Abstract
Due to the high cost of the cranberry extract, there have been several reported cases of adulteration. The aim of our study was to find markers to authenticate extracts or cranberry-based food supplements. Cranberry fruits from 7 countries, 17 cranberry extracts and 10 cranberry-based food supplements were analysed by UPLC-DAD-Orbitrap MS. Procyanidins were assessed by DMAC method. Anthocyanin fingerprint and epicatechin/catechin, procyanidin A2/total procyanidin and procyanidin/anthocyanin ratios were used as markers, and PCA carried out to check for similarity. Approximately 24% and 60% of the extracts and food supplements, respectively, differed significantly from the fruits. One seemed adulterated with Morus nigra and two with Hibiscus extract. Six food supplements were non-compliant and five contained mainly cyanidin-glucoside and cyanidin-rutinoside, suggesting adulteration with M. nigra extract. Only four products contained the procyanidin amount declared on the package, and only one provided the daily dose deemed effective for treating a urinary tract infection.
Keywords: Food science, Food analysis, Cranberry authentication, Anthocyanin fingerprint, Flavan-3-ols, PCA, UHPLC-Orbitrap MS, Quality markers
Food science; Food analysis; Cranberry authentication; Anthocyanin fingerprint; Flavan-3-ols; PCA; UHPLC-Orbitrap MS; Quality markers
1. Introduction
Cranberry juice and cranberry-based products have been used in many clinical investigations of recurrent urinary tract infection (UTI) treatment (Fu et al., 2017), and a positive effect has been reported in critical reviews and meta-analyses (Jepson and Craig, 2008; Luis et al., 2017). Nevertheless, the EFSA Panel stated that “the evidence provided is insufficient to establish a cause and effect relationship between the consumption of proanthocyanidins from cranberry (V. macrocarpon Aiton) fruit and defence against bacterial pathogens in the lower urinary tract” (EFSA, 2009, 2011).
Concerning non-pharmacological treatment of UTI, a multicentric randomized double blind study appears to show that the effective amount of proanthocyanidins (PAC) is 36 mg twice daily (Howell et al., 2010). Moreover, PAC with A-type linkages seem to be responsible for this activity. Indeed, research has proven that B-type PAC do not provide bacterial anti-adhesion activity in urine as do cranberry PAC with A-type linkages (Howell et al., 2005).
Despite this evidence, Jepson et al. (2012) suggested that methodological deficiency in the design of clinical studies and a lack of standardization of the cranberry-based products administered hamper a reliable assessment. Thus, insufficient qualitative and quantitative description of cranberry-based extracts contributes to the impossibility both of establishing the most effective cranberry preparation and of comparing clinical results. Addressing this issue will result in a more successful and wider use of cranberry-based products.
The PAC content of fresh cranberry ranges from 0.1–0.7%, while the most used commercial extracts are standardized to 15% and 33% PAC. This higher content has made cranberry one of the most expensive natural extracts and this, unfortunately, has led to fraudulent practice such as the adulteration of cranberry with similar, but much less expensive, berries or plant species. Thus, cases of adulteration with extract of grape seed, red peanut, pine bark, black bean, black rice, plum, mulberry and hibiscus have been reported (Boudesocque et al., 2013; Boudesocque-Delaye et al., 2016; Gafner et al., 2016; Lee, 2016; Navarro et al., 2014; Sánchez-Patán et al., 2012; Upton and Brendler, 2016; Wei et al., 2011).
Several analytical approaches to detect adulteration have been reported over the years such as the ratio of quinic to malic acid (Nagy and Wade, 1995), anthocyanin fingerprinting by LC (Lee, 2016), PAC determination by LC-MS (Jungfer et al., 2012; Tarascou et al., 2011), HPTLC-densitometry (Boudesocque-Delaye et al., 2018), DNA analysis (Yang et al., 2019) and, more recently, the evaluation of anti-adhesion activity. However, some of these have drawbacks. For example, quinic and malic acid can be used to determine the quality of the cranberry juice; but unfortunately, these acids are not present only in cranberry. The spectrophotometric determination of anthocyanin or PAC is not able to determine adulteration with anthocyanin-rich or PAC-rich extracts, respectively. High-resolution mass spectrometry (HR-MS) gives structural information, making it possible to discriminate PAC-A from PAC-B, and coupled to LC it provides a polyphenolic profile (Barbosa et al., 2018; Feliciano et al., 2012). However, even this technology alone may not be sufficient to detect adulteration of cranberry extract with other extracts containing A-type PAC. Boudesocque-Delaye et al. (2018) suggested a procyanidin A2/epicatechin ratio greater than 2 as an appropriate indicator of the quality of cranberry ingredients. This marker may not be sufficient to detect adulteration performed with extracts rich in PAC but devoid of or low in procyanidin A2 and epicatechin, such as, for example, pine bark extracts. Genetic methods may be of limited use in processed materials such as extracts. Indeed, depending on the type of processing, cranberry extract might not contain DNA or it might be so deteriorated that it does not allow the identification of adulteration by genetic test. Finally, anti-adhesion tests provide real positive results if the cranberry extract has been adulterated with matrices containing PAC-A such as, for example, grape seeds or peanut skin extracts. Thus, this test should be used to determine effectiveness but not authenticity.
Regarding anthocyanins (ACNs), cranberry seems to have a unique qualitative profile (Brown and Shipley, 2011; Lee, 2016). Therefore, comparison with certified standard material allows the identification of possible adulterations of both cranberry extract and cranberry-based products. However, anthocyanin fingerprinting does not allow the detection of cranberry extract with added PAC-A from matrices devoid of anthocyanin such as grape seed or pine bark extracts.
It thus appears that to detect the adulteration of cranberry extract or cranberry-based products, more than one marker is necessary. Thus, the aim of our study was to find markers able to detect frauds in the authentication of cranberry-based extracts. For that purpose, the total amount of anthocyanin and PAC in authentic cranberry fruits, commercially available cranberry extracts and cranberry-based food supplements was determined spectrophotometrically by differential pH and DMAC assays, respectively. Then, polyphenols belonging to different families such as anthocyanins and flavanols were identified and quantified by LC-DAD-HR-MS. An accurate mass database was built from such spectral and chromatographic data. Finally, the results obtained were employed as descriptors to achieve the classification of samples by principal components analysis (PCA).
2. Materials and methods
2.1. Chemicals
Standards of cyanidin (Cy)-, delphinidin (D)-, petunidin (Pet)-, peonidin (Peo)-, malvidin (Mv)-, pelargonidin (Pel)- and their 3-O-glucoside (glc), Cy-3-O-galactoside (Cy-gal), Peo-gal, Cy-arabinoside (Cy-ara), Peo-ara, Cy-rutinoside (Cy-rut), D-rut, Cy-3,5-di-glucoside (Cy-di-glc), Peo-di-glc, Mv-di-glc, Cy-sambubioside (Cy-sam), D-sambubioside (D-sam) and Cy-3-O-sophoroside (Cy-sop) were purchased from Polyphenols Laboratory (Sandnes, Norway). Potassium chloride, hydrochloric acid, methanol, acetonitrile, acetone, phosphoric and trifluoroacetic acid (TFA) were from Merck (Darmstadt, Germany). Delphinidin-3-galactoside (D-gal), 4-dimethylamino-cinnamaldehyde (DMAC), ammonium acetate and acetic acid were provided by Sigma-Aldrich (St. Louis, MO, USA). Extrasynthese (Genay, France) supplied catechin (CAT), epicatechin (EC), procyanidin C1 (PC1) and procyanidin A2 (PA2). Water was obtained from Arium pro apparatus (Sartorius, Milan, Italy).
2.2. Cranberry fruits and commercial cranberry-based products
Fully ripe cranberries (V. macrocarpon Ait.) were from Slovenia (F1, cultivar Ben Lear), USA (F2, cultivar Howes; F3, cultivar Stevens), Canada (F4, cultivar Ben Lear; F5, cultivar Bergman), Chile (F6, cultivar Ben Lear) and Poland (F7, cultivar Stevens). Samples were shipped frozen on dry ice and kept at −80 °C before use.
European and North American producers provided cranberry extracts (E1–E17) over the period 2015–2018. Details regarding the plant origins and manufacturing processes of the extracts are not available.
Food supplements (S1–S10) containing cranberry extract, all produced by Italian company, were acquired in herbal shops and local markets. Table 1 gives an overview of the commercial products selected for the study.
Table 1.
Main features of commercial food supplements selected for the study.
| Sample | PAC and other sources of polyphenols | Daily dose | Declareda | Founda |
|---|---|---|---|---|
| S1 | Cranberry 15% and 30% PAC, birch | 1 stick | 45 | 44 ± 2 |
| S2 | Cranberry 15% PAC, berry flavour, beetroot red | 1 stick | 36 | 30 ± 1 |
| S3 | Cranberry 40% PAC, uva ursi, Solidago, propolis, grapefruit | 2 sticks | 40 | 17 ± 1 |
| S4 | Cranberry | 1 tablet | n.d. | 2 ± 0 |
| S5 | Cranberry 36% PAC, ononis | 2 tablet | 86 | 60 ± 2 |
| S6 | Cranberry 80% PAC, propolis | 1 tablet | 72 | 25 ± 1 |
| S7 | Cranberry 2.77% PAC | 1 stick | 36 | 25 ± 1 |
| S8 | Cranberry 36% PAC, beetroot red | 1 stick | 47 | 41 ± 1 |
| S9 | Cranberry 36% PAC, quercetin, curcuma | 1 tablet | 43 | 29 ± 1 |
| S10 | Cranberry 30% PAC | 2 capsules | 72 | 71 ± 2 |
mg PAC/day determined by DMAC method. n.d.: not declared.
The extract of possible adulterants, such as chokeberry (n = 3, Aronia melanocarpa), elderberry (n = 4), blackberry (n = 2), blackcurrant (n = 4), red raspberry (n = 3), sweet cherry (n = 2), black bean (n = 4) and black soybean (n = 5), grape seed (n = 4), pine bark (n = 6), karkadè (n = 3, Hibiscus sabdariffa) and the fruits of mulberry (n = 4, Morus nigra) were a gift from Specchiasol (Bussolengo, VR, Italy).
2.3. Anthocyanin determination
2.3.1. Cranberry fruit extraction
Fruits were extracted as described by Gardana et al. (2014), with slight modifications. Briefly, approximately 10 g of whole fruits was mixed with 20 mL of a solution of methanol : 2% aqueous TFA (10 : 90, v/v) and homogenized using a T-25 Ultra-Turrax (IKA-Werke, Staufen, Germany) for 1 min. The homogenate was extracted for 20 min under agitation in the dark at room temperature. The suspension was centrifuged at 1000 × g for 10 min at 4 °C, and the supernatant recovered. The residue was extracted again until disappearance of the red colour (3 × 20 mL) with a solution of methanol : 2% aqueous TFA (10 : 90, v/v) and treated as described above. The supernatants were combined, the volume adjusted to 100 mL using solution of 2% aqueous TFA and the extracts stored at −20 °C. Before the UHPLC-DAD-MS analysis the extracts were centrifuged at 3000 × g for 1 min.
2.3.2. Cranberry extract extraction
Approximately 20 mg of powder was dissolved in 15 mL of a solution of methanol : 2% aqueous TFA (20 : 80, v/v). The suspension was sonicated for 10 min at room temperature, centrifuged at 1000 × g for 5 min and the supernatant transferred to a 20 mL flask. The volume was then adjusted using a solution of 2% aqueous TFA.
2.3.3. Food supplement extraction
Approximately 200 mg of powder was dissolved in 8 mL of a solution of methanol : 2% aqueous TFA (20 : 80, v/v), sonicated for 5 min and centrifuged at 1000 × g for 5 min. The supernatant was transferred to a 10 mL flask and the volume adjusted using a solution of 2% TFA in water. The juice (2 g) was diluted in 7 mL of solution of methanol : 2% aqueous TFA (10 : 90, v/v), sonicated for 5 min and centrifuged at 1000 × g for 5 min. The supernatant was transferred to a 10 mL flask and the volume adjusted using a solution of 2% aqueous TFA.
2.3.4. Spectrophotometric determination of total anthocyanin
The total content of anthocyanin (ACN) was determined spectrophotometrically (Evolution 201, Thermo Scientific, Rodano, Italy) as described by Lee et al. (2005). Briefly, the samples were diluted by a solution of 1% aqueous H3PO4; the absorbance (A) was measured twice for each sample at 520 and 700 nm, and calculated by the Eq. (1).
| (1) |
Then, the total percentage of ACN was determined by means of Eq. (2).
| (2) |
where: 26900 = Cy-glc molar extinction coefficient, 449 = Cy-glc MW, W = sample weight (mg), V = volume (mL), D = dilution factor.
2.3.5. Chromatographic determination of anthocyanins
Anthocyanin analysis was carried out as reported by Spinardi et al. (2019), with slight modifications. Briefly, an HSS T3 column (150 × 2.1 mm, 1.8 μm, Waters), maintained at 50 °C, carried out the separation. The flow rate was 0.5 mL/min, and the eluents were (A) 0.2% TFA and (B) 0.2% TFA in CH3CN. The elution gradient was as follows: 0–20 min 5–20% B; 20–25 min from 20% to 45% B; 25–30 min from 45% to 90% B; 90% B for 3 min; and then from 90% to 5% B in 1 min. The acquisition was made in the full-scan mode in the range (m/z)+ 200–2000 u, using an isolation window of ±2 ppm. The MS data were processed using Xcalibur software (Thermo Scientific). Working solutions (n = 5) were prepared in the range of 2–50 μg/mL, and 5 μL was injected into the UHPLC system. Each analysis was carried out in triplicate.
2.4. Flavan-3-ol determination
2.4.1. Cranberry fruit extraction
Fruits were extracted as described by Ye et al. (2016), with slight modifications. Briefly, 100 mL of a solution containing acetone : 2% CH3COOH in water (70 : 30, v/v) was added to 30 g cranberries; the mixture was blended in a Waring blender and extracted for 10 min in an ultrasonic water bath. The mixture was centrifuged at 600 × g and after 20 min the supernatant was transferred into a flask. The residue was extracted using 50 mL of a solution containing acetone : 2% CH3COOH in water (70 : 30, v/v) and treated as described below. The supernatants were mixed, freeze-dried and the residue dissolved in 10 mL of water. The extract (5 mL) was loaded onto a 20 mL Strata C18-E 5 g SPE cartridge (Phenomenex, Torrence, CA) pre-activated with methanol (15 mL) and then washed with water (30 mL). Afterwards, the cartridge was washed with 20 mL of water to remove polar compounds such as sugar; the PAC were then eluted from the cartridge using 20 mL of methanol. Organic fractions from multiple columns were combined, evaporated and freeze-dried. Dry extract was stored at −20 °C before use.
2.4.2. Cranberry extract and food supplement extraction and spectrophotometric determination of total proanthocyanidins
Fruits were extracted as described by Gardana and Simonetti (2019), with slight modifications. Briefly, cranberry extract (25 mg) or food supplement (600mg)were dissolved in 40 mL of a solution of acetone : 2% aqueous CH3COOH (70 : 30, v/v). The mixture was vortexed for 30 s, sonicated for 10 min and then the volume adjusted to 50 mL by a solution of acetone : 2% aqueous CH3COOH (70 : 30, v/v).
The diluted extract was analysed colorimetrically by the BL-DMAC assay as described by Gardana and Simonetti (2019), with slight variations. The reaction was monitored using an Evolution 201 spectrophotometer (Thermo Scientific). For the calibration, six working solutions of PA2 in the range 2–50 μg/mL were prepared.
2.4.3. Flavan-3-ol monomer, dimer and trimer determination by UHPLC-DAD-Orbitrap MS
Cranberry extract (20 mg) and food supplement (0.5 g) were extracted with 10 mL of a solution of methanol : water (50 : 50, v/v). The mixture was centrifuged at 500 × g for 10 min and the supernatant transferred to a 20 mL volume flask. The residue, if present, was washed with 8 mL of a solution of methanol : water (50 : 50, v/v) and the mixture treated as described above. The supernatants were mixed, and water added to adjust the volume. The solution was centrifuged at 1000 × g for 2 min, and 5 μL was injected into the UHPLC system. The analysis was carried out according to Gardana and Simonetti (2019). Peaks were identified by evaluating the accurate mass, the fragments obtained in the HCD and the on-line UV spectra. For the calibration, five working solutions containing CAT, EC, PA2 and PC1 were prepared in the range of 0.2–20 μg/mL and stored at 4 °C. Analysis was carried out in duplicate. The amount of dimeric and trimeric flavanols not available was estimated using PA2 and PC1 calibration curve equations, respectively.
2.5. Statistical analysis
Statistical analysis was performed by means of Excel software. PCA was performed using R statistical software 3.1.2 by means of the function PRCOMP. To verify the similarity or difference between the reference chromatograms (fruits) and target products (extracts, commercial preparations), the total deviation percentage (TD%) was introduced and calculated as reported by Gardana et al. (2014).
3. Results and discussion
3.1. Anthocyanins
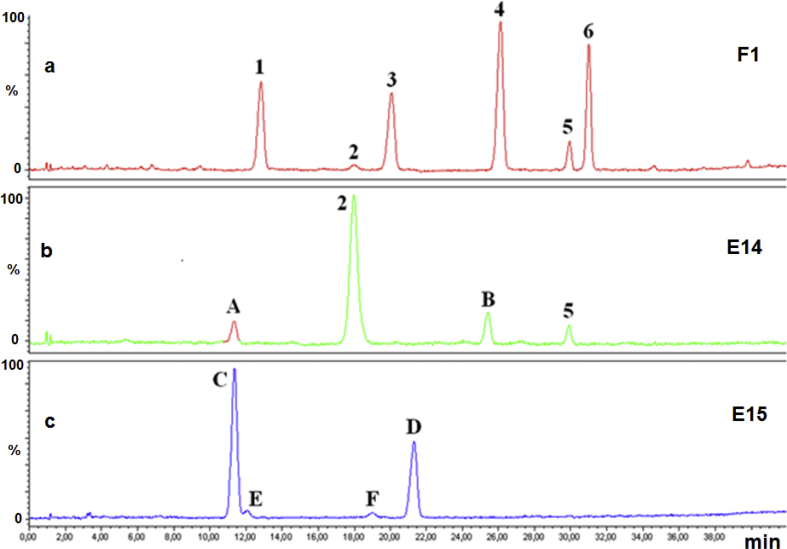
Anthocyanins in reference products (fruits), extracts and food supplements cranberry-base were identified by co-chromatography, on-line UV-Vis spectra, molecular weight and fragment ions identification obtained by collision induced dissociation (CID). The chromatograms relating to fruits showed the presence of four main ACNs corresponding to Peo-gal, Peo-ara, Cy-ara and Cy-gal, respectively (Figure 1a, sample F1), and a lower amount of Peo-glc and Cy-glc was also detected. The respective aglycones were not detected in any samples.
Figure 1.
Chromatograms integrated at 520 nm of cranberry fruit F1 (a) and cranberry extracts E14 (b) and E15 (c). 1, Delphinidin-galactoside; 2, Cyanidin-glucoside; 3, Cyanidin-arabinoside; 4, Peonidin-galactoside; 5, Peonidin-glucoside; 6, Peonidin-arabinoside; A, Delphinidin-glucoside; B, Cyanidin-rutinoside; C; Delphinidin-glucose-xylose; D, Cyanidin-glucose-xylose; E, Delphinidin-hexose-pentose-pentose; F, Cyanidin-hexose-pentose-pentose. The chromatographic profile of samples E14 and E15 was comparable to that of mulberry and Hibiscus extract, respectively.
The individual and total ACN amount in cranberries from different countries is reported in Table 2. The monomeric ACN content of the cranberry samples ranged from 240 to 560 mg/100 g DW with a mean of 405 ± 85 mg/100 g DW, and galactosides, arabinosides and glucosides comprised approximately 53%, 42% and 5% of the total ACN, respectively. The average contents of these ACNs were comparable to those reported by different authors (Brown and Shipley, 2011; Česonienė et al., 2009; Prior et al., 2001).
Table 2.
Total amount (mean ± SD, mg/100g DW) and relative percentage of anthocyanins in authentic cranberry (F1–F7), commercial cranberry extracts (E1–E17) and cranberry-based food supplements (S1–S10).
| Sample | Total | 1 | 2 | 3 | 4 | 5 | 6 | A | B | C | D | E | F | TD% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 387 ± 32 | 19.7 | 3.5 | 21.7 | 30.1 | 7.3 | 17.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 63 |
| F2 | 507 ± 51 | 17.3 | 0.7 | 17.5 | 46.2 | 0.8 | 17.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 44 |
| F3 | 490 ± 39 | 17.6 | 1.1 | 20.0 | 32.8 | 6.5 | 22.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 34 |
| F4 | 265 ± 23 | 18.5 | 1.7 | 14.8 | 49.0 | 0.5 | 15.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 46 |
| F5 | 336 ± 34 | 9.2 | 1.8 | 28.3 | 21.2 | 5.4 | 34.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 49 |
| F6 | 414 ± 35 | 10.3 | 0.2 | 11.2 | 47.1 | 1.1 | 30.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 60 |
| F7 | 440 ± 36 | 22.2 | 0.8 | 19.2 | 27.6 | 3.1 | 27.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 34 |
| E1 | 1286 ± 49 | 11.6 | 2.3 | 33.7 | 11.4 | 3.3 | 37.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 56 |
| E2 | 2400 ± 98 | 18.8 | 1.2 | 16.6 | 32.9 | 4.1 | 26.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14 |
| E3 | 77 ± 4 | 31.0 | 0.0 | 20.5 | 36.8 | 0.0 | 11.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 58 |
| E4 | 3230 ± 113 | 14.6 | 1.1 | 20.0 | 32.8 | 5.5 | 26.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 19 |
| E5 | 3191 ± 115 | 15.9 | 0.9 | 24.5 | 27.8 | 4.4 | 26.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21 |
| E6 | 2492 ± 105 | 13.4 | 1.5 | 27.6 | 25.9 | 4.7 | 27.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 24 |
| E7 | 510 ± 27 | 18.6 | 0.0 | 14.4 | 39.9 | 4.8 | 22.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 31 |
| E8 | 2211 ± 88 | 13.2 | 1.1 | 28.6 | 25.4 | 4.8 | 26.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 29 |
| E9 | 2823 ± 110 | 11.1 | 1.3 | 25.0 | 25.6 | 6.2 | 30.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 34 |
| E10 | 2770 ± 111 | 23.1 | 0.9 | 18.2 | 37.1 | 4.2 | 16.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21 |
| E11 | 1810 ± 76 | 16.9 | 1.5 | 26.1 | 26.1 | 5.4 | 24.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 22 |
| E12 | 124 ± 6 | 17.8 | 0.9 | 19.9 | 34.2 | 5.0 | 22.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 17 |
| E13 | 160 ± 8 | 9.0 | 1.7 | 32.6 | 18.4 | 6.4 | 31.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50 |
| E14 | 825 ± 40 | 0.0 | 73.6 | 0.0 | 0.0 | 5.0 | 0.0 | 8.3 | 13.2 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| E15 | 810 ± 42 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 68.0 | 30.0 | 0.9 | 1.1 | ----- |
| E16 | 920 ± 46 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 61.9 | 35.9 | 1.1 | 1.1 | ----- |
| E17 | 51 ± 3 | 0.0 | 49.2 | 50.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S1 | 145 ± 6 | 18.3 | 1.0 | 24.5 | 29.4 | 4.9 | 21.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S2 | 140 ± 2 | 22.2 | 1.4 | 24.3 | 26.9 | 4.5 | 20.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 33 |
| S3 | 47 ± 2 | 21.2 | 0.0 | 22.1 | 32.7 | 4.4 | 19.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 44 |
| S4 | 30 ± 2 | 11.9 | 1.3 | 29.1 | 21.5 | 4.8 | 31.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 41 |
| S5 | 38 ± 2 | 0.0 | 68.3 | 0.0 | 0.0 | 3.4 | 0.0 | 3.2 | 25.1 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S6 | 24 ± 1 | 0.0 | 54.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 45.5 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S7 | 151 ± 7 | 0.0 | 58.0 | 36.6 | 0.0 | 2.4 | 0.0 | 11.0 | 3.0 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S8 | 20 ± 1 | 0.0 | 57.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 42.2 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S9 | 27 ± 1 | 0.0 | 73.3 | 0.0 | 0.0 | 5.2 | 0.0 | 6.6 | 14.8 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
| S10 | 516 ± 24 | 93.0 | 0.0 | 0.0 | 0.0 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ----- |
1, Delphinidin-galactoside; 2, Cyanidin-glucoside; 3, Cyanidin-arabinoside; 4, Peonidin-galactoside; 5, Peonidin-glucoside; 6, Peonidin-arabinoside; A, Delphinidin-glucoside; B, Cyanidin-rutinoside; C; Delphinidin-glucose-xylose (Delphinidin-sambubioside); D, Cyanidin-glucose-xylose (Cyanidin-sambubioside); E, Delphinidin-hexose-pentose-pentose; F, Cyanidin-hexose-pentose-pentose.
Quantitative analysis of the cranberry extracts showed marked differences among them (Table 2). The spectrophotometric determinations did not provide different quantitative data than those obtained by chromatography, suggesting that other pigments, polymerized ACNs or both were not present.
The chromatographic analyses showed that four (E14–E17) out of 17 extracts tested had a chromatographic profile different from that obtained analysing cranberry fruits. Figure 1b and c show the chromatograms corresponding to samples E14 and E15, respectively. The chromatographic profile of sample E14 (Figure 1b) showed the presence of four ACNs, the main one being Cy-glc (Peak 2). Peaks A and B had (m/z)+ 465.0911 and 595.1510 u, respectively, and at low collision energy values gave fragments with (m/z)+ 303.0423 and 287.0478 u, respectively, likely corresponding to the aglycone moiety. Thus, Peaks A and B were D-glc and Cy-rut, respectively. Their identity was then confirmed by the reference standard. Cyanidin-rutinoside was not detected in the reference products (cranberry fruits) but was present M. nigra (black mulberry) berries.
The chromatographic profile of extract E15 (Figure 1c), similar to E16, differed significantly from that of cranberry fruits. In fact, they mainly contained ions with (m/z)+ 597.1439 (Peak C) and 581.1491 u (Peak D), and smaller quantities of ACNs with (m/z)+ 729.1305 (Peak E) and 713.1505 u (Peak F). Peak C gave fragments with (m/z)+ 465.0911 and 303.0423 u, while for Peak D they were 449.1078 and 287.0478 u. Peak D and C was Cy-glc-xyl (Cy-sam) and D-glc-xyl (D-sam), respectively. Their identity was confirmed by the reference standard. Peaks E and F were D-hexose-pentose-pentose and Cy-hexose-pentose-pentose, respectively. Neither the identity nor the position of the sugars could be determined. Cyanidin- and D-sam have been detected, with the same relative percentage, also in Hibiscus extract. Therefore, extracts E15 and E16 do not appear to contain cranberry but an extract of Hibiscus. Unfortunately, this is not the first case of adulteration of cranberry by Hibiscus (Wei et al., 2011).
Regarding commercial cranberry-based products, four out of ten (S1–S4) had a chromatographic profile similar to that obtained when analysing cranberry fruits, unlike the other six (S5–S10). We highlight that products S5–S9 contained mainly Cy-glc and Cy-rut, like extract E14, while S10 contained almost exclusively Cy-gal (Table 2).
The last column of Table 2 shows the TD% values of the commercial products versus the reference cranberries (F1–F7). The TD% data for the commercial cranberry extracts E1–E13 (31 ± 15) and food supplements S1–S4 (38 ± 5) were similar to the given reference (47 ± 12), but the others differed significantly from it. On the whole, the TD% provides an index, dimensionless, and not a quantitative data, but nevertheless it can be a valid tool to verify the conformity of a product.
3.2. Flavan-3-ol monomers, oligomers and proanthocyanidins
The total PAC content in fruit samples (F1–F7) harvested in 2018 was determined using the DMAC method and is reported in Table 3. The repeatability and inter-day precision were5.4 ± 1.4 and 5.8 ± 1.6 %, respectively.
Table 3.
Total amount of procyanidin (mean ± SD, mg/100g DW) and value of the markers (M1, M2 and M3) determined in cranberry fruits (F1–F7), commercial cranberry extracts (E1–E17) and cranberry-based food supplements (S1–S10).
| Sample | Total PACa | M1 | M2 | M3 |
|---|---|---|---|---|
| F1 | 2294 ± 216 | 6.8 | 9.3 | 8.7 |
| F2 | 6563 ± 635 | 15.7 | 10.4 | 7.6 |
| F3 | 3688 ± 320 | 8.7 | 7.7 | 9.5 |
| F4 | 1471 ± 235 | 6.6 | 9.6 | 6.3 |
| F5 | 3500 ± 355 | 12.6 | 8.1 | 5.4 |
| F6 | 2333 ± 240 | 6.4 | 4.4 | 4.6 |
| F7 | 3529 ± 319 | 9.5 | 6.6 | 3.6 |
| E1 | 15680 ± 520 | 12.2 | 9.0 | 7.0 |
| E2 | 27020 ± 950 | 11.3 | 12.3 | 3.0 |
| E3 | 320 ± 10 | 3.9 | 7.6 | 5.5 |
| E4 | 25101 ± 750 | 7.8 | 9.5 | 3.7 |
| E5 | 13320 ± 550 | 4.2 | 11.2 | 4.7 |
| E6 | 13520 ± 540 | 5.4 | 14.2 | 4.0 |
| E7 | 2910 ± 150 | 5.7 | 9.0 | 8.3 |
| E8 | 12640 ± 490 | 5.7 | 11.0 | 7.1 |
| E9 | 13730 ± 600 | 4.9 | 6.2 | 6.5 |
| E10 | 11920 ± 570 | 4.3 | 9.2 | 7.5 |
| E11 | 12720 ± 530 | 7.0 | 9.8 | 8.1 |
| E12 | 340 ± 20 | 2.7 | 9.5 | 7.5 |
| E13 | 1120 ± 060 | 6.9 | 7.0 | 3.8 |
| E14 | 33120 ± 1680 | 40.0 | 0.29 | 0.6 |
| E15 | 31720 ± 1360 | 39.1 | 0.32 | 0.6 |
| E16 | 29110 ± 1220 | 31.6 | 0.34 | 0.5 |
| E17 | 1130 ± 060 | 21.7 | 0.51 | 1.3 |
| S1 | 1330 ± 070 | 9.0 | 7.8 | 4.7 |
| S2 | 610 ± 40 | 4.3 | 6.0 | 6.8 |
| S3 | 520 ± 30 | 10.5 | 3.1 | 4.0 |
| S4 | 200 ± 10 | 6.7 | 7.0 | 5.0 |
| S5 | 2620 ± 130 | 69.3 | 0.28 | 0.4 |
| S6 | 2030 ± 100 | 83.6 | 0.35 | 1.0 |
| S7 | 610 ± 30 | 4.0 | 0.33 | 0.2 |
| S8 | 1420 ± 60 | 68.5 | 0.16 | 1.1 |
| S9 | 2200 ± 110 | 80.7 | 0.19 | 0.7 |
| S10 | 17180 ± 530 | 33.4 | 0.22 | 0.1 |
M1, PAC : ACN; M2, EC : CAT; M3, (PA2 : PAC) × 100.
mg/100g DW.
The fruits with the highest PAC content (6563 mg/100 g DW) were those of the cultivar Howes (F2), followed by Stevens (F3), Ben Lear (F7) and Bergman (F5). Sample F4, Ben Lear from Canada, had a significantly lower PAC content than all other cultivars on average (1471mg/100 g DW). These results are in agreement with those reported by several authors (Carpenter et al., 2014: Gu et al., 2003) and higher than those of Lu et al. (2017). In all berries, PA2 was the main dimer and it was present in the range from 110 to 550 mg/100 g dried berries (247 ± 161 mg/100 g DW). Moreover, the relative percentage of PA2 in berries was in the range from 3.6% to 9.5% (6.5 ± 2.2%), in agreement with the 6.1 ± 2.3% reported by van Dooren et al. (2018). Regarding monomers, the only ones found in the berries were EC and CAT. The main one was EC, representing more than 90% of the total monomers, and the EC : CAT ratio was 8 ± 2. Thus, varieties of the same species have a similar EC : CAT ratio independent of the area of origin (Table 3), as already reported by Jungfer et al. (2012).
Quantitative analysis of the cranberry extracts showed marked differences among them (Table 3). The total PAC amount was in accordance with that declared by the producers except for E7, whose content was approximately 80% lower. Regarding extracts E3, E12 and E17, the PAC amount was not included on the manufacturer's certificate.
The EC : CAT ratio in commercial cranberry extracts determined by the UPLC-HR-MS method was 7.5 ± 4.5, a value comparable to that obtained for fresh fruits (8 ± 2). However, evaluation of the EC : CAT ratio in the individual products showed a significant difference between the values. In particular, the EC : CAT ratio in extracts E1–E13 and E14–E17 was 9.7 ± 2.2 and 0.4 ± 0.1, respectively. Therefore, while for samples E1–E13 the EC : CAT ratio was comparable with that obtained by analysing the cranberry fruits, the one relative to extracts E14–E17 was significantly different. Thus, EC was the main monomer in E1–E13, as in fruits. In contrast, CAT was the main monomer in E14–E17, representing more than 80% of the total monomers.
Procyanidin A2 was the main dimer in E1–E13, representing approximately 90% of total dimers, and the relative percentage was 5.9 ± 2.2. On the contrary, PA2 was not the main dimer in E14–E17, constituting only about 20–30% of the total dimers, and the relative percentage of PA2 (0.8 ± 0.4) was significantly different from those obtained from fruits and E1–E13 samples.
Regarding the commercial products containing cranberry, S1, S2, S8 and S10 contained the PAC amount mentioned on the package, while samples S3 (−57%), S5 (−30%), S6 (−65%), S7 (−30%) and S9 (−32%) contained quantities lower than those declared. The package of sample S4 did not report the PAC content. Taking into account that for the prevention of UTI in women, the suggested daily dose of cranberry PAC should be at least 36 mg (Asma et al., 2018), only products S1 (44 ± 2 mg), S5 (60 ± 2 mg), S8 (41 ± 1 mg) and S9 (71 ± 2 mg) provided this amount or more. To underline that, among these, only S1 seemed to contain cranberry.
Speaking of the EC : CAT ratio, for S1–S4 the value was 6.0 ± 2.1, comparable to that found in berries and extracts E1–E13, while for S5–S10 it was much lower (0.3 ± 0.1). It is to be noted that this last value was comparable to that found by analysing extracts E14–E17.
Low EC : CAT ratios were also found in H. sabdariffa (0.4), pine bark (0.9), M. nigra (1.1), blackcurrant (0.7), elderberry (1.3), grape seed (1.1), chokeberry (1.9) and black bean (0.3) extract. On the contrary, the EC : CAT ratio for blackberry and sweet cherry was 27.3 and 4.3, respectively.
A similar consideration was made regarding the percentage ratio between the main dimer found in cranberry berries, PA2, and total PAC. Indeed, while in samples S1–S4 the ratio was 5.1 ± 1.2, in products S5–S10 it was 0.6 ± 0.4.
3.3. Multivariate statistical analysis
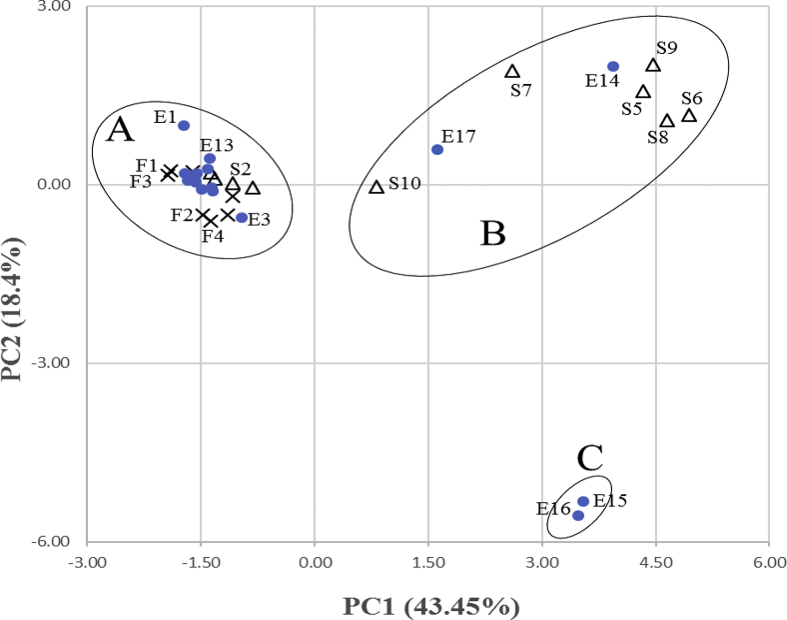
Principal component analysis was performed to check for similarity and differences between samples according to the parameters measured. The PCA score plot of the two first components shown in Figure 2 explained up to 62% of the total variance, of which PC1 and PC2 contributed 43.5% and 18.4%, respectively. As highlighted in Figure 2, PCA analysis showed a clear discrimination between the different cranberry products, which were clustered into three separate groups (A–C) based on the levels or presence of their constituents.
Figure 2.
Bi-plot of principal component analysis of the parameters of the 7 cranberry cultivars (F1–F7), 17 commercial cranberry extracts (E1–E17) and 10 cranberry-based food supplements (S1–S10). Principal component analysis on levels of individual anthocyanins, EC/CAT, PA2/PAC and PAC/ACN ratios for 7 cranberry cultivars (×, F1–F7), 17 commercial cranberry extracts (•, E1–E17) and 10 cranberry-based food supplements (Δ, S1–S10). PC, principal component. Group A: compliant products. This group includes cranberry fruits (F1–F7), extracts and commercial products signed E1–E13 and S1–S4, respectively. Group B and C: non-compliant samples. These groups were separated from group A by their ACN content and the PAC:ACN ratio, and group C was separated from group B because of its particular ACN content.
Group A included the cranberry fruits (F1–F7), extracts E1–E13 and products S1–S4. These samples were positioned on the negative semi-axis of PC1 due to their content of ACN (Peo-gal, Peo-ara, Cy-ara and Cy-gal) and for the EC : CAT and PA2 : PAC ratios. The other samples (groups B and C), positioned on the positive semi-axis, were separated from group A by their ACN content and for the PAC : ACN ratio. Moreover, group C was separated from group B due to its particular content of ACN (D-glc-xyl and Cy-glc-xyl).
Thus, the composition of products E1–E13 and S1–S4 was close to that of the reference fruits, whereas products E14–E17 and S5–S10 were indiscriminate. Therefore, extracts E1–E13 and food supplements S1–S4 appeared as the best-quality products containing cranberry.
Overall, the ACN profile and the ratios EC : CAT, PAC : ACN and PA2 : PAC seemed to be more indicative of the quality of cranberry ingredient than total PAC amount. Thus, they could be used as markers, in particular the EC : CAT ratio and ACN profile, to authenticate cranberry.
Regarding the PA2 : EC ratio greater than 2 as a marker of the quality of cranberry proposed by Boudesocque-Delaye et al. (2018), in non-compliant extracts E14–E17 and cranberry-based food supplements S5–S10, the ratio was in the range 8–11 and 6–16, respectively. On the contrary, in some compliant extracts (i.e. E2, E4–E6 and E9–E10) and food supplements (i.e. S3) the value was lower than 2, while in the cranberry fruits the ratio was in the range 1.2–2.2. Thus, our data do not seem to support the PA2 : EC ratio as a quality indicator.
4. Conclusion
A targeted analysis was performed by UHPLC-DAD-HR-MS to determine ACN, EC, CAT and dimers in cranberry fruits, commercial cranberry extracts and cranberry-based food supplements. The ACN fingerprint and EC : CAT, PAC : ACN and PA2 : PAC ratios were used as markers for the evaluation of product authenticity. Based on these markers and PCA, four extracts and six food supplements were not compliant; one extract seemed adulterated with M. nigra and two with Hibiscus.
Overall, the picture is discouraging and, therefore, it would be appropriate for producers to standardize their extracts with chromatographic techniques to make sure that the product contains cranberry. In this regard, the markers reported in this study could be a potential tool as a reliable analytical method for cranberry authentication.
Declarations
Author contribution statement
Claudio Gardana: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Antonio Scialpi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Christian Fachechi: Performed the experiments.
Paolo Simonetti: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare the following conflict of interests: Two authors, Dr. Antonio Scialpi and Dr. Christian Fachechi, are employees for a company that produce cranberry-based food supplements. The company produces cranberry-based food supplements but not cranberry extracts. Like other companies, it buys cranberry extracts from various suppliers to make their commercial products. Thus, the purpose of the company is to make sure that the product purchased is actually cranberry extract and that it has the correct title in PACs.
Additional information
No additional information is available for this paper.
References
- Asma B., Vicky L., Stephanie D., Yves D., Amy H., Sylvie D. Standardised high dose versus low dose cranberry proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women (PACCANN): a double blind randomised controlled trial protocol. BMC Urol. 2018;18:29. doi: 10.1186/s12894-018-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa S., Pardo-Mates N., Hidalgo-Serrano M., Saurina J., Puignou L., Núñez O. Detection and quantitation of frauds in the authentication of cranberry-based extracts by UHPLC-HRMS (Orbitrap) polyphenolic profiling and multivariate calibration methods. J. Agric. Food Chem. 2018;66:9353–9365. doi: 10.1021/acs.jafc.8b02855. [DOI] [PubMed] [Google Scholar]
- Boudesocque L., Dorat J., Pothier J., Gueiffier A., Enguehard-Gueiffier C. High performance thin layer chromatography-densitometry: a step further for quality control of cranberry extracts. Food Chem. 2013;139:866–871. doi: 10.1016/j.foodchem.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Boudesocque-Delaye L., Dorat J., Lanoue A., Ceniviva E., Bruyere F., Enguehard-Gueiffier C. Solid/liquid extraction as key step for quality assessment of commercial cranberry products using HPTLC-densitometry. Planta Med. 2016;81:P1080. [Google Scholar]
- Boudesocque-Delaye L., Lanoue A., Dorat J., Bruyère F., Gueiffier A., Enguehard-Gueiffier C. Quality control of commercial cranberry products: HPTLC-densitometry a new deal. Food Contr. 2018;86:214–223. [Google Scholar]
- Brown P.N., Shipley P.R. Determination of anthocyanin in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: single-laboratory validation. J. AOAC Int. 2011;94:459–466. [PMC free article] [PubMed] [Google Scholar]
- Carpenter J.L., Caruso F.L., Tata A., Vorsac N., Neto C.C. Variation in proanthocyanidin content and composition among commonly grown North American cranberry cultivars (Vaccinium macrocarpon) J. Sci. Food Agric. 2014;94:2738–2745. doi: 10.1002/jsfa.6618. [DOI] [PubMed] [Google Scholar]
- Česonienė L., Jasutienė I., Šarkinas A. Phenolics and anthocyanin in berries of European cranberry and their antimicrobial activity. Medicina. 2009;45:992–996. [PubMed] [Google Scholar]
- EFSA Scientific Opinion on the substantiation of health claims related to proanthocyanidins from cranberry (Vaccinium macrocarpon Aiton) fruit and defence against bacterial pathogens in the lower urinary tract (ID 1841, 2153, 2770, 3328), “powerful protectors of our gums” (ID 1365), and “heart health” (ID 2499) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2011;9:2215. [Google Scholar]
- EFSA Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies on a request from Ocean Spray International Services Limited (UK), related to the scientific substantiation of a health claim on Ocean Spray Cranberry Products® and reduced risk of urinary tract infection in women by inhibiting the adhesion of certain bacteria in the urinary tract. EFSA J. 2009;943:1–16. [Google Scholar]
- Feliciano R.P., Krueger C.G., Shanmuganayagam D., Vestling M.M., Reed J.D. Deconvolution of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry isotope patterns to determine ratios of A-type to B-type interflavan bonds in cranberry proanthocyanidins. Food Chem. 2012;135:1485–1493. doi: 10.1016/j.foodchem.2012.05.102. [DOI] [PubMed] [Google Scholar]
- Fu Z., Liska D., Talan D., Chung M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: a systematic review and meta-analysis. J. Nutr. 2017;147:2282–2288. doi: 10.3945/jn.117.254961. [DOI] [PubMed] [Google Scholar]
- Gafner S., Blumenthal M., Foster S., Cardellina J.H., Khan I.A., Upton R. Presented at 16th International Conference on the Science of Botanicals, Oxford, MS. 2016. Botanical ingredient adulteration: efforts by the ABC-AHP-NCNPR Botanical Adulterants Program to raise awareness of current issues and provide solutions to the problem. [Google Scholar]
- Gardana C., Simonetti P. Evaluation of the degree-of-polymerization of the proanthocyanidins in cranberry by molecular sieving and characterization of the low-molecular weight fractions by UHPLC-Orbitrap mass spectrometry. Molecules. 2019;24:1504. doi: 10.3390/molecules24081504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardana C., Ciappellano S., Marinoni L., Fachechi C., Simonetti P. Bilberry adulteration: identification and chemical profiling of anthocyanins by different analytical methods. J. Agric. Food Chem. 2014;62:10998–11004. doi: 10.1021/jf504078v. [DOI] [PubMed] [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Prior R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- Howell A.B., Botto H., Combescure C., Blanc-Potard A.B., Gausa L., Matsumoto T., Tenke P., Sotto A., Lavigne J.P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect. Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A.B., Reed J.D., Krueger C.G., Winterbottom R., Cunningham D.G., Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jepson R.G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2008;1:CD001321. doi: 10.1002/14651858.CD001321.pub4. [DOI] [PubMed] [Google Scholar]
- Jepson R.G., Williams G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungfer E., Zimmermann B.F., Ruttkat A., Galensa R. Comparing procyanidins in selected Vaccinium species by UHPLC-MS2 with regard to authenticity and health effects. J. Agric. Food Chem. 2012;60:9688–9696. doi: 10.1021/jf303100q. [DOI] [PubMed] [Google Scholar]
- Lee J. Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci. Nutr. 2016;4:742–752. doi: 10.1002/fsn3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Durst R.W., Wrolstad R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Lu Y., Pekerti B.N., Toh Z.S., Broom F., Savage G., Liu S.Q., Huang D. Physico-chemical parameters and proanthocyanidin profiles of cranberries cultivated in New Zealand. J. Food Compos. Anal. 2017;63:1–7. [Google Scholar]
- Luis A., Dominguez F., Pereira L. Can cranberries contribute to reduce the incidence of urinary tract infections? A systematic review with meta-analysis and trial sequential analysis of clinical trials. J. Urol. 2017;198:614–621. doi: 10.1016/j.juro.2017.03.078. [DOI] [PubMed] [Google Scholar]
- Nagy S., Wade R.L. Vol. 1. Agscience, Inc; Auburndale, FL: 1995. (Methods to Detect Adulteration of Fruit Juice Beverages). [Google Scholar]
- Navarro M., Núñez O., Saurina J., Hernández-Cassou S., Puignou L. Characterization of fruit products by capillary zone electrophoresis and liquid chromatography using the compositional profiles of polyphenols: application to authentication of natural extracts. J. Agric. Food Chem. 2014;62:1038–1046. doi: 10.1021/jf404776d. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Lazarus S.A., Cao G., Muccitelli H., Hammerstone J.F. Identification of procyanidins and anthocyanin in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001;49:1270–1276. doi: 10.1021/jf001211q. [DOI] [PubMed] [Google Scholar]
- Sánchez-Patán F., Bartolomé B., Martin-Alvarez P.J., Anderson M., Howell A., Monagas M. Comprehensive assessment of the quality of commercial cranberry products. Phenolic characterization and in vitro bioactivity. J. Agric. Food Chem. 2012;60:3396–3408. doi: 10.1021/jf204912u. [DOI] [PubMed] [Google Scholar]
- Spinardi A., Cola G., Gardana C., Mignani I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019;10:1045. doi: 10.3389/fpls.2019.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarascou I., Mazauric J.P., Meudec E., Souquet J.M., Cunningham D., Nojeim S., Cheynier V., Fulcrand H. Characterisation of genuine and derived cranberry proanthocyanidins by LC–ESI-MS. Food Chem. 2011;128:802–810. [Google Scholar]
- Upton R., Brendler T., editors. American Herbal Pharmacopoeia and Therapeutic Compendium: Cranberry Fruit: Vaccinium Macrocarpon Aiton. American Herbal Pharmacopoeia; Scotts Valley, CA: 2016. Monograph revision. [Google Scholar]
- van Dooren I., Foubert K., Theunis M., Naessens T., Pieters L., Apers S. Advantages of a validated UPLC-MS/MS standard addition method for the quantification of A-type dimeric and trimeric proanthocyanidins in cranberry extracts in comparison with well-known quantification methods. J. Pharmaceut. Biomed. Anal. 2018;148:32–41. doi: 10.1016/j.jpba.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Wei Y., Sardar M.R., Sutherland I.A., Fisher D. Separation of delphinidin-3-O-sambubioside, cyanidin-3-O-sambubioside and p-coumaric acid from cranberry by CCC followed by prep-HPLC using robotic CCC solvent system selection. Chromatographia. 2011;74:367–373. [Google Scholar]
- Yang Y., Liu M., Niu N., Wang H., Wang B., Li M., Wang Y., Wu Y. Identification of small berry species in food and juice using TaqMan-based real-time PCR. J. AOAC Int. 2019 doi: 10.5740/jaoacint.17-0492. [DOI] [PubMed] [Google Scholar]
- Ye L., Neilson A., Sarnoski P., Ray W.K., Duncan S., Boyer R., O’Keefe S.F. Comparison of A-type proanthocyanidins in cranberry and peanut skin extracts using matrix assisted laser desorption ionization-time of flight mass spectrometry. J. Mol. Genet. Med. 2016;10:1–7. [Google Scholar]