-g heart, renal nephrosclerosis, tubular fan-shaped crystals (kidneys), a nodular thyroid, and obesity. Sections from the heart showed no evidence of myocarditis. In the final autopsy report, the cause of death was listed as “complications of hepatic cirrhosis,” with muscular dystrophy, aspiration pneumonia, and COVID-19 listed as other significant conditions. The manner of death was listed as natural.
Discussion
These autopsies are the first to be performed in decedents who have tested positive for SARS-CoV-2 in Oklahoma, and to our knowledge this manuscript represents the first published report of pathologic findings based on complete autopsies in COVID-19 decedents in the English literature. Our observations add to the sparse worldwide pathology literature on this entity. The older of the two decedents had multiple comorbidities and died of DAD, which is an expected pathologic finding in fatal viral infection. The other decedent had progressive myotonic muscular dystrophy and died of acute bacterial bronchopneumonia likely caused by aspiration. Therefore, this patient likely died with COVID-19, not from COVID-19. These cases illustrate the challenges that pathologists and the medical community at large will face in determining the cause of death in decedents who test positive for SARS-CoV-2. Some findings will represent true virus-related pathology, while others will reflect superimposed processes or unrelated illnesses. Separating bona fide virus-related pathology from potential confounders and red herrings in these complex scenarios will benefit from the experience and expertise of forensic pathologists and pulmonary pathologists.
Although initial reports based on postmortem core biopsies have pointed to DAD as the underlying pathology in cases of COVID-19, complete autopsies offer some advantages over more limited samples. First, autopsies allow study of multiple organs and procurement of adequate tissue for diagnosis and research. Second, because they allow adequate sampling of affected tissues, they minimize the chances of missing an accurate diagnosis due to sampling error. There is an emerging conversation around myocardial injury in COVID-19 patients, and many in the medical community are wondering whether tissue examination will reveal evidence of myocarditis in these patients. With the caveat that ours is a very limited sample based only on two fatal cases, we have not observed evidence of myocarditis in these decedents. We have also not observed evidence of potentially reversible pathologic findings in the lungs, such as mucus plugs, tissue eosinophilia, or organizing pneumonia.
Since the beginning of COVID-19 circulation in Oklahoma (early March 2020), we have performed swabs on decedents where the history included symptoms suspicious for COVID-19, such as cough, fever, and shortness of breath. We are fortunate to have capabilities to perform full body postmortem radiography prior to autopsy and perform the autopsies in a safe facility with appropriate PPE. However, we recognize that most hospital autopsy suites and medical examiner facilities are not equipped to handle a highly infectious disease. As we are continuing to receive unattended decedents with a history of suspected COVID-19, we are trying to increase the efficiency of our work flow and decrease the risk of exposure by utilizing the Toshiba computed tomography scanner more frequently and often using core needle biopsy tools to retrieve lung specimens, rather than opening the body. We will be collecting and analyzing COVID-19 information to help understand trends, triage decedents appropriately, and give families an accurate and specific cause of death. We are collecting specimens for potential further research, including the development of specific stains for diagnosis, analysis of the cellular characteristics of the virally infected tissue, and the profiles of the immune response to the viral infection, which may prove to be valuable.
We acknowledge the limitations of this report. Our observations are based on only two cases, and it is likely that a wider spectrum of disease will emerge as pathologic findings in larger numbers of cases are reported. It is important to stress that ARDS develops only in a subset of severely ill patients with COVID-19. It is likely, therefore, that the tissue response is different in individuals with COVID-19 who are asymptomatic or have only mild symptoms. Because we are in the midst of a pandemic, we are answering the call to action and reporting these findings to understand the pathology of COVID-19. We express our sincere gratitude to all the people who are working tirelessly during this crisis, and we hope that our description of the precautions taken during these autopsies will be helpful to others.
Acknowledgments
We would like to acknowledge the staff of the Office of the Chief Medical Examiner, Oklahoma City, OK, for responding to the call for the COVID-19 pandemic.
References
- 1. World Health Organization. Coronavirus disease (COVID-2019) situation reports https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed April 3, 2020.
- 2. Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153:420-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;396:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukhopadhyay S, Philip AT, Stoppacher R. Pathologic findings in novel influenza A (H1N1) virus (“Swine Flu”) infection: contrasting clinical manifestations and lung pathology in two fatal cases. Am J Clin Pathol. 2010;133:380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukhopadhyay S, Mehrad M, Dammert P, et al. Lung biopsy findings in severe pulmonary illness associated with E-cigarette use (vaping). Am J Clin Pathol. 2020;153:30-39. [DOI] [PubMed] [Google Scholar]
- 6. Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381:1780-1781. [DOI] [PubMed] [Google Scholar]
- 7. Diffuse alveolar damage. In: Katzenstein A-LA. ed. Katzenstein and Askin’s Surgical Pathology of Non-Neoplastic Lung Disease. 4th ed. Philadelphia, PA: WB Saunders; 2006:29-31. [Google Scholar]
- 8. Parambil JG, Myers JL, Aubry MC, et al. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest. 2007;132:50-57. [DOI] [PubMed] [Google Scholar]
- 9. Mukhopadhyay S, Parambil JG. Acute interstitial pneumonia (AIP): relationship to Hamman-Rich syndrome, diffuse alveolar damage (DAD), and acute respiratory distress syndrome (ARDS). Semin Respir Crit Care Med. 2012;33:476-485. [DOI] [PubMed] [Google Scholar]
- 10. Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer: special report. J Thorac Oncol. 2020;DOI: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;DOI: 10.1016/S2213-2600(20)30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Preprints. 2020:2020030311. doi: 10.20944/preprints202003.0311.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson GF, Clark SC; National Association of Medical Examiners Forensic autopsy performance standards. Am J Forensic Med Pathol. 2006;27:200-225. [DOI] [PubMed] [Google Scholar]
- 14. Collins KA. Autopsy Performance and Reporting. 3rd ed. Northfield, IL: CAP Press; 2017. [Google Scholar]
- 15. Hanley B, Lucas SB, Youd E, et al. J Clin Pathol. 2020. DOI: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 16. Iwen PC, Stiles KL, Pentella MA. Safety considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Clin Pathol. 2020. DOI: 10.1093/AJCP/AQAA047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Center for Disease Control and Prevention: National Vital Statistics System. Guidance for certifying deaths due to coronavirus disease 2019 (COVID-19) Available at https://www.cdc.gov/nchs/nvss/covid-19.htm. Accessed April 7, 2020.