Abstract
Adolescence is a critical developmental period marked by an increase in risk behaviors, including nonsuicidal self-injury (NSSI). Heightened reward-related brain activation and relatively limited recruitment of prefrontal regions contribute to the initiation of risky behaviors in adolescence. However, neural reward processing has not been examined among adolescents who are at risk for future engagement for NSSI specifically, but who have yet to actually engage in this behavior. In the current fMRI study (N = 71), we hypothesized that altered reward processing would be associated with adolescents’ thoughts of NSSI. Results showed that NSSI youth exhibited heightened activation in the bilateral putamen in response to a monetary reward. This pattern of findings suggests that heightened neural sensitivity to reward is associated with thoughts of NSSI in early adolescence. Implications for prevention are discussed.
Adolescence is a critical developmental period marked by an increase in risk behaviors, including nonsuicidal self-injury (NSSI). NSSI comprises a variety of behaviors in which an individual intentionally inflicts harm upon his or her body without suicidal intent, including cutting or carving one’s skin, burning, hair-pulling, and bruising one’s self (Favazza, 1998). NSSI behavior usually first occurs in early-to-middle adolescence, and rates of NSSI increase throughout adolescence (Hankin & Abela, 2011; Nock, Joiner, Gordon, Lloyd-Richardson, & Prinstein, 2006). NSSI is recurrent across the lifespan and is associated with internalizing disorders, externalizing disorders, suicide attempts, and completed suicides among adolescents and adults (Lloyd-Richardson, Perrine, Dierker, & Kelley, 2007). In addition, NSSI ideation can occur among early adolescents and thoughts of completing NSSI have been shown to predict future engagement in NSSI behaviors, in addition to suicide. Alarmingly, although NSSI and suicide attempts constitute distinct constructs and behavioral phenomena, they often co-occur within individuals (e.g., Whitlock et al., 2011) and many have found that NSSI may serve as “practice” for subsequent suicide attempts and completions (e.g., Hamza, Stewart, & Willoughby, 2012; Joiner, 2005). In sum, it is critical to identify risk factors for NSSI thoughts and behaviors in adolescence.
In addition to increases in NSSI thoughts and behaviors observed during this time period, youth also undergo a number of changes in brain function during adolescence. During early-to-middle adolescence, there are increases in activation in emotion-and reward-related brain regions including regions in the striatum (nucleus accumbens [NAcc], caudate, and putamen) and, at the same time, relatively limited recruitment of prefrontal regions (Braams, van Duijvenvoorde, Peper, & Crone, 2015; Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Galvan, Hare, Voss, Glover, & Casey, 2007; Van Leijenhorst et al., 2010). This heightened reward activation, coupled with limited regulatory region activation, has been theorized to contribute to the initiation of risky behaviors in adolescence (Steinberg, 2010). However, despite several studies documenting elevated reward-related brain activation (Forbes et al., 2010; Galvan, 2010; Galvan et al., 2006) and other studies finding increases in NSSI behaviors during adolescence (Hankin & Abela, 2011; Nock et al., 2006), there has been very little research on reward-related brain activation as it relates to risk for NSSI among youth. It is important to examine neurobiological predictors of adolescent NSSI in order to better target and refine programs to prevent the initiation of NSSI. To this end, in this study we examined the association between reward-related brain activation and NSSI thoughts in early adolescence, the developmental period just before known initiation and increases in NSSI behavior.
THEORETICAL MODELS OF THE ROLE OF REWARD IN NSSI
Adolescents’ reward-related brain activation may be an important brain-based risk factor for NSSI thoughts and behaviors. Theories of the development of NSSI behavior, including the four-function model of NSSI (Nock & Prinstein, 2004), support this potential link. The four-function model of NSSI provides a framework for understanding the ways in which NSSI is reinforced and cuts across two dimensions: (1) positive versus negative valence and (2) automatic (intrapersonal) versus social (interpersonal) reinforcement. Although many individuals engage in NSSI as a means of alleviating negative affect (i.e., automatic negative reinforcement) or escaping from stressful social situations (i.e., interpersonal negative reinforcement), individuals also engage in NSSI for automatic positive reinforcement. The automatic positive reinforcement function proposes that individuals engage in NSSI to generate a desirable, ostensibly rewarding sensation. For example, some youth may engage in self-injury to combat feelings of numbness or anhedonia (e.g., cutting to “feel something”), to feel in control, or otherwise generate emotion. Indeed, Nock, Prinstein, and Sterba (2010) found that, while the majority of adolescents (64.7%) engaged in NSSI to decrease negative affect (i.e., automatic negative reinforcement), a substantial minority of youth (24.5%) engaged in self-injury in order to “feel something” (i.e., automatic positive reinforcement). Finally, Chapman, Derbidge, Cooney, Hong, and Linehan (2009) found that reward dependence, a temperamental characteristic defined by enhanced responsivity to rewarding stimuli, was a prospective predictor of self-injury among a sample of adults with borderline personality disorder (BPD). Given this evidence, it is likely that adolescents considering engaging in NSSI may show alterations in reward processing at the neurobiological level, with possible increases in activation in reward-related brain regions.
Empirical Evidence for Role of Neural Reward Processing in NSSI
Empirical evidence from functional MRI (fMRI) studies—predominantly with adult samples—has found that NSSI behavior is associated with altered brain activation in reward regions. First, in a study of adults with BPD, those with NSSI showed enhanced activity in reward-related regions in the striatum (e.g., putamen, caudate) and in the orbitofrontal cortex, a frontal region implicated in reward processing, in response to a monetary reward task compared to BPD patients without NSSI and healthy controls (Moreno, 2015). Second, a study by Osuch, Ford, Wrath, Bartha, and Neufeld (2014) found that, in a sample of 16-to 24-year-olds, compared to noninjurers, adolescent and young adult self-injurers showed increased activation in reward-related brain regions (e.g., ventromedial prefrontal cortex [vmPFC]) in response to the self-administration of a painfully cold compress (which served as a proxy for NSSI). Taken together, these studies found evidence for heightened brain activation in reward-related regions in adults and adolescents who engaged in NSSI behaviors. However, a third study of reward processing and NSSI found the opposite: self-injuring 13–19-year-old adolescent girls showed less activation in reward-related striatal and prefrontal regions (e.g., putamen, orbitofrontal cortex) during the anticipation of a monetary reward compared to controls (Sauder, Derbidge, & Beauchaine, 2016). In sum, initial fMRI studies have found that NSSI is associated with altered reward-related brain activation; however, the direction of effects is less clear. There is a need for further research on reward-related brain activation and NSSI risk, particularly among early adolescents, which is the focus of the current study. As noted above, while a few studies have examined NSSI and reward-related brain activation in middle-to-older adolescents and adults, no studies have examined reward system processing among younger adolescents who may be at risk for future NSSI. Given that mechanisms of positive reinforcement are theorized to play a role in the initiation of self-injurious behaviors, it is important to examine reward processing among early adolescents considering NSSI in order to better understand how thoughts may lead to future NSSI behaviors.
Current Study
The goal of this study was to examine how early adolescents’ activation in reward-related brain regions in response to the receipt of reward is associated with thoughts of NSSI. Importantly, NSSI thoughts or ideation may be a marker for future risk of adolescent NSSI behavior (Janis & Nock, 2008; Nock et al., 2010) and so are investigated here as a proxy for future risk of self-harm. Due to the habitual nature of NSSI, it is critically important to examine brain function factors that are related to thoughts of NSSI during early adolescence before youth actually hurt themselves in order to inform identification and prevention efforts. We hypothesized that altered activation in reward-related regions of interest (ROIs: caudate, putamen, nucleus accumbens [NAcc], vmPFC) would be associated with adolescents’ thoughts of NSSI. Due to mixed evidence and a dearth of fMRI research on early adolescent reward processing as it relates to NSSI, we did not hypothesize a direction of effect (i.e., exaggerated vs. attenuated responsivity).
METHODS
Participants
Participants were 71 adolescents, ages 12–14 (M age = 12.56; 42 boys), with normal or corrected-to-normal vision recruited from a larger, community-based study of emotion and adolescent risk behavior. For the larger study, adolescents were recruited via mailings to local households and flyers distributed at middle schools in a suburban area in the mid-Atlantic United States. Inclusion criteria for the larger study were as follows: (1) child IQ ≥ 80 and (2) both parent and child possessed adequate English proficiency to complete questionnaires. Exclusion criteria included the following: (1) a history of psychotic disorders, intellectual disability, or low-functioning autism or (2) current need for acute psychiatric or medical treatment. Of the 197 youth enrolled in the larger study, adolescents who were interested and met MRI inclusion criteria were invited to participate in an additional fMRI session. MRI-eligible adolescents included adolescents who were (1) MRI-safe (e.g., no metal braces), (2) not currently pregnant, and (3) had no history of a congenital brain defect or severe traumatic brain injury.
Of the 197 adolescents who participated in the larger study, 89 adolescents were interested in and eligible for the fMRI session. Of these, six youth began the fMRI scan but left the scanner early due to feeling anxious, two youth left early due to allergies or developing a headache, and one youth did not complete the reward task because of a computer malfunction. Of the 80 remaining participants, nine were excluded due to psychiatric medication use. Thus, the final sample included 71 adolescents (M age = 12.52; 38 boys).
The majority of the adolescents were identified as White (75%) and were of middle-to-upper-middle socioeconomic status (for demographic information, see Table 1). These youth were not significantly different from the full fMRI sample on thoughts of self-injury or demographic variables. Four of the 71 participants included in MRI analyses met criteria for a DSM-IV disorder as assessed by the Diagnostic Interview for Children and Adolescents (DICA; Reich, 2000), see Table 1.
TABLE 1.
Demographics and Adolescent NSSI Thoughts and Diagnosis Information
| Adolescent gender: number (%) male | 38 (47.5) |
| Adolescent age (years): mean (SD); range | 12.56 (.65); 12–14 |
| Child race: number (%) | |
| White | 60 (75.0) |
| More than one race | 8(12.1) |
| Asian | 5 (6.3) |
| Other | 5 (6.3) |
| African American | 2 (2.5) |
| Adolescent Hispanic ethnicity number (%) | 8 (7.7) |
| Family income: number (%) | |
| $25,000–34,999/year | 2 (2.5) |
| $50,000–59,999/year | 1 (1.3) |
| $60,000–74,999/year | 8 (10.0) |
| $75,000–100,000/year | 8 (10.0) |
| >$100,000/year | 60 (75.0) |
| –Not reported | 1 (1.3) |
| Adolescent thoughts of NSSI: number (%) | 19 (23.8) |
| aAdolescent DICA diagnoses: number (%) | |
| No diagnosis | 67 (94.4) |
| Major depressive disorder (MDD) | 2 (2.8) |
| Generalized anxiety disorder (GAD) | 1 (1.4) |
| Oppositional defiant disorder (ODD) | 1 (1.4) |
DICA, Diagnostic Interview for Children and Adolescents.
These diagnoses only include the 71 participants included in MRI sessions. We excluded youth who were currently on psychiatric medication from MRI analyses.
Procedures
Adolescents attended two behavioral assessment sessions as part of the larger study. During the first behavioral session, adolescents completed questionnaires, interviews, and other tasks, including a structured interview on NSSI. The 71 MRI-eligible and interested adolescents also completed an MRI session. The study was described to the parent and adolescent, and a research assistant (RA) obtained their informed consent and assent, respectively.
fMRI Session Procedure Overview
The fMRI sessions were scheduled approximately 2 to 6 weeks from the initial questionnaire/interview sessions (M time difference = 3.37 weeks, SD = 3.18). Adolescents were asked to refrain from substance use (e.g., alcohol, tobacco) on the day of the fMRI session; all confirmed compliance the day of the session. An experienced MRI technologist operated the scanner with the assistance of a trained RA. Upon arrival for the fMRI session, participants were rescreened for MRI safety by the MRI technologist via an interview with the adolescent and parent. Next, adolescents met with the RA and completed practice task items on a laptop to become familiarized with the scanner tasks and button response pad. Afterward, the RA described the scan to the adolescents and offered the adolescent an opportunity to lie down in a mock scanner (11 youth did so).
Adolescents then completed a 60-minute fMRI session, including four functional task-based scans, a resting-state scan, and a T1-weighted structural scan. The current study includes the first functional task-based scan only. Functional MRI scans and structural images were acquired on a Siemens 3T Allegra MR scanner. Ear buds, headphones, and head support/padding were provided to reduce noise and minimize movement. While in the scanner, adolescents viewed stimuli via a high-resolution rear projection system at the head of the bore. Mirrors attached to the head coil allowed the participant to view the display. Stimuli for the reward task were presented via E-Prime software. All participants were compensated $50 for participating in the fMRI session.
Reward Task.
The reward paradigm was a card-guessing task developed and used by Forbes et al. (2009) with adolescents. The task was a single run, slow event-related design comprising 24 trials, 12 of which were potential win trials (six win outcomes, six neutral outcomes) and 12 of which were potential loss trials (six loss outcomes, six neutral outcomes). Trials were presented in a pseudorandom order. Although outcomes were fixed, adolescents were told that outcomes were random.
With respect to the trial structure (see Figure 1), during each 20-second trial, adolescents were first presented with a question mark and had 4 seconds to guess via button press (index finger = low, middle finger = high) whether the number of a subsequently presented card—with possible values ranging from 1 to 9—would be lower or higher than 5. After his or her prediction, the adolescent viewed shuffling cards accompanied by either an up or down arrow for 6 seconds to indicate whether the current trial was a possible win or possible loss trial, respectively. Next, adolescents viewed the “actual” card (500 ms) and then viewed feedback on the outcome of the trial (win: green upward-facing arrow; loss: red downward-facing arrow; neutral/no change: yellow circle; 500 ms). Afterward, they viewed a crosshair, which served as an intertrial interval (9 s). Participants were told that they would earn $1 for each win, lose $.50 for each loss, and experience no change in their earnings for neutral outcomes. The participants were unaware of the predetermined outcome probabilities and were led to believe that their compensation was directly tied to their performance on the task. Of note, participants were told that the RA would calculate their winnings at the end of the task, and their current total earnings were not displayed in each trial of the task. All participants who completed the reward paradigm “won” 6 and were provided with $6 in cash at the end of the session, in addition to the $50 compensation for participating in the MRI session. Analyses focused on win outcome minus neutral outcome contrasts in potential win trials (win outcome–neutral outcome).
Figure 1.
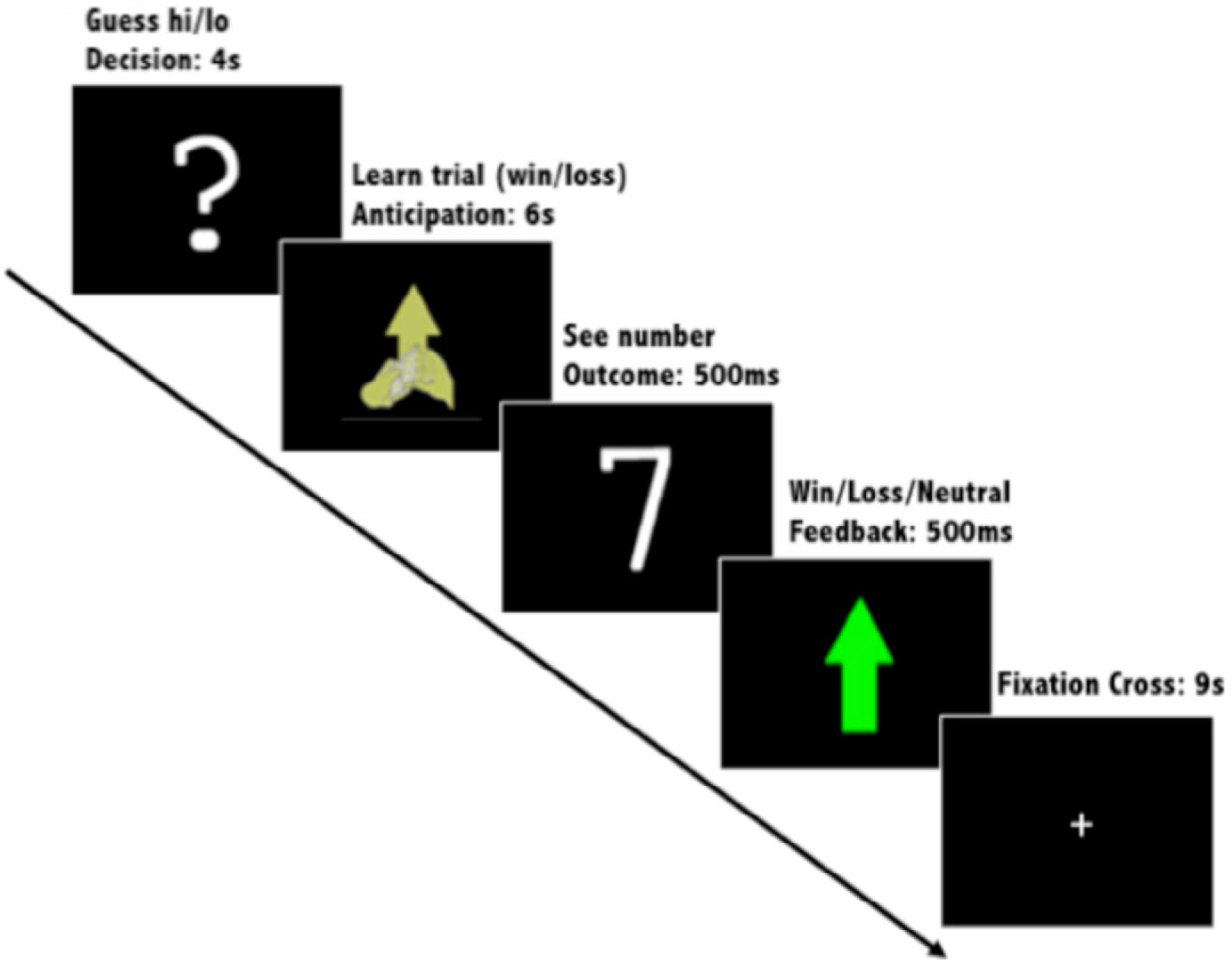
Reward task.
Image Acquisition.
Functional images of the blood oxygen level-dependent (BOLD) response during the reward task were collected using gradient-echo echoplanar images (EPI) (TR/TE: 2250/30 ms; flip = 70°; FOV: 192 mm; matrix size: 64 × 64; 40 axial 3 mm thick/1 mm gap slices). For structural imaging, we acquired T1-weighted MPRAGE anatomical images (TR/TE = 2,300/3 ms; FOV = 260 mm; matrix size = 256 9 256; 160 1-mm-thick slices).
Measures
Nonsuicidal Self-Injury.
Adolescents’ thoughts of NSSI were assessed via the NSSI-specific portions of the Self-Injurious Thoughts and Behaviors Interview (SITBI; Nock, Holmberg, Photos, & Michel, 2007) during the initial assessment session. The SITBI is a widely used measure that assesses the presence, frequency, and severity of a range of self-injurious thoughts and behaviors that has demonstrated strong interrater reliability (κ = .99), test–retest reliability (κ = .70), and convergent validity (Nock et al., 2007). Adolescents’ thoughts of NSSI were assessed with two items from the scale. The first item asked about past thoughts (“Have you ever had thoughts of purposely hurting yourself without wanting to die (e.g., cutting or burning)?” yes/no). The second item asked about future thoughts (“How much do you believe that you will think about NSSI in the future?” 0 = no chance; 4 = very much). For analyses, we created a dichotomous variable that combined responses on these two items to reflect overall past and future thoughts of NSSI (0 = no to all, 1 = yes or > 0 on either). These items were highly correlated and overlapping (r = .60, p < .000). Approximately 25% (n = 19) of youth reported experiencing past or future NSSI thoughts. Finally, we examined NSSI thoughts in this study as a proxy for risk for potential NSSI behaviors (as described in the introductory text), but we also measured past NSSI behaviors. Only one child reported past behaviors, so we were unable to examine that variable in analyses.
Data Analytic Plan
MRI Processing and Preliminary Analyses.
The fMRI data were analyzed with FSL 5.0 (FMRIB, Oxford, UK) and MATLAB (MathWorks, Natick, MA, USA). Data were slice timing and motion corrected. We inspected the data for motion. None of the youth had motion > 6 mm in any direction for one TR or motion > 1.5 mm for 80% of TRs; therefore, no youth were excluded. For the 12 youth who had motion spikes greater than1.5 mm (< 6 mm) for one TR in any direction, we censored these timepoints in the linear regression analysis using temporal masks created by fsl_motion_outliers. Data were coregistered to each participant’s mean MPRAGE image from all timepoints, and then to the Montreal Neurological Institute (MNI) template, and smoothed with a 6 mm FWHM Gaussian kernel. Regressors for the onset and duration of events of interest were convolved with double gamma functions and temporal derivative to create explanatory variables. Motion parameters (and temporal masks, when appropriate) were included in the first-level analysis. Linear regression at each voxel, using generalized least squares with a voxel-wise, temporally and spatially regularized autocorrelation model, drift fit with a Gaussian-weighted running line smoother (96s FWHM). Contrasts of parameter estimates (COPEs) for win outcome and neutral outcome were calculated for each participant.
Covariates.
We considered including child age, sex, race, handedness, and diagnosis as potential covariates in analyses. Although the presence of a diagnosis was positively correlated with thoughts of NSSI (r = .35, p = .003), DICA diagnosis was not correlated with activation in any of the reward-related ROIs; therefore, we did not include this as a covariate in analyses. None of them were significantly associated with NSSI thoughts or activation in reward-related regions and were therefore not included in analyses (see Table 2). Given prior findings of different patterns of activation in samples with girls only versus samples with both boys and girls, we also examined gender as a moderator of the association between reward-related brain activation and NSSI.
TABLE 2.
Intercorrelations, Among, Demographic Predictor and Dependent Variables
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Child age | — | −.08 | −.11 | −.03 | .03 | −.05 | −.06 | .01 | −.06 | −.13 | −.05 | −.06 | −.06 |
| 2. | Child sex | — | −.02 | .04 | −.11 | .06 | .03 | .00 | .02 | .07 | −.07 | −.02 | −.11 | |
| 3. | Handedness | — | .00 | .00 | −.08 | .02 | −.01 | −.08 | −.03 | .03 | .03 | −.07 | ||
| 4. | Race | — | −.03 | −.16 | −.04 | −.13 | −.17 | −.04 | −.01 | −.01 | −.09 | |||
| 5. | NSSI thoughts | — | .07 | .20*** | .34** | .14 | .23* | .19 | .32** | .22*** | ||||
| 6. | L NAcc | — | .66** | .62** | .68** | .76** | .64** | .59** | .75** | |||||
| 7. | L caudate | — | .61** | .41** | .69** | .85** | .71** | .61** | ||||||
| 8. | L putamen | — | .65** | .67** | .71** | .85** | .75** | |||||||
| 9. | L vmPFC | — | .56** | .48** | .56** | .82** | ||||||||
| 10. | R NAcc | — | .78** | .70** | .75** | |||||||||
| 11. | R caudate | — | .82** | .73** | ||||||||||
| 12. | R putamen | — | .71** | |||||||||||
| 13. | R vmPFC | — |
L NAcc, left nucleus accumbens activation; L caudate, left caudate activation; L putamen, left putamen activation; L vmPFC, left ventromedial prefrontal cortex activation; R NAcc, right nucleus accumbens activation; R caudate, right caudate activation; R putamen, right putamen activation; R vmPFC, right ventromedial prefrontal cortex activation.
Note. Race = coded as White (0)/non-White (1).
Statistical significance denotes as follows:
p < .10,
p < .05,
p < .01.
Analyses.
Analyses focused on a priori ROIs related to reward processing (L & R NAcc, L & R caudate, L & R putamen, L & R vmPFC). Subcortical ROIs were created from the Harvard-Oxford atlas in FSL, and cortical ROIs (L&R vmPFC) were created from the AAL atlas. Mean COPE values from the first-level analyses of the win trials and neutral trials were extracted from the ROIs and win–neutral scores were calculated. Independent-samples t tests were conducted to compare BOLD activation in reward-related regions upon the receipt of money between adolescents who did and did not report thoughts of NSSI. We also used logistic regressions to test gender as a moderator of activation in reward regions and adolescents’ thoughts of NSSI. To control for multiple comparisons, a Benjamini–Hochberg false discovery rate (FDR) correction was applied at p < .05. Both FDR-corrected and uncorrected p values, in addition to estimates of effect size, are reported.
RESULTS
Data Inspection
Region of interest activation data were examined for outliers (values > 3 SDs above the mean). L and R NAcc activation and R putamen each had one outlier case, and the L caudate had 2 outliers in the win–neutral condition. Outliers were set to 3 SDs above or below the mean; similar procedures have been used in prior fMRI studies.
Adolescent NSSI Thoughts and Reward-Related Activation
Putamen.
Early adolescents’ thoughts of NSSI were significantly associated with both L and R putamen activation in response to the reward outcome condition with FDR correction and with large effect sizes (see Table 3, Figure 2). This finding suggests that adolescents who have endorsed current or past thoughts of NSSI show elevated bilateral activation in the putamen compared to their non-NSSI thinking counterparts.
TABLE 3.
Differences Between Reward-Related ROI Activation Among NSSI Ideators and Non-ideators in Win–Neutral Reward Outcome Condition
| No NSSI thoughts M (SD) | NSSI thoughts M (SD) | t value | Adjusted p value | p value | Cohen’s d | |
|---|---|---|---|---|---|---|
| L NAcc | 11.87 (23.62) | 15.34(22.05) | −.55 | .586 | .586 | .15 |
| L caudate | 1.32 (23.47) | 12.26 (22.45) | −1.73 | .142 | .089 | .48 |
| L putamen | .26 (15.79) | 14.40 (20.66) | −3.03 | .024 | .003 | .77 |
| L vmPFC | 6.36 (20.20) | 13.51 (29.56) | −1.15 | .291 | .255 | .28 |
| R NAcc | 7.65 (18.40) | 18.39(23.25) | −2.00 | .132 | .05 | .52 |
| R caudate | 1.94 (20.99) | 11.10(20.79) | −1.60 | .192 | .114 | .44 |
| R putamen | −0.58 (15.04) | 11.92 (19.96) | −2.79 | .028 | .007 | .71 |
| R vmPFC | 2.64 (13.66) | 10.06 (16.96) | −1.87 | .132 | .066 | .48 |
L NAcc, left nucleus accumbens; L caudate, left caudate; L putamen, left putamen; L vmPFC, left ventromedial prefrontal cortex; R NAcc, right nucleus accumbens; R caudate, right caudate; R putamen, right putamen; R vmPFC, right ventromedial prefrontal cortex.
Figure 2.
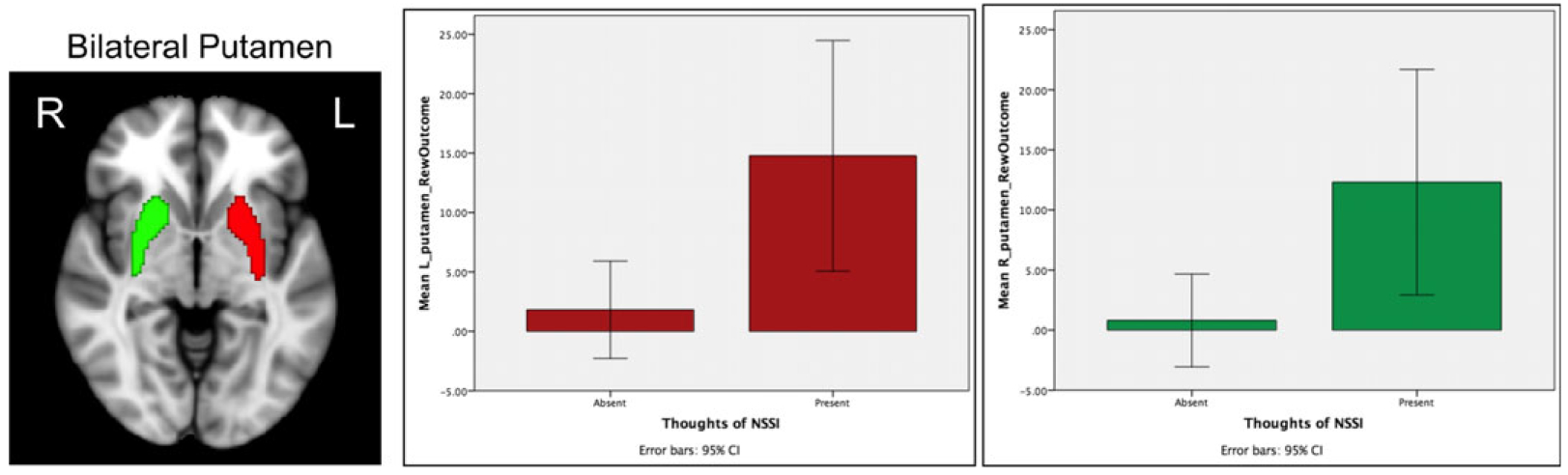
Significant brain activation to reward outcome (reward win–reward neutral) between those who did and did not endorse thoughts of nonsuicidal self-injury (NSSI) for the L and R putamen. Brain image displays bilateral putamen ROI masks from the Harvard-Oxford subcortical atlas.
Caudate.
Adolescents’ thoughts of NSSI were not significantly associated with either L or R caudate activation in the reward outcome condition after applying the FDR correction (see Table 3). Although not statistically significant, there was a medium effect size for adolescents who endorsed thoughts of NSSI to have higher mean levels of bilateral caudate activation than those without thoughts of NSSI.
NAcc.
Independent-samples t-test analyses showed that early adolescents’ thoughts of NSSI were not significantly associated with either L or R NAcc activation. Although not statistically significant, there was a medium effect size for adolescents who endorsed thoughts of NSSI to have higher mean levels of R NAcc activation than those without thoughts of NSSI.
vmPFC.
Independent-samples t tests showed that thoughts of NSSI were not associated with either L or R vmPFC activation to the win–neutral outcome condition (see Table 3). Although not statistically significant, there was a medium effect size for adolescents who endorsed thoughts of NSSI to have higher mean levels of R vmPFC activation than those without thoughts of NSSI.
Moderation by Gender
Gender was not found to be a moderator of adolescents’ thoughts of NSSI and reward-related activation in any of the ROIs.
DISCUSSION
The present study was the first to examine associations between reward-related brain activation and thoughts of NSSI in early adolescence. Based on prior research implicating aspects of positive affectivity and reward sensitivity—including abnormal reward processing—in NSSI behaviors (e.g., Schreiner et al., 2015), we hypothesized that altered activation of reward-related regions would be associated with early adolescents’ thoughts of NSSI. The results showed that youth who endorse thoughts of NSSI exhibited heightened bilateral activation in the putamen, a reward-related region in the striatum, in response to a monetary reward compared to those who did not report having such thoughts. In addition, although they did not survive FDR correction, there were nonsignificant differences between NSSI thinkers versus nonthinkers in the L and R caudate, R NAcc, and R vmPFC, with medium effect sizes. These nonsignificant patterns of findings were in the same direction as the putamen, with NSSI ideators showing heightened activation in these reward-related brain regions in response to monetary reward.
The results suggest that early adolescents who have considered NSSI—but who have not yet acted on these thoughts (with the exception of one child)—may be especially sensitive at the neural level to rewarding stimuli. Compared to children and adults, adolescents show increased striatal activation in response to the anticipation and receipt of rewards (Braams et al., 2015; Chein et al., 2011; Galvan et al., 2006), and reward hypersensitivity has been theoretically and empirically linked to the increase in risky behaviors observed among adolescents (Van Leijenhorst et al., 2010). In the context in which suicide-related experiences —including NSSI thoughts and behaviors— may be considered risky or impulsive behaviors, our findings are consistent with these theories (e.g., Youssef et al., 2016). Our findings are also consistent with past behavioral research showing that many adolescents engage in NSSI for reasons related to positive affect (e.g., cutting to “feel something”; Nock et al., 2010) and that NSSI promotes the release of endorphins, which are experienced as a rewarding “rush” or “high” following acts of self-injury (Stanley et al., 2010).
Our findings were consistent with two neuroimaging studies (one with adults and one with a sample of adolescents and adults) which found heightened activity in reward-related striatal regions (and vmPFC) in response to a cold compress and a monetary reward was associated with greater NSSI behaviors (Moreno, 2015; Osuch et al., 2014). Notably, one of the striatal regions found by the Moreno study to be associated with NSSI was the putamen, also found in our study. However, it is important to note that the current study employed a generic reward task; it is unclear whether these findings would generalize to more NSSI-specific rewards. Our findings are inconsistent with one study that found associations between lowered brain activation in striatal regions and vmPFC to anticipation of monetary reward in female 13-to 19-year-old adolescents with NSSI versus controls (Sauder et al., 2016). However, in contrast to the present study, Sauder et al. (2016) examined adolescents who were slightly older (M age = 15.8 years), who were female, and who had already engaged in NSSI behaviors. The current study’s results replicate and extend past studies’ findings by including a younger sample of youth who have considered NSSI but who have yet to engage in actual self-harm behaviors (and thus who have not yet experienced consequent neurobiological effects of self-injury). Gender was not found to be a significant moderator of the association between brain activation and NSSI thoughts, suggesting that our inclusion of boys was likely not the differentiating factor (but should be considered in future research with larger samples of boys and girls). However, developmental level and progress of NSSI severity may be an explanation.
From a developmental perspective, one way to reconcile these seemingly discrepant findings (with NSSI linked to blunted and exaggerated reward system responses in studies with different age groups) involves considering developmental factors. For example, it is possible that heightened reward system activation (perhaps particularly in the putamen) may be associated with thoughts of future NSSI during early adolescence, which may subsequently lead youth to engage in NSSI behaviors in middle-to-late adolescence. Then, the act of engaging in self-harm may alter reward system responsivity, perhaps by desensitizing middle-to-late adolescents and adults to rewarding stimuli more generally and leading to a blunted reward system response over time. Although theories likening NSSI to an addiction have generally not received empirical support (Victor, Glenn, & Klonsky, 2012), repeatedly engaging in NSSI may have the unintended consequence (similar to addictive behaviors) of altering reward circuitry, perhaps dampening reward sensitivity and reactivity.
Developmentally, in childhood and early adolescence, some children may show elevated reward system sensitivity, making it more likely that they will engage in a myriad of risky—albeit rewarding—behaviors, including NSSI. Interestingly, a similar “blunting” pattern has been shown in the early childhood stress literature, wherein some children who repeatedly engage the HPA axis in early childhood when experiencing chronic stress may over time show a pattern of blunted or hyporesponsive HPA axis activation by adolescence (e.g., Gunnar & Vazquez, 2001; Tarullo & Gunnar, 2006). Longitudinal studies are especially needed to fully understand the developmental patterns of whether reward-processing abnormalities precede or follow from engaging in NSSI, and how these trajectories change across time. It also may be that our findings are different because we included both boys and girls, whereas most other adolescent fMRI studies of NSSI only include girls (Sauder et al., 2016). It is possible that NSSI may be related to more sensation-seeking reward sensitivity for boys and dampened reward sensitivity for girls (or vice versa). However, as previously mentioned, we did not find gender moderation of links between brain function and NSSI; future studies should explore the role of gender on the relationship between neural reward function and NSSI in larger samples of adolescents, including both boys and girls.
Notably, our strongest findings for associations between brain activation to reward and NSSI thoughts were found for the putamen. Neurobiologically, the putamen and caudate may be particularly functionally related to risk behaviors in that they both, along with the nucleus accumbens, comprise the striatum, a critical component of the reward system involved in motor and action planning, risky decision making, motivation, reinforcement, and reward perception. Heavily anatomically connected to the premotor and primary motor areas of the brain (i.e., the basal ganglia), the putamen has been particularly implicated (more so than the NAcc and caudate) in evaluating actions in terms of sensory contexts and rewards. Furthermore, the putamen is particularly involved in the learning of stimulus–action–response associations and the preparation and execution of reward-oriented motor movements (Haruno & Kawato, 2006). Perhaps because the putamen has been found to integrate information regarding the expectation of a reward with associated action tendencies, the putamen has also been linked to the transition from goal-directed to habitual drug use behavior in animal models (McKim, Shnitko, Robinson, & Boettiger, 2016; Zapata, Minney, & Shippenberg, 2010). Thus, it is possible that the putamen may be involved in the transition from the consideration of self-injury to actual engagement in NSSI behaviors and, thus, it may have been particularly sensitive to our measure of NSSI, which involved questions regarding one’s actual intent to carry out future NSSI behaviors.
Limitations and Future Directions
Despite the intriguing pattern of findings, the limitations of the present study provide many avenues for future research. First, our sample consisted of predominantly White, middle-socioeconomic status early adolescent participants recruited from community settings; these results may not generalize to older, clinical, and/or ethnically and socioeconomically diverse samples of adolescents. Notably, it is still useful to understand these processes in younger, non-clinical samples—ideally before the onset and escalation of risk behaviors and associated psychopathology—to better understand processes underlying NSSI before NSSI behaviors occur. In addition, our sample included youth who endorsed thoughts of self-injury, as opposed to actual NSSI behaviors. Although these adolescents may be at risk for future NSSI, it is likely that neural patterns of reward activation may differ between those who do and do not engage in self-harm. The current study was also limited in that our paradigm only allowed us to assess adolescents’ responses to generic, monetary rewards. Future studies should employ different paradigms that include rewards that may be more salient for youth at risk for NSSI, such as social or NSSI-specific rewards.
Given that the act of engaging in self-injury may alter reward pathways, future studies should employ longitudinal analyses that follow early adolescents through young adulthood in order to better elucidate how changes in reward sensitivity contribute to the etiology and maintenance of NSSI and other suicide-related behaviors over time. Furthermore, given the importance of social– contextual factors on adolescents’ reward responsivity as well as their susceptibility to deviant risk behavior, future studies should consider examining what developmental factors (such as environmental factors) lead to altered reward system responsivity. Finally, it remains unclear whether aberrant reward processing underlying NSSI differs from that underlying other risky behaviors, many of which share similarities such as high impulsivity and reward sensitivity. High striatal activation to reward may be a “transdiagnostic” factor underlying substance use, risky sex, and a myriad of other risk-taking behaviors including NSSI. However, it is also possible that there are alterations in reward processing specific to NSSI. For example, NSSI commonly co-occurs with depression, which has been linked to a blunted reward system response in adolescents (Forbes et al., 2009). Given this, reward system activation among those who engage in NSSI may be slightly attenuated compared to other types of risky behaviors. Future studies should seek to elucidate which, if any, aspects of reward processing are NSSI-specific.
The present study offers a number of promising implications aimed at the identification, prevention, and treatment of individuals at risk for self-harm. First, results of the current study suggest that interventions may target youth with increased reward sensitivity for programs to prevent self-harm. Second, our findings suggest that interventions should focus on decreasing reward sensitivity in at-risk youth or channeling at-risk adolescents’ heightened reward responsiveness toward more positive alternative behaviors instead of risky behaviors such as NSSI. Future studies should consider examining other structural and functional brain factors among early adolescents who endorse thoughts of NSSI to inform targeted prevention efforts for those at risk for engaging in these behaviors.
Contributor Information
Erika E. Forbes, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Tara M. Chaplin, Department of Psychology, George Mason University, Fairfax, VA, USA;
REFERENCES
- BRAAMS BR, VAN DUIJVENVOORDE AC, PEPER JS, & CRONE EA (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN AL, DERBIDGE CM, COONEY E, HONG PY, & LINEHAN MM (2009). Temperament as a prospective predictor of self-injury among patients with borderline personality disorder. Journal of Personality Disorders, 23, 122–140. [DOI] [PubMed] [Google Scholar]
- CHEIN J, ALBERT D, O’BRIEN L, UCKERT K, & STEINBERG L (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVAZZA A (1998). The coming of age of self-mutilation. Journal of Nervous and Mental Disease, 186, 259–268. [DOI] [PubMed] [Google Scholar]
- FORBES EE, HARIRI AR, MARTIN SL, SILK JS, MOYLES DL, FISHER PM,et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES EE, RYAN ND, PHILLIPS ML, MANUCK SB, WORTHMAN CM,MOYLES DL, et al. (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVAN A (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVAN A, HARE TA, PARRA CE, PENN J, VOSS H, GLOVER G, et al. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience, 26, 6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVAN A, HARE T, VOSS H, GLOVER G, & CASEY BJ (2007). Risk-taking and the adolescent brain: Who is at risk? Developmental Science, 10(2), F8–F14. [DOI] [PubMed] [Google Scholar]
- GUNNAR MR, & VAZQUEZ DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. [DOI] [PubMed] [Google Scholar]
- HAMZA CA, STEWART SL, & WILLOUGHBY T (2012). Examining the link between non-suicidal self-injury and suicidal behavior: A review of the literature and an integrated model. Clinical Psychology Review, 32, 482–495. [DOI] [PubMed] [Google Scholar]
- HANKIN BL, & ABELA JR (2011). Nonsuicidal self-injury in adolescence: Prospective rates and risk factors in a 2-year longitudinal study. Psychiatry Research, 186, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARUNO M, & KAWATO M (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology, 95, 948–959. [DOI] [PubMed] [Google Scholar]
- JANIS IB, & NOCK MK (2008). Behavioral forecasts do not improve the prediction of future behavior: A prospective study of self-injury. Journal of Clinical Psychology, 64, 1164–1174. [DOI] [PubMed] [Google Scholar]
- JOINER TE (2005). Why people die by suicide. Cambridge, MA: Harvard University Press. [Google Scholar]
- LLOYD-RICHARDSON EE, PERRINE N, DIERKER L, & KELLEY ML (2007). Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychological Medicine, 37, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKIM TH, SHNITKO TA, ROBINSON DL, & BOETTIGER CA (2016). Translational research on habit and alcohol. Current Addiction Reports, 3, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORENO D, BELTRI R, & RODR IGUEZ A (2015). Neurophysiological correlates of reward processing and cognitive control in borderline personality disorder patients with and without self-harm history. Unpublished doctoral dissertation. University of Barcelona, Barcelona, Spain. [Google Scholar]
- NOCK MK, HOLMBERG EB, PHOTOS VI, & MICHEL BD (2007). Self-injurious thoughts and behaviors interview: Development, reliability, and validity in an adolescent sample. Psychological Assessment, 19, 309–317. [DOI] [PubMed] [Google Scholar]
- NOCK MK, JOINER TE, GORDON KH, LLOYD-RICHARDSON E, & PRINSTEIN MJ (2006). Non-suicidal self-injury among adolescents: Diagnostic correlates and relation to suicide attempts. Psychiatry Research, 144, 65–72. [DOI] [PubMed] [Google Scholar]
- NOCK MK, & PRINSTEIN MJ (2004). A functional approach to the assessment of self-mutilative behavior. Journal of Consulting and Clinical Psychology, 72, 885. [DOI] [PubMed] [Google Scholar]
- NOCK MK, PRINSTEIN MJ, & STERBA SK (2010). Revealing the form and function of self-injurious thoughts and behaviors: A real-time ecological assessment study among adolescents and young adults. Journal of Violence, 1, 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSUCH E, FORD K, WRATH A, BARTHA R, & NEUFELD R (2014). Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Research: Neuroimaging, 223(2), 104–112. [DOI] [PubMed] [Google Scholar]
- REICH W (2000). Diagnostic Interview for Children and Adolescents (DICA). Journal of the American Academy of Child and Adolescent Psychiatry, 39, 59–66. [DOI] [PubMed] [Google Scholar]
- SAUDER CL, DERBIDGE CM, & BEAUCHAINE TP (2016). Neural responses to monetary incentives among self-injuring adolescent girls. Development and Psychopathology, 28, 277–291. [DOI] [PubMed] [Google Scholar]
- SCHREINER MW, KLIMES-DOUGAN B, BEGNEL ED, & CULLEN KR (2015). Conceptualizing the neurobiology of non-suicidal self-injury from the perspective of the Research Domain Criteria Project. Neuroscience & Biobehavioral Reviews, 57, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY B, SHER L, WILSON S, EKMAN R, HUANG YY, & MANN JJ (2010). Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. Journal of Affective Disorders, 124, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG L (2010). A behavioral scientist looks at the science of adolescent brain development. Brain and Cognition, 72, 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARULLO AR, & GUNNAR MR (2006). Child maltreatment and the developing HPA axis. Hormones and Behavior, 50, 632–639. [DOI] [PubMed] [Google Scholar]
- VAN LEIJENHORST L, ZANOLIE K, VAN MEEL CS, WESTENBERG PM, ROMBOUTS SA, & CRONE EA (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–69. [DOI] [PubMed] [Google Scholar]
- VICTOR SE, GLENN CR, & KLONSKY ED (2012). Is non-suicidal self-injury an “addiction”? A comparison of craving in substance use and non-suicidal self-injury. Psychiatry Research, 197, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITLOCK J, MUEHLENKAMP J, PURINGTON A, ECKENRODE J, BARREIRA P, BARAL ABRAMS G, et al. (2011). Nonsuicidal self-injury in a college population: General trends and sex differences. Journal of American College Health, 59, 691–698. [DOI] [PubMed] [Google Scholar]
- YOUSSEF GJ, WHITTLE S, ALLEN NB, LUBMAN DI, SIMMONS JG, & YÜCEL M (2016). Cognitive control as a moderator of temperamental motivations toward adolescent risk-taking behavior. Child Development, 87, 395–404. [DOI] [PubMed] [Google Scholar]
- ZAPATA A, MINNEY VL, & SHIPPENBERG TS (2010). Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. The Journal of Neuroscience, 30, 15457–15463. [DOI] [PMC free article] [PubMed] [Google Scholar]


