Abstract
A novel coronavirus, SARS-CoV-2, emerged in December 2019, leading within a few months to a global pandemic. COVID-19, the disease caused by this highly contagious virus, can have serious health consequences, though risks of complications are highly age-dependent. Rates of hospitalization and death are less than 0.1% in children, but increase to 10% or more in older people. Moreover, at all ages, men are more likely than women to suffer serious consequences from COVID-19. These patterns are familiar to the geroscience community. The effects of age and sex on mortality rates from COVID-19 mirror the effects of aging on almost all major causes of mortality. These similarities are explored here, and underscore the need to consider the role of basic biological mechanisms of aging on potential treatment and outcomes of COVID-19.
Keywords: Biology of aging, Immunosenescence, Mortality, SARS-CoV-2, COVID-19
In December 2019, a new coronavirus, now known as SARS-CoV-2, emerged in Wuhan, China. This highly infectious virus leads to Coronavirus Disease 2019, or COVID-19, and has given rise to a global pandemic. As of April 28, 2020, there had been over 2M confirmed cases, and over 200,000 deaths due to the disease (1).
The COVID-19 pandemic is unlike anything we have experienced in our lives, but there are some patterns to this catastrophic disease that are very familiar to those engaged in aging research. Epidemiological analysis of cases and fatalities has identified numerous risk factors both for mortality from complications arising from COVID-19. Specifically, the risk of serious complications and mortality increases dramatically at later ages (2–4), and is higher in males than in females at all ages (2,4). As a recent editorial in this journal noted, COVID-19 could be considered a disease of older people (5).
The effect of age on risk of death due to COVID-19 increases exponentially with age. Case fatality rates (the risk of dying among those diagnosed with COVID-19) are on the order of 0.1% (1 in 1,000) in children, but as high as 14.8% in older individuals in China (3) and even higher in older Italians (4) and Americans (6). This striking increase—over 100-fold—in risk over the life span, and the fact that men are more likely than women to die of COVID-19, has caught the attention of the public.
To fully understand the implications of these patterns of increased risk with age and in men versus women, we need to place them in the context of how age and sex affect the risk of chronic diseases. It has long been known not only that the risk of dying increases exponentially over the course of adult life, but also that this exponential increase holds for individual causes of death (7). We also observe a male bias in total mortality rates (8), and in death due to specific diseases (9).
In this note, I consider age and sex as epidemiological risk factors for COVID-19 in the context of other age-specific diseases, and suggest some insights that we might gain from this comparison.
Materials and Methods
COVID-19 Infection and Mortality
Data on COVID-19 cases and deaths were obtained for outbreaks in China (3), Italy (4), and New York City (10). The data for Italy provided estimates for case fatality rates (CFR). Chinese data included corrections for several biases to provide a more accurate estimate of “Infection Fatality Ratio” (IFR). New York City (10) provides measures in terms of deaths per 100,000 in the population, which measures the risk of dying of COVID-19 for anyone in a particular age class, regardless of infection status. Age classes for Italy and China were calculated in 10-year intervals. In the plots for these sources, age shown is the youngest age in the interval. For New York City, there are fewer, larger age classes. For these data, death rates are plotted as a function of the mean age within each age class (eg, for age 0–17 years, the age shown is 8.5 years).
Age-Specific Disease Mortality Rates
Information on age- and sex-specific death rates (per 100,000 population in specified group) for the United States were obtained from the CDC’s 2017 National Vital Statistics Report (11). Death rates are typically averaged across 5-year intervals, and in the plots, age shown is the youngest age in the interval.
Data Analysis
All data analysis was carried out using the R statistical package. Mortality rate doubling time (MRDT) is calculated as the time in years (Δx) that it would take for a mortality rate at age x, µx, to double, such that µx+Δx = 2µx. Adult mortality rates increase exponentially with age, following a Gompertz trajectory, µx = αeβx, so on a semi-log plot of log10(mortality) versus age, where the intercept is given by log(α), and the slope is equal to β, we can calculate the MRDT as Δx = log10 (2)/β. The 95% confidence intervals for MRDT are provided as direct derivations of the confidence intervals for β, determined using the confint function in R.
To calculate β, we used the lm function in R to fit least-squares regression lines to plots of log10(mortality) versus age over the range of mid-adult life (ages 20–70 years), during which time we see consistently linear patterns of log-transformed mortality versus age. Where fitted lines are shown, they are drawn over the range of ages that were used in the regression model.
Results
Age-Specific Mortality Rates
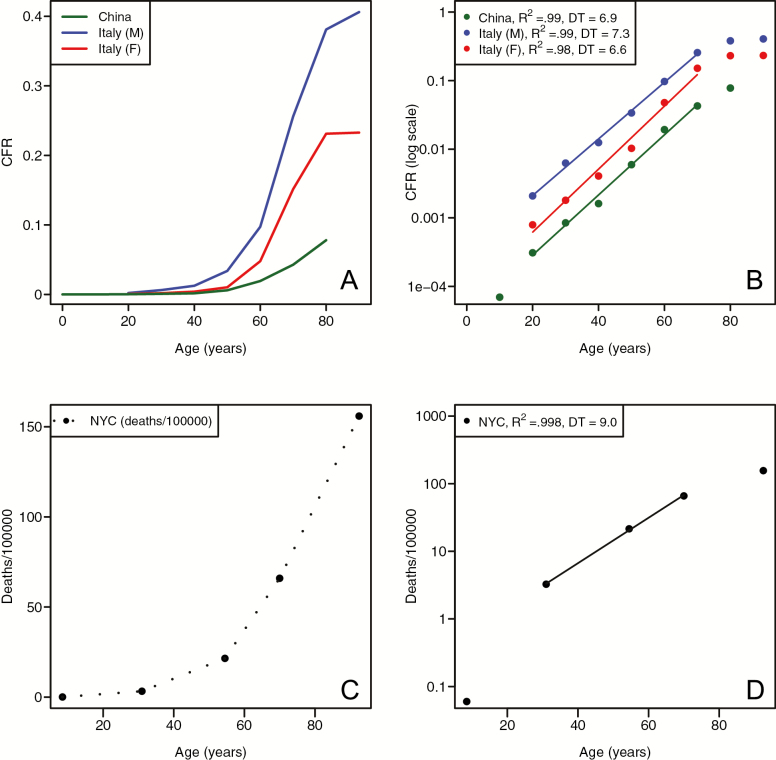
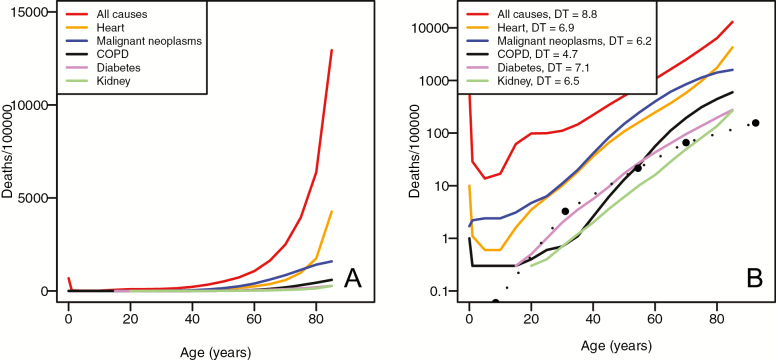
COVID-19 fatality rates, whether calculated on a per-case basis (CFR in Italy, IFR in China), or in terms of deaths per 100,000 in the population (for New York City), all exhibit a clear pattern of exponential increase with age (Figure 1), for both males and females. We also observe male-biased mortality at all ages, a pattern that is clearly seen when CFR is plotted on a semi-log scale (Figure 1B). The pattern of age-related severity in outcomes of COVID-19 is mirrored in the overall mortality rate as a function of age. As previous studies have shown, CDC vital rate data (11) show an exponential increase with age for all-cause and cause-specific mortality (Figure 2).
Figure 1.
Age-related risk of death from COVID-19. (A) Case fatality rate for COVID-19 in China and Italy, plotted on an absolute scale. (B) Case fatality rate for COVID-19 in China and Italy, plotted on a log10 scale. Straight lines represent fitted values from least-squares fitted regression from ages 20–70. (C) Deaths per 100,000 from COVID-19 in New York City plotted on an absolute scale. (D) Deaths per 100,000 from COVID-19 in New York City plotted on a log10 scale. The straight line is the fitted linear regression.
Figure 2.
Age-related risk of mortality from multiple causes. (A) Deaths per 100,000 from specific causes, as well as all-cause mortality, for both sexes in the United States, 2017. (B) Deaths per 100,000 from specific causes and all-cause mortality, plotted on a log10 scale. The numbers in the legend refer to the mortality rate doubling time (MRDT). The dotted line is deaths per 100,000 from COVID-19 in New York City. The New York City MRDT = 9.0 years (95% confidence interval [CI] = 6.6–13.9). For specific causes of mortality, MRDT 95% CI: all causes (8.6–9.1); heart disease (6.4–7.6); malignant neoplasms (5.7–7.0); Chronic obstructive pulmonary disease (COPD) (4.5–5.0); diabetes (6.7–7.5); and kidney disease (6.4–6.7).
COVID-19 and Other Sources of Mortality
Over the range of ages for which we calculate the slopes of log10(CFR) versus age, we see a consistently strong linear relationship, with R2 ≥ 0.98 (Figure 1B and D). For all-cause mortality, mortality rates in the United States appear to double approximately every 9 years (Figure 2). For the subset of age-related causes of mortality shown in Figure 2, we see MRDTs in years from a range of 4.7 (for chronic obstructive pulmonary disease [COPD], 95% confidence interval [CI] = 4.5–5.0) to 7.1 (for diabetes, 95% CI = 6.7–7.5). For COVID-19 CFR, MRDTs were found to be 6.6 (Italian females, 95% CI = 5.6–7.9), 7.3 (Italian males, 95% CI = 6.8–7.9), and 6.9 (China, 95% CI = 6.1–7.8). The New York data, calculated as death rate per 100,000, exhibit a shallower slope, leading to a doubling time of 9 years.
Sex Differences
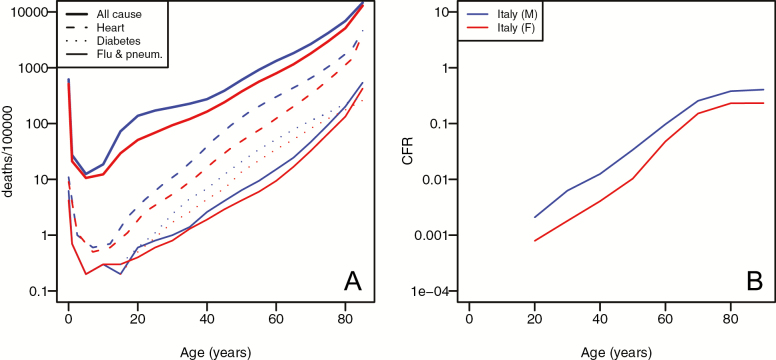
For all major causes of mortality, males tend to have a higher risk of dying at all ages (Figure 3). This pattern is seen for risk of death from COVID-19, and the scale of difference between sexes is consistent with that seen for more common causes of mortality. The Y axis in Figure 3A and B shows log-transformed mortality, with tick marks separating 10-fold differences. Thus, although Figure 3A shows deaths per 100,000 individuals, and Figure 3B shows CFR, an equal distance between males and females at a given age would suggest an equally increased risk for males. The data from Italy show a clear male bias in CFR at all ages above 30, with CFR between 1.7 and 2.6 times greater in males than in females. Although the bias is seen at all ages, there appears to be a reduction in the relative risk for males at later ages. The degree of male bias appears comparable to what we see for heart disease, but greater than that seen for death due to diabetes, or combined influenza and pneumonia (Figure 3A).
Figure 3.
Sex-differences in age-related mortality. (A) Age-specific deaths per 100,000 population in the United States in 2017 for males (blue) and females (red). (B) Age-specific case fatality rate for COVID-19 for men (blue) and women (red) in Italy in 2020.
Discussion
Scientists (2,3,12) and the popular press have made much of the fact that age is the greatest risk factor for COVID-19. The dynamics of age-specific mortality, including both the exponential increase with age and the similar doubling times, as well as the sex-bias in mortality seen for COVID-19, mirror other major causes of mortality.
A possible explanation for this age-related increase in risk of mortality could be the age-related decline in immune and inflammatory responses, which can lead, in turn, to a cytokine storm (13). This is a likely contributing factor, given that in general, age leads to a decline in adaptive immune function, and an increase in proinflammatory activity (14). However, this is unlikely to be the complete story, for at least two reasons: First, virtually all physiological systems and cellular processes decline with age, not limited to immune and inflammatory pathways (15), and second, there is a diversity of pre-existing conditions that appear to increase the risk of COVID-19, including hypertension, coronary heart disease, diabetes (16), and likely high body mass index (17). As people age, the average number of comorbid conditions steadily increases, and the greater the number of comorbid conditions, the lower the life expectancy (18).
The geroscience concept holds that treatments that target the underlying molecular pathways of aging biology that shape this physiological decline could, in turn, decrease risk for many different age-related diseases and improve outcomes in those that succumb to disease (19,20). In light of the coronavirus pandemic, researchers have also suggested that putative ‘geroprotective’ treatments, such as rapamycin or senolytics, might be used to treat or prevent COVID-19 (21,22). For example, it would be of great interest to understand the role of widely-known aging-related molecular pathways—mTOR signaling, IIS signaling, cell senescence—in the response to coronavirus infection and recovery from COVID-19. Geroscience has seen major strategic advances for the discovery of treatments to increase health span in laboratory organisms (23), and there is some evidence for the efficacy of longevity-derived interventions in treating acute symptoms of age-related disease at least in the lab (24,25). There can be no doubt as to the interaction of aging biology and COVID-19 mortality, and its seems likely that a better understanding of this relationship would inform not only the biology of viral susceptibility and progression to life-threatening outcomes, but also how aging creates a pernicious environment that augments the negative consequence of viral load.
Funding
The author was supported in part by grants from the National Institutes of Health (R01 AG049494 and U19 AG057377).
Acknowledgments
Mitchell Lee, Alex Genshaft and Rozalyn Anderson provided helpful comments on an earlier draft of this manuscript. Caleb Finch identified a seminal early reference. Leslie Kean, Carly Ziegler, and Alex Genshaft provided essential support in the midst of the coronavirus pandemic.
Conflict of Interest
None reported.
References
- 1. Coronavirus Resource Center, Johns Hopkins University. https://coronavirus.jhu.edu/; 2020. Accessed April 2, 2020. [Google Scholar]
- 2. Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis [published online ahead of print April 28, 2020]. Lancet Infect Dis. 2020. doi: 10.1016/S1473-3099(20)30257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Istituto Superiore di Sanità R. Epidemia COVID-19. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-aprile-2020.pdf; 2020. Accessed April 2, 2020.
- 5. Le Couteur DG, Anderson RM, Newman AB. COVID-19 through the lens of gerontology. J Gerontol A Biol Sci Med Sci. 2020.doi: 10.1093/gerona/glaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3external [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones HB. A special consideration of the aging process, disease, and life expectancy. Adv Biol Med Phys. 1956;4:281–337. doi: 10.1016/b978-1-4832-3110-5.50012-1 [DOI] [PubMed] [Google Scholar]
- 8. Austad SN. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1 [DOI] [PubMed] [Google Scholar]
- 9. Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. 1984;5:433–458. doi: 10.1146/annurev.pu.05.050184.002245 [DOI] [PubMed] [Google Scholar]
- 10. NYC Department of Health and Mental Hygiene. Coronavirus-data.https://github.com/nychealth; 2020. Accessed April 2, 2020.
- 11. Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: final data for 2017.https://stacks.cdc.gov/view/cdc/79486; 2019.
- 12. Spiegelhalter D. How much ‘normal’ risk does Covid represent.https://medium.com/wintoncentre/how-much-normal-risk-does-covid-represent-4539118e1196; 2020.
- 13. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print April 28, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Márquez EJ, Chung CH, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan DH, Ravussin E, Heymsfield S et al. COVID 19 and the patient with obesity–the Editors speak out. Obesity. 2020;28:847. doi: 10.1002/oby.22808 [DOI] [PMC free article] [PubMed]
- 18. DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, Anderson GF. Multiple chronic conditions and life expectancy: a life table analysis. Med Care. 2014;52:688–694. doi: 10.1097/MLR.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 19. Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015;16:640–647. doi: 10.1016/j.jamda.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhavoronkov A. Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections [published online ahead of print March 31, 2020]. Aging (Albany NY). 2020;12. doi: 10.18632/aging.102988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection [published online ahead of print March 30, 2020]? Aging (Albany NY). 2020;12 . doi: 10.18632/aging.103001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myers A, Lithgow GJ. Drugs that target aging: how do we discover them? Expert Opin Drug Discov. 2019;14:541–548. doi: 10.1080/17460441.2019.1597049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson SC, Yanos ME, Kayser EB, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nogueira-Recalde U, Lorenzo-Gómez I, Blanco FJ, et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019;45:588–605. doi: 10.1016/j.ebiom.2019.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]