Abstract
Delayed neurodevelopment is a common outcome in perinatally HIV-infected children. Our aim was to assess the intellectual profile of our cohort, considering both the infection and socio-environmental related variables. A cross-sectional cohort study was undertaken at seven major hospitals in Spain belonging to the CoRISpeS cohort (n = 97). Patients were followed up according to a standard protocol. Intellectual measures, psychosocial profile and HIV infection-related data have been analysed. The average patient age was 15 years. The median CD4 cell percentage was 35% (1,59). Viral load was undetectable in 80% of the patients and 27% were on AIDS category; 38% of whom had encephalopathy. The average composite score of both crystallized intelligence (CI) and intelligence quotient (IQ) for the cohort was lower than that of the general population (p < 0.001). Results revealed a significant difference of 38% between crystallized and fluid intelligence. There was a clear association between IQ and age of diagnosis (p = 0.022); CI and CDC classification (p = 0.035), CD4 count (p = 0.011) and CD4 nadir (p = 0.001). Higher parental education was associated with better performance across all intelligence scales (p < 0.002). A regression model showed that CI was influenced by the academic level of caregivers (p = 0.002), age at start of cART (p = 0.050) and primary language (p = 0.058). Findings revealed significant differences in verbal and non-verbal intellectual scales resulting in a misleading IQ Composite score. Crystallized intelligence demonstrated the highest level of impairment despite adequate treatment and good immunovirological status, while fluid intelligence results were average. Caregiver level of education was the strongest factor across all intelligence measures.
Keywords: Virology, Psychology, Cognitive psychology, Cognition, Learning and memory, Vertical transmission, IQ, HIV, Intelligence
Virology; Psychology; Cognitive psychology; Cognition; Learning and memory; Vertical transmission; IQ; HIV; Intelligence
1. Introduction
Since the introduction of highly active antiretroviral therapy in 1996, there has been a reduction in the mortality associated with HIV infection in children (Sánchez et al., 2003), with improvements to long-term growth and immunological health (Picat et al., 2013). Upon advancement to a chronic infection, new questions have emerged regarding the effects of long-term infection in the combination antiretroviral therapy (cART) era thereafter. Namely concerns over how the neurodevelopmental outcomes of these patients will impact their future quality of life.
A consistent finding observed in the majority of studies carried out on HIV-infected children was delayed neurodevelopment (Benki-Nugent et al., 2015; Chase et al., 2000; Kerr et al., 2014). The intellectual profile has been studied in this population, with results often reflecting general intellectual functioning below the normative average in high rates. The effects of HIV infection can be difficult to assess. The populations included in these studies were heterogeneous with a necessity to consider a predisposition to certain social risks, such as low socioeconomic status (SES), children reared by their grandparents or extended family, and inaccessibility to health services (Coscia et al., 2001; Medin et al., 2016; Puthanakit et al., 2010). Potential confounders for the analysis, such as psychiatric and behavioural problems, were also common in HIV infected children and youth (Mellins et al., 2006; Nozyce et al., 2006). Both the early exposure to the effects of the virus in an immature central nervous system (Sirois et al., 2013), as well as the long term impact during central nervous system development in childhood and adolescence, make these children a particularly vulnerable population. Previous reports have also suggested that educational problems were common in perinatally HIV-infected (PHIV) youth regardless of virologic suppression (García-Navarro et al., 2014; Puthanakit et al., 2010).
The purpose of this study was to assess the intellectual profile of our cohort of children and adolescents with perinatally-acquired HIV infection, considering their social-environmental characteristics and those related to the infection.
2. Materials and methods
2.1. Participants and procedures
A cross-sectional cohort study was carried out. PHIV subjects and their parents/legal guardians were invited to participate during their follow-up visits between May 2012 and May 2014. The study was performed at seven major hospitals included in the Spanish National Cohort of HIV-Infected children and adolescents (CoRISpeS). A complete standard protocol which had been previously approved by the ethics committee of the hospitals was conducted by the neuropsychologist and psychologist of the team, including intellectual measures and a semi-structured interview model to determine psychosocial profile. HIV-related data were obtained from clinical records as well as gathering self-reported adherence to antiretroviral therapy (ART). All participants were required to provide written assent/consent for this research (together with caregiver consent for participants aged under 18 years old).
2.2. Instruments
For a general intellectual screening a Spanish version of the Kaufman Brief Intelligence Test (K-BIT) (Kaufman and Kaufman, 1997) was administered on each subject. This is a standardised test that is widely utilised in clinical and research settings to obtain an efficient intelligence estimate. K-BIT scores correlate moderately well with other intelligence methods (correlations reported between K-BIT Composite and Wechsler Full-Scale IQ scores range from .61 to .89). Nevertheless, K-BIT and longer intelligence testing methods such as Wechsler scores are not interchangeable (Strauss et al., 2006).
The K-BIT provides both verbal and non-verbal scales, from which an overall Intelligence Quotient (IQ) Composite score is computed. Large scale variations between them, provided in the manual, highlight a discrepancy in the development of both abilities. This could be related to the presence of neurocognitive deficits or suggestive of structural damage. In such cases IQ value should not be considered with diagnostic purposes nor should it determine the intellectual capacity of an individual. The interpretive framework of the K-BIT is based on the Cattell model of intelligence, with the verbal scale as Crystallized intelligence (CI) and the non-verbal scale as Fluid intelligence (FI). To ensure continuity of the proposed terminology, we are going to use these terms indistinctly throughout the paper. The verbal scale measures are related to knowledge of the language; the formation of verbal concepts and information flow. The non-verbal scale assesses the capacity of reasoning and problem-solving based on visual analogies and matrices that rely on pattern completion.
The test provides Spanish normative data for people ranging from four to 90 years old, matched by age.
Non-native patients were excluded if they were not fluent in Spanish.
The psychosocial aspects registered in the semi-structured interview included; caregiver education, primary language, number of repeated grades, and reported academic performance. The primary language of the gipsy population in our cohort was registered as different from the typical European Spanish population owing to their use of the caló dialect.
2.3. Data analysis
Raw scores were converted by means of tables provided in the K-BIT handbook to age-based standard scores (Mean = 100, SD = 15) for both CI and FI scales and the total IQ scale. For statistical purposes, we classified the scores in three categories simplified from the manual: low (40–79), low-average (80–89), average or higher (≥90).
Data were analysed using SPSS 18.0 (SPSS Inc., Chicago, IL). General descriptive statistics were used to obtain the profile of the sample. Sociodemographic, clinical and immunovirological quantitative and categorical variables were described with medians/ranges and absolute or relative frequencies, respectively. The one-sample t-test was used to compare the means of intellectual performance of our patients with that of the general population. In addition, we subdivided the sample into: 1) Two groups depending on the patient's age (G1: 5–16, G2: 17–23) to study if differences existed between school-age and young adults groups; 2) Two groups, split by the presence of large differences provided in the manual between CI and FI scores (0.05 level of significance) to compare the intellectual profile in patients with homogeneous and discordant results. Chi-square tests were used to examine the association between categorical HIV-related and intelligence variables. Spearman's correlation was used to analyse the association between quantitative intelligence measures and educational variables. Kruskal-Wallis tests were used to assess differences in quantitative HIV-related variables and categorised intelligence variables. Univariate and multivariate linear regression models were constructed to study the relationship between intellectual measures and clinical and psychosocial variables. To assess the effect of potential confounding variables, we performed an analysis adjusting for CD4 nadir, age at start of cART, adherence, primary language and parents' level of education. Age and gender were also included as covariates in a previous model. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Sociodemographic characteristics
Ninety-seven patients followed in the centers agreed to participate in the study (Table 1). The mean age was 15 years old; 66% of whom were female. Of the patients and their families who participated, 63% were Caucasian. At the time of the study 85% were still attending school and 70% had repeated one grade (31%) or more (39%).
Table 1.
Characteristics of 97 HIV-infected patients' sample.
| Mean age (years, SD) | 15 (4.51) | |
| 5–16 years (%, n) | 60% (58) | |
| 17–23 years (%, n) | 40% (39) | |
| Sociodemographic characteristics (%, n) | ||
| Female Gender | 66% (64) | |
| Ethnicity | ||
| Caucasian | 64% (62) | |
| Sub Saharan Africa | 11% (11) | |
| South America | 8% (8) | |
| Other | 17% (16) | |
| Family structure | ||
| Biologic parent/s | 49% (47) | |
| Grandparents or extended family | 16% (15) | |
| Foster care or adoption | 29% (28) | |
| Other | 7% (7) | |
| Parental education (most educated caregiver) | ||
| No education/Read and write | 17% (16) | |
| Elementary/Secondary school | 31% (30) | |
| High school or higher degrees | 47% (46) | |
| Unknown | 5% (5) | |
| Attending at school | 85% (82) | |
| Number of repeated grades | ||
| 0 | 27% (26) | |
| 1 | 28% (27) | |
| ≥2 | 35% (34) | |
| No data | 10% (10) | |
| Reported academic performance | ||
| Good | 29% (28) | |
| Poor | 41% (40) | |
| With difficulties | 20% (19) | |
| No data | 10% (10) | |
| Immunological and virological characteristics at study entry | ||
| AIDS (%, n) | 27% (26) | |
| HIV-Encephalopathy (%, n) | 10% (10) | |
| Hepatitis C virus (%, n) | 6% (6) | |
| Median CD4 cells/mm3 (IQR) | 770 (570, 1004) | |
| Median CD4 % (IQR) | 35 (30, 40) | |
| Median CD4/CD8 (IQR) | 1.00 (0.7, 1.40) | |
| Viral load <50 cop/ml (%, n) | 80% (78) | |
| Median Nadir CD4 cells/mm3 (IQR) | 360 (185, 463) | |
| Median Nadir CD4 % (IQR) | 15% (10, 22) | |
| Median age at diagnosis (IQR) | 0.46 (0, 12.24) | |
| Antiretroviral treatment (years, IQR) | ||
| Median age at the start of ART (IQR) | 1.34 (0.01, 14) | |
| Median age at the start of cART (IQR) | 2.62 (0.01, 15) | |
| Time of treatment with ART (IQR) | 12.45 (0.98, 21) | |
| Time of treatment with cART (IQR) | 11.10 (0.51, 17) | |
| Median number of cART regimens (IQR) | 4 (0, 12) | |
| Current treatment situation (%, n) | ||
| Patients without ART | 6% (6) | |
| Patients with cART | 89% (86) | |
| Good adherence to treatment | 86% (78) | |
Abbreviations: SD: Standard Deviation, AIDS: Acquired Immune Deficiency Syndrome, HIV: Human Immunodeficiency Virus, CD4: CD4 T lymphocytes, IQR: Interquartile range, ART: Antiretroviral therapy, cART: Combination Antiretroviral Therapy.
3.2. HIV-related characteristics
Table 1 shows immunological, virological and treatment data. The median age at diagnosis of HIV infection was 0.5 years old (IQR: 0.2, 2.4). At the initial stages, the majority of children exhibited a good clinical and immunovirological situation. The median CD4 cell percentage was 35% (IQR: 30, 40). Viral load was undetectable in 80% of the patients.
Regarding ART, 89% were on cART and only 6% of the patients were not on therapy. The remaining were on monotherapy or dual therapy. The most common combination was NRTI + NNRTI (42%), followed by NRTI + PI (40%). Patients displayed high level of adherence; 86% of them (≥95% of doses).
3.3. Intellectual abilities
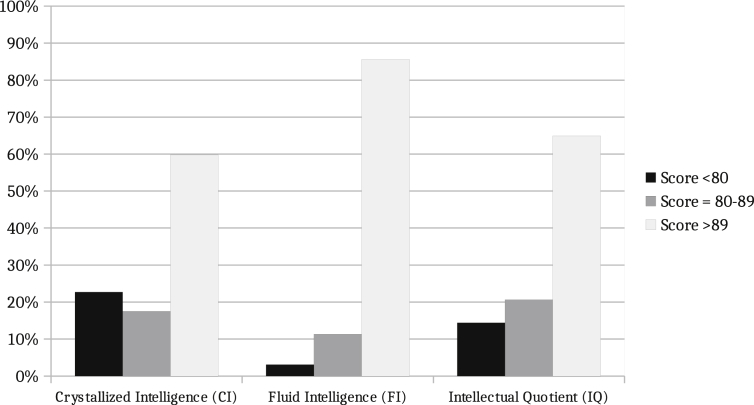
We initially performed the analysis of the whole sample. Children and adolescents' average performances were within the average range on the K-BIT classification for every intelligence measure (Figure 1). The mean of both CI and IQ Composite score for the cohort was however lower than that of the general population (one-sample t-test for means: CI t = -5.979, p < 0.001; IQ t = -6.025, p < 0.001). The results revealed no homogeneity between measures, with verbal scale more affected than non-verbal. According to the normative data provided in the manual, 38% of the patients obtained large differences between crystallized and fluid intelligence scales. For IQ Composite score, 65% of the cohort were within average range, 21% in the low-average range and 14% in the low range. We found poorer performance in CI, with 23% in the low range, while performance in FI remained within ranges close to the average, and only 3% of scores within the low range (<79). Eighty-six percent of the patients scored within average range of the FI scale.
Figure 1.
Intellectual performance of perinatally HIV-infected patients. Whole sample. Mean value (SD), CI: 91 (15); FI: 100 (10); IQ: 93 (12); Normative data: 100 (15). The mean of both CI and IQ Composite score was lower than in the general population (one-sample t-test for means: CI t = -5.979, p < 0.001; IQ t = -6.025, p < 0.001).
Secondly, we divided the sample depending on the existence of large differences between CI and FI with the aim of comparing performance in both groups. We found higher CI scores (p = 0.001) in the patients who obtained homogeneous measures. In contrast with this group which presented all the intelligence scales in the average range, the mean of CI and IQ scales was of low-average in the group with discordant measures (Table 2). No significant differences were found in FI.
Table 2.
Intellectual performance of perinatally HIV-infected subjects. Differences between patients who obtained homogeneous measures in both verbal and non-verbal scale and those who did not.
| Parameter | Cohort (n = 97) |
Homogeneous measures group (n = 60) |
Discordant measures group (n = 37) |
Homogeneous vs discordant measures group |
|
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean difference | p-valuea | |
| Crystallized intelligence | 91 (15) | 95 (12) | 84 (17) | 10.872 | 0.001 |
| Fluid intelligence | 100 (10) | 99 (10) | 101 (10) | -2.231 | 0.290 |
| IQ Composite score | 93 (12) | 94 (12) | 90 (13) | 4.816 | 0.058 |
Normative data, Mean value (SD): 100 (15). P-values less than 0.05 were considered statistically significant.
Independent Samples T Test, two-sided.
3.4. Age and gender
Findings revealed no association between age at evaluation and performance on the tests.
No significant differences were observed between school-aged patients and young adults except in fluid intelligence, though this was of no clinical significance (School-aged patient mean (SD) = 98 (10); Young adult patients mean (SD) = 103 (9); p = 0.025).
Males tended to score above females in CI, though this was neither statistically significant (p = 0.094) both in this instance and when we performed the multivariable analysis (p = 0.141).
3.5. Cognitive functioning and clinical and immunovirological HIV related variables
We did find a relationship between IQ and age at HIV-diagnosis (p = 0.022), though neither age at the beginning of cART (p = 0.286) nor total time on ART (p = 0.367) were associated.
Patients with better clinical CDC classification had higher performance levels in CI (p = 0.035). CI scores were greater in those patients with better immunological parameters including baseline CD4 total count (p = 0.011) and CD4 nadir (p = 0.001). There was no statistically significant relationship with age at start of cART, AIDS, encephalopathy nor Hepatitis C virus co-infection (Table 3). It is worth noting however that few patients were co-infected and scarce data were available for analysis.
Table 3.
Associations between intelligence measures and HIV clinical data.
| Crystallized Intelligence | Fluid Intelligence | IQ Composite Score | |
|---|---|---|---|
| aBaseline CD4 cells/mm3 | 0.011 | 0.712 | 0.794 |
| aCD4 nadir cells/mm3 | 0.001 | 0.149 | 0.110 |
| bUndetectable viral load | 0.529 | 0.640 | 0.978 |
| bCDC stage | 0.035 | 0.283 | 0.512 |
| bAIDS | 0.059 | 0.313 | 0.270 |
| bEncephalopathy | 0.077 | 0.130 | 0.181 |
| bHepatitis C virus | 0.067 | 0.835 | 0.689 |
| aAge at diagnosis | 0.115 | 0.889 | 0.022 |
| aAge at start of cART | 0.089 | 0.844 | 0.286 |
| bAdherence | 0.901 | 0.827 | 0.157 |
Abbreviations: CDC: U.S. Centers for Disease Control and Prevention classification system for HIV infection. AIDS: Acquired Immune Deficiency Syndrome. cART: Combination Antiretroviral Therapy. P-values less than 0.05 were considered statistically significant.
Kruskal-Wallis Test p-value.
Contingency tables, chi-square test p-value.
None of these virological or immunological parameters were associated with better FI scores (Table 3).
A significant difference still existed between CI and FI scores when we studied patients with undetectable viral load (p = 0.025). We did not find any relationship with any ART nor adherence.
3.6. Caregiver influence and school performance
Performance across all intelligence measures was better in those patients whose caregivers had achieved higher grades (p < 0.002) (Figure 2). Academic performance of the patient and the number of grades repeated at school were principally correlated with the CI and IQ score, as well as with FI in the case of patients' academic performance (Table 4).
Figure 2.
Intellectual performance of PHIV patients depending on their caregiver's level of schooling. Performance across all intelligence measures was better in those patients whose caregivers had achieved higher grades (p < 0.002).
Table 4.
Relationship between intelligence measures and education-related variables.
| Crystallized Intelligence |
Fluid Intelligence |
Intelligence Quotient |
||||
|---|---|---|---|---|---|---|
| Spearman correlation | p-value | Spearman correlation | p-value | Spearman correlation | p-value | |
| Academic performance | r = 0.354 | 0.001 | r = 0.289 | 0.007 | r = 0.361 | 0.001 |
| Repeated grades | r = -0.304 | 0.004 | r = -0.121 | 0.260 | r = -0.263 | 0.013 |
| Caregiver level of education | r = 0.458 | <0.001 | r = 0.313 | 0.002 | r = 0.459 | <0.001 |
P-values less than 0.05 were considered statistically significant.
Patients who had been brought up by grandparents or extended family scored slightly lower than patients who were raised by their own or adoptive parents in the three measures (mean T-score of CI/FI/IQ; Biological parents: 91/101/93; Grandparents and extended family: 85/97/88; Foster care or adoption: 95/99/94). This was not statistically significant however (CI p = 0.133; FI p = 0.259; IQ p = 0.267). Educational performance was lower in the group of grandparents and extended family compared to the other groups (p = 0.001).
Primary language was a factor in CI performance observing higher scores in those patients whose primary language was European Spanish (p = 0.002).
3.7. Multivariable analysis
An analysis was performed with which we adjusted variables that could potentially affect the performance in CI scale, both clinical and psychosocial. Results revealed the influence of: academic level of caregivers less than elementary school compared to secondary/higher education (-18.171 (Confidence Interval 95% -25.588–10.754, p < 0.001), elementary/compulsory education compared to secondary/higher education (-9.634 (CI 95% -15.759–3.509, p = 0.002), and age at start of cART (-0.741 years (CI 95% -1.485–0.002, p = 0.050). There was a trend in worse performance of those patients who didn't speak European Spanish as primary language (CI 95% -9.446–0.315, p = 0.058) (Table 5).
Table 5.
Associations with crystallized intelligence in the multivariate linear regression model. Whole sample.
| Parameter | B | p-value | Confidence Interval (95%) |
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Primary language | -9.446 | 0.058 | -19.207 | 0.315 |
| Adherence | -0.495 | 0.883 | -7.188 | 6.198 |
| aNo estudies (CG) | -18.171 | <0.001 | -25.588 | -10.754 |
| bElementary/Compulsory education (CG) | -9.634 | 0.002 | -15.759 | -3.509 |
| Age at start of cART | -0.741 | 0.050 | -1.485 | 0.002 |
| Nadir CD4 (%) | 0.195 | 0.201 | -0.106 | 0.497 |
Abbreviations: CG: Caregiver. cART: Combination Antiretroviral Therapy. CD4: CD4 T lymphocytes. P-values less than 0.05 were considered statistically significant.
Elementary school vs Secondary or higher education (Caregiver education).
Elementary/Compulsory education vs Secondary or higher (Caregiver education).
4. Discussion
The aim of this study was to examine global cognitive function in a HIV-infected cohort of children and adolescents, and to explore its relationship with both HIV- and environment-related factors. We concluded with the below findings. Firstly, the HIV-infected patients of our cohort showed good performance in fluid intelligence tests, related to their ability to think logically and solve problems in novel situations. This was however mediated by poorer performance in crystallized intelligence, which is influenced by school learning, cultural experience and previous knowledge acquired. The IQ Composite Score was in this case lower than that of the general population. The results then suggest that the environment where the children are raised is more of a factor than the effects produced by the virus in achieving optimal intellectual development. Given these findings, providing our patients with nurturing and supportive home environments and access to relevant information and culture-specific knowledge could play an important role in the intellectual development of the patient.
Studies which have reported prevalences of IQ in HIV-infected children are variable, as are their population characteristics. Results often reflect however general intellectual functioning below the normative average in high rates (Cohen et al., 2015; Musindo et al., 2018; Nozyce et al., 2006; Puthanakit et al., 2013; Smith et al., 2012; Thomaidis et al., 2010). Exceptions do exist as Tardieu et al. (1995) and Weber et al. (2017) show mean IQ within average range whilst studying patients of the French and German cohort. Similarly, Martin et al. (2006) found composite scores of intelligence in the average range when no brain abnormalities existed.
This variability in the results of other cohorts could be related to our findings. Patients with homogeneous performance showed average scores while patients with discordant measures obtained statistically significant lower IQ, consistently mediated by deficits in the verbal area. Our research could be further developed by studying if a certain structural basis underlies the discrepancy found between verbal and non-verbal abilities, similar to Martin et al.
Secondly, we observed that IQ, which is frequently used to assess the intelligence of the person, was not valid as a representative measure of capacity in our patients nor as a diagnostic tool, due to large differences between both CI and FI measures. This is a major finding as it could serve as a deterrent against using this sole measure in the categorisation of HIV-infected patient ability. The consideration of IQ alone could negatively impact the future of HIV-infected patients through a wrong prognosis, which could subsequently impact on academic results and future development. Indeed, earlier studies have found a keener deficit in the verbal area (Puthanakit et al., 2010), specifically in the expressive language skills (Wolters et al., 1997). Noteworthy research by Koekkoek et al. (2008) found general intellectual measures within the average range for their age through non-verbal tests. Puthanakit et al. (2010) described a decline in verbal IQ after 2 years following the initial assessment carried out through a longitudinal study, though this decline did not occur in the performance scale. These results were congruent to Wolters et al. (1997) who previously indicated the need for investigating the genesis of receptive and expressive language discrepancies, both of which had significantly declined after two years, despite ART. A recent review by Le Doaré et al. (2012) pointed out that deficits in language skills were most widely reported in children older than three. Simultaneously, language skill development occurred at a slower rate than in HIV-exposed uninfected children, irrespective of ART, when the effects of home circumstances or caregiver arrangements were taken into account. These findings point to the possibility of a marked and continuous deficit in this area, even if intellectual skills are preserved, as our results in FI seem to indicate. Given the significance of the formative years spent at school and the implication for future intellectual performance, career development and possibly successful integration into society, it is clear that greater knowledge and understanding of this area is needed.
Poor performance exhibited by these children could be affected by several factors related to the presence of the virus, family or socioeconomic characteristics. According to HIV characteristics, we have seen that CD4 count, CD4 nadir and CDC stage are implicated. These variables are linked to the progression of the disease. Literature has shown an inverse association between CD4 nadir and neurocognitive impairment, having been recently defined as a robust predictor in both combination ART and previous era (Heaton et al., 2011; Muñoz-Moreno et al., 2014; van den Dries et al., 2017). It has also been linked to regional white matter deficits across the brain (Hua et al., 2013). Previous studies suggest that increased HIV viral load has been related to poorer cognitive performance in HIV-infected children (Crowell et al., 2015; Ruel et al., 2012; Smith et al., 2012; Weber et al., 2017). We did not find evidence of this relationship though it is worth taking into consideration that the majority of our patients were undetectable.
With regard to non-HIV related variables, family situation played a significant role in determining the general profile, irrespective of cART and its adherence. This point was previously described in the introduction, and prior studies have detailed the importance of the caregivers and family characteristics in the child's development (Garvie et al., 2014; Puthanakit et al., 2010; Smith et al., 2012). The study found that having a primary language other than European Spanish was an influential factor, even though speaking fluent Spanish was a selection criteria for this study. This is important as CI is obtained through a verbal test that is closely connected to educational and cultural aspects. Thus, CI results may have been influenced by the poorer ability of HIV-infected children. After adjusting both the HIV-related and non-related variables in the regression model, the association remained. Youths whose caregivers had low level of education obtained lower score in CI than those whose caregivers had secondary or higher education (-18.171 (Confidence Interval 95% -25.588–10.754) when caregivers had less than elementary school; -9.634 (Confidence interval 95% -15.759–3.509) when they had elementary or compulsory education).
In our study, all intelligence measures were associated with the educational achievement of the caregiver, revealing better performance in those patients whose parents achieved higher grades compared to those with lower levels of education. We chose parental education as indicator of SES since it has been linked with cognitive functioning (Bradley and Corwyn, 2002; Christensen et al., 2014; McLoyd, 1998). These findings are encouraging, as they indicate a major bearing on environmental variables not related to the infection. These results must however be interpreted with the consideration of the absence of a healthy matched group, noting especially recent studies performed in HIV-infected children. Cohen et al. (2015) found poorer cognitive performance in PHIV children compared to SES-matched controls, a group who also scored lower than the national general group. Similarly, HIV-infected children in the PREDICT Neurodevelopmental Study displayed lower performance in every Wechsler scale (full-Scale IQ, verbal and manipulative) compared to perinatally exposed uninfected children when differences in the caregiver's education and low income between both groups didn't exist (Puthanakit et al., 2013).
We cannot however ignore the existence of subtle neurocognitive deficits that could be influencing a lower CI. In this study, we have solely analyzed intellectual performance, but our results suggest the presence of neurocognitive deficits. Almost 40% of our cohort presented poor performance in the verbal scale. Although environmental factors are most likely a factor in these results, large differences between FI and CI indicate the need for further neurocognitive assessment. Attentional and executive functions play a crucial role in learning, especially as children grow up and learning processes become more demanding and complex. Neurocognitive deficits are being increasingly studied and there is no consensus on the deficits present in HIV infection. Nevertheless, the prevalence of impaired profiles is frequent, and several studies pinpoint attention and executive processes as the main deficits in this population (Judd et al., 2016; Koekkoek et al., 2008; Laughton et al., 2013; Phillips et al., 2016). Further assessments with a more specific focus are needed, as neurocognitive processes underlying global functions may be more sensitive to developing mild difficulties.
The present study does have some limitations. Namely that our results belong to a cohort of individuals from a limited geographical region with specific characteristics. A further limitation is the lack of a similar non-HIV-infected control group to compare intellectual skills and educational achievement. Despite these limitations, our findings respond to an exhaustive study performed in our cohort, providing valuable data. It has been successful in assessing the influence of HIV-related variables versus environmental aspects, based on the comparison of a HIV-infected population with national standards.
In conclusion, our main finding is that IQ value doesn't act as a representative measure of the intellectual capacity in our cohort. Crystallized intelligence (more affected by culture, education and experience) was most affected despite adequate treatment and good immunovirological status, while fluid intelligence (related to their ability to think logically and solve problems in novel situations) results were similar to those of the general population. Remarkably, this deficit in the verbal area disappeared when the patients obtained homogeneous scores on both scales. To conclude, socioeconomic status measured through the caregiver level of education was the strongest factor related to all intelligence measures. The high prevalence of verbal deficits found in our cohort and their possible future implications, both clinical and related to the personal and professional life of the patients, is a clear indication that further study in the area is required.
Declarations
Author contribution statement
C García-Navarro: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
S Jimenez de Ory, C. Velo Higueras: Analyzed and interpreted the data; Wrote the paper.
B. Zamora: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
L Prieto, L Escosa-García, R Jurado-Barba, Dolores Falcón, David Moreno: Contributed reagents, materials, analysis tools or data.
JT Ramos, ML Navarro: Conceived and designed the experiments; Analyzed and interpreted the data.
MI González-Tomé: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Instituto de Salud Carlos III [FIS 15/00694] which is co-funded by Fondo Europeo de Desarrollo Regional(FEDER): “Una manera de hacer Europa”; Fundación para la Investigación y Prevención de SIDA en Espana (FIPSE) [24691/07,3608229/09, 240800/09, 361910/10 and 36-0910-1]. CorispeS is integrated in AIDS research network RIS (RED RIS) [RD16/0025/0017-ISCIII-FEDER,RD16/0025/0019-ISCIII-FEDER, RD16/0025/0024-ISCIII-FEDER and RIS EPICLIN-12/2012). CV was granted by Comunidad de Madrid and fondos FEDER (Ayudas para la contratación de ayudantes de Investigación y técnicos de laboratorio/Orden 2524/2016, de 1 de agosto, BOCM núm. 188, de 8 de agosto de 2016).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thank you to all the patients and their families for their collaboration and patience. We extent our thanks to Maite Fernández, Manuela Moya, Bene Cobacho, Teresa García, Carla Hallabrin and Michelle Fritz for their valuable help. Also to the members of NeuroCoRISpeS group.
Contributor Information
C. García-Navarro, Email: cristinagcn@gmail.com.
M.I. González-Tomé, Email: maribelgt73@gmail.com.
References
- Benki-Nugent S., Eshelman C., Wamalwa D., Langat A., Tapia K., Okinyi H.M., John-Stewart G. Correlates of age at attainment of developmental milestones in HIV-infected infants receiving early antiretroviral therapy. Pediatr. Infect. Dis. J. 2015;34(1):55–61. doi: 10.1097/INF.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Chase C., Ware J., Hittelman J., Blasini I., Smith R., Llorente A. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics. 2000;106(2):E25. doi: 10.1542/peds.106.2.e25. [DOI] [PubMed] [Google Scholar]
- Christensen D.L., Schieve L.A., Devine O., Drews-Botsch C. Socioeconomic status, child enrichment factors, and cognitive performance among preschool-age children: results from the Follow-Up of Growth and Development Experiences study. Res. Dev. Disabil. 2014;35(7):1789–1801. doi: 10.1016/j.ridd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Ter Stege J.A., Geurtsen G.J., Scherpbier H.J., Kuijpers T.W., Reiss P. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin. Infect. Dis. 2015;60(7):1111–1119. doi: 10.1093/cid/ciu1144. [DOI] [PubMed] [Google Scholar]
- Coscia J.M., Christensen B.K., Henry R.R., Wallston K., Radcliffe J., Rutstein R. Effects of home environment, socioeconomic status, and health status on cognitive functioning in children with HIV-1 infection. J. Pediatr. Psychol. 2001;26(6):321–329. doi: 10.1093/jpepsy/26.6.321. [DOI] [PubMed] [Google Scholar]
- Crowell C.S., Huo Y., Tassiopoulos K., Malee K.M., Yogev R., Hazra R., PACTG 219C Study Team and the Pediatric HIVAIDS Cohort Study (PHACS) Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015;29:295–304. doi: 10.1097/QAD.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Navarro C., García I., Medín G., Ramos-Amador J.T., Navarro-Gómez M., Mellado-Peña M.J., González-Tomé M.I. Psychosocial aspects in a cohort of vertically transmitted human immunodeficiency virus-infected adolescents. Enferm. Infecc. Microbiol. Clín. 2014;32(10):631–637. doi: 10.1016/j.eimc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Garvie P.A., Zeldow B., Malee K., Nichols S.L., Smith R.A., Wilkins M.L., Williams P.L., Pediatric HIVAIDS Cohort Study (PHACS) Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. Pediatr. Infect. Dis. J. 2014;33(9):e232–e238. doi: 10.1097/INF.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Boyle C.P., Harezlak J., Tate D.F., Yiannoutsos C.T., Cohen R., HIV Neuroimaging Consortium Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. Neuroimage Clin. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd A., Le Prevost M., Melvin D., Arenas-Pinto A., Parrott F., Winston A., Adolescents and Adults Living With Perinatal HIV (AALPHI) Steering Committee Cognitive function in young persons with and without perinatal HIV in the AALPHI cohort in England: role of non-HIV-related factors. Clin. Infect. Dis. 2016;63(10):1380–1387. doi: 10.1093/cid/ciw568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A., Kaufman N. TEA; Madrid: 1997. K-BIT Test Breve de Inteligencia [K-BIT. Kaufman Brief Intelligence Test] [Google Scholar]
- Kerr S.J., Puthanakit T., Vibol U., Aurpibul L., Vonthanak S., Kosalaraksa P., SEARCH 012 Study Team Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26(11):1327–1335. doi: 10.1080/09540121.2014.920949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek S., de Sonneville L.M., Wolfs T.F., Licht R., Geelen S.P. Neurocognitive function profile in HIV-infected school-age children. Eur. J. Paediatr. Neurol. 2008;12(4):290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Laughton B., Cornell M., Boivin M., Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J. Int. AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doaré K., Bland R., Newell M.L. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130(5):e1326–e1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- Martin S.C., Wolters P.L., Toledo-Tamula M.A., Zeichner S.L., Hazra R., Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev. Neuropsychol. 2006;30(2):633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Medin G., García-Navarro C., Navarro Gomez M., Ramos Amador J.T., Mellado M.J., Jimenez S. Disease disclosure, treatment adherence, and behavioural profile in a cohort of vertically acquired HIV-infected adolescents. NeuroCoRISpeS study. AIDS Care. 2016;28(1):124–130. doi: 10.1080/09540121.2015.1071768. [DOI] [PubMed] [Google Scholar]
- Mellins C.A., Brackis-Cott E., Dolezal C., Abrams E.J. Psychiatric disorders in youth with perinatally acquired human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 2006;25:432–437. doi: 10.1097/01.inf.0000217372.10385.2a. [DOI] [PubMed] [Google Scholar]
- Muñoz-Moreno J.A., Pérez-Álvarez N., Muñoz-Murillo A., Prats A., Garolera M., Jurado M.À. Classification models for neurocognitive impairment in HIV infection based on demographic and clinical variables. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0107625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musindo O., Bangirana P., Kigamwa P., Okoth R., Kumar M. Neurocognitive functioning of HIV positive children attending the comprehensive care clinic at Kenyatta national hospital: exploring neurocognitive deficits and psychosocial risk factors. AIDS Care. 2018 doi: 10.1080/09540121.2018.1426829. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozyce M.L., Lee S.S., Wiznia A., Nachman S., Mofenson L.M., Smith M.E. A behavioural and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- Picat M.Q., Lewis J., Musiime V., Prendergast A., Nathoo K., Kekitiinwa A. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in Sub-Saharan Africa: a cohort-based modelling study. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N., Amos T., Kuo C., Hoare J., Ipser J., Thomas K.G., Stein D.J. HIV-associated cognitive impairment in perinatally infected children: a meta-analysis. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanakit T., Ananworanich J., Vonthanak S., Kosalaraksa P., Hansudewechakul R., van der Lugt J. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr. Infect. Dis. J. 2013;32(5):501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanakit T., Aurpibul L., Louthrenoo O., Tapanya P., Nadsasarn R., Insee-ard S., Sirisanthana V. Poor cognitive functioning of school-aged children in Thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24(3):141–146. doi: 10.1089/apc.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel T.D., Boivin M.J., Boal H.E., Bangirana P., Charlebois E., Havlir D.V. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin. Infect. Dis. 2012;54(7):1001–1009. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez J.M., Ramos Amador J.T., Fernández de Miguel S., González Tomé M.I., Rojo Conejo P., Ferrnado Vivas P. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 2003;22(10):863–867. doi: 10.1097/01.inf.0000091282.70253.5f. [DOI] [PubMed] [Google Scholar]
- Sirois P.A., Huo Y., Williams P.L., Malee K., Garvie P.A., Kammerer B., Pediatric HIVAIDS Cohort Study Safety of perinatal exposure to antiretroviral medications: developmental outcomes in infants. Pediatr. Infect. Dis. J. 2013;32(6):648–655. doi: 10.1097/INF.0b013e318284129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Chernoff M., Williams P.L., Malee K.M., Sirois P.A., Kammerer B., Pediatric HIV/AIDS Cohort Study (PHACS) Team Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr. Infect. Dis. J. 2012;31(6):592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E.M.S., Spreen O. third ed. Oxford University Press; New York: 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- Tardieu M., Mayaux M.J., Seibel N., Funck-Brentano I., Straub E., Teglas J.P., Blanche S. Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J. Pediatr. 1995;126(3):375–379. doi: 10.1016/s0022-3476(95)70451-5. [DOI] [PubMed] [Google Scholar]
- Thomaidis L., Bertou G., Critselis E., Spoulou V., Kafetzis D.A., Theodoridou M. Cognitive and psychosocial development of HIV pediatric patients receiving highly active anti-retroviral therapy: a case-control study. BMC Pediatr. 2010;10:99. doi: 10.1186/1471-2431-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries L.W.J., Wagener M.N., Jiskoot L.C., Visser M., Robertson K.R., Adriani K.S., van Gorp E.C.M. Neurocognitive impairment in a chronically well-suppressed HIV-infected population: the Dutch TREVI cohort study. AIDS Patient Care STDS. 2017;31(8):329–334. doi: 10.1089/apc.2017.0038. [DOI] [PubMed] [Google Scholar]
- Weber V., Radeloff D., Reimers B., Salzmann-Manrique E., Bader P., Schwabe D., Königs C. Neurocognitive development in HIV-positive children is correlated with plasma viral loads in early childhood. Medicine (Baltim.) 2017;96(23) doi: 10.1097/MD.0000000000006867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters P.L., Brouwers P., Civitello L., Moss H.A. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS. 1997;11(9):1135–1144. doi: 10.1097/00002030-199709000-00009. [DOI] [PubMed] [Google Scholar]