Abstract
Introduction
5-aminolevulinic acid induced protoporphyrin IX (5-ALA-PpIX) fluorescence guidance has emerged as a valuable surgical adjunct for resection of intracranial tumors.
Methods
Here we present a focused review on 5-ALA-PpIX fluorescence guidance for meningiomas.
Results
We discuss the clinical studies and specific applications to date as well as the two main intraoperative fluorescence technologies applied to meningiomas.
Conclusions
The use of 5-ALA-PpIX in meningiomas holds promising potential so neurosurgeons can improve surgical outcomes for patients with meningiomas as well as be pioneers in developing improved fluorescence imaging technologies.
Keywords: Fluorescence-guided surgery, 5-Aminolevulinic acid, Protoporphyrin IX, Quantitative fluorescence, Meningioma, Spectroscopy
Introduction
Meningiomas account for approximately 20% of all intracranial tumors and depending on their location, biology and prior treatment pose a surgical challenge to the neurosurgeon [1–3]. Maximizing the extent of resection plays a critical role in minimizing tumor recurrence as well as overall disease morbidity and mortality [4]. In recent years, 5-aminolevulinic acid induced protoporphyrin IX (5-ALA-PpIX) fluorescence guidance has emerged as a valuable surgical adjunct for resection of high grade gliomas [5]. Nevertheless, the role of 5-ALA-PpIX as a surgical adjunct for meningiomas has had a more limited experience.
Here, we present a focused discussion on 5-ALA-PpIX fluorescence guidance for meningiomas in neurosurgery. We provide the reader with an overview of the major studies using 5-ALA-PpIX for meningiomas, including key applications of this technology for aspects of meningioma resection which are specifically challenging and particular to this pathology. The goal of this study is to provide the reader a thorough overview of the current clinical experience highlighting the surgical implementations, intraoperative technologies, and possible role of 5-ALA-PpIX fluorescence in the surgical management of meningiomas.
Technology
Technologies in clinical use for 5-ALA-PpIX fluorescence guided surgery of meningiomas can be described as those for visible fluorescence imaging (vFI) [5–9] and those for quantitative fluorescence imaging (qFI) [3, 10–12]. Briefly, tumor tissue will selectively accumulate the fluorescent compound, PpIX, following administration of 5-ALA [13]. Tissues are illuminated with violet light (i.e., 405 nm wavelength light) to excite PpIX molecules, which causes emission of red-pink fluorescent light (i.e., 620–710 nm wavelength light). The emitted red-pink fluorescent light is subsequently visualized by the surgeon through the oculars of the surgical microscope, or collected by a digital camera integrated to the surgical microscope and displayed on a screen [11, 14, 15]. The surgeon may then judge whether or not the tissue fluoresces, some instances characterizing further as faint or robust and in some investigative studies, assigning a qualitative score to what they see in either a four-tier classification system (0 = no fluorescence to various intensities of red-pink fluorescence up to 3 = very bright fluorescence) or a threetier classification system 0 = no fluorescence, 1 = vague fluorescence, 2 = strong fluorescence (Fig. 1) [9, 15–20]. This qualitative or visual assessment we call visual fluorescence imaging (vFI), because the surgeon assesses fluorescence, or assigns a fluorescence score, based on what he or she sees prior to any specific processing of the fluorescence data.
Fig. 1.
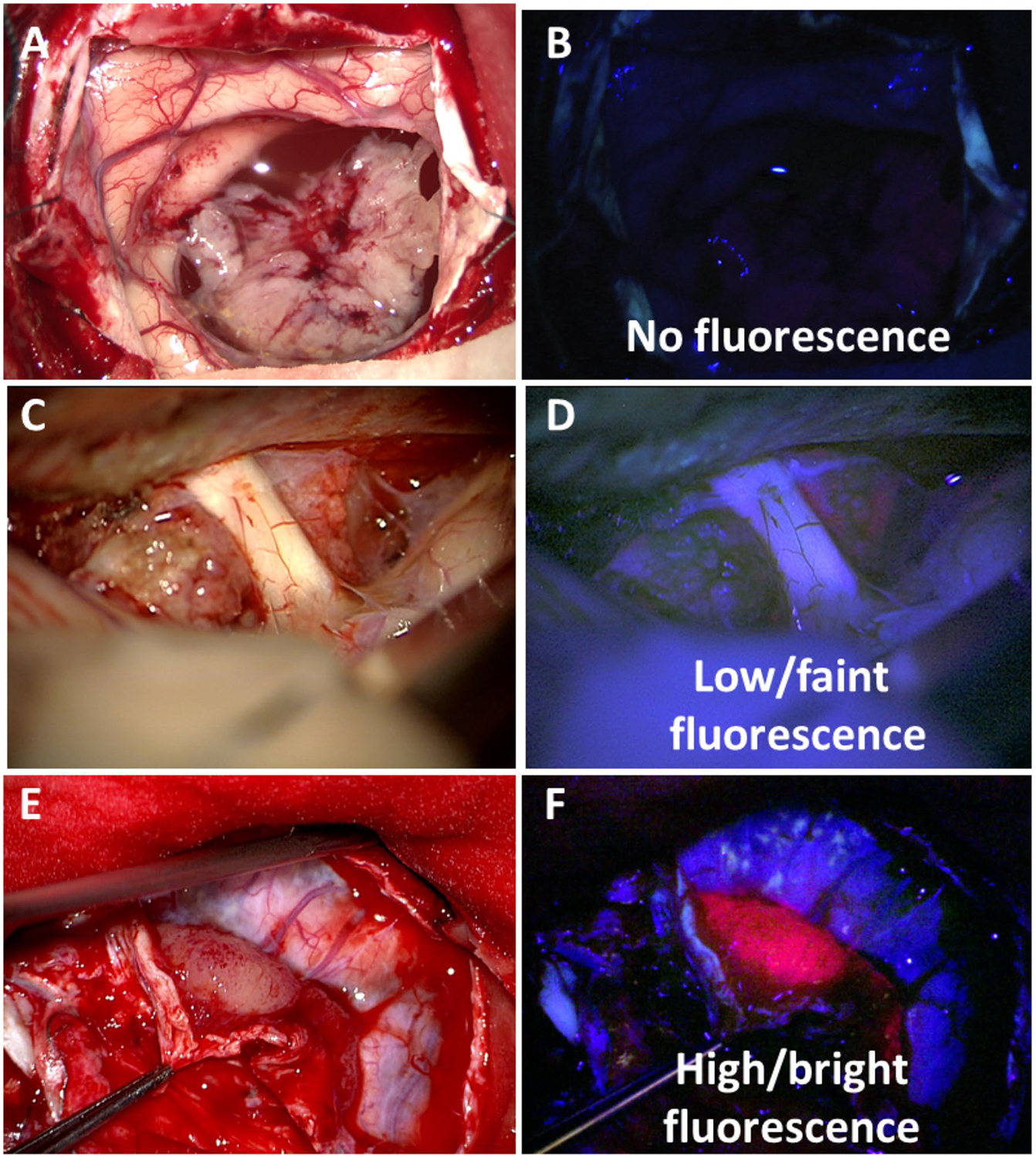
Qualitative intraoperative visible fluorescence scores. Representative intraoperative white and violet-blue light images with a, b tumor with no visible fluorescence, c, d low or faint visible fluorescence in underlying tumor, and e, f high or bright visible fluorescence in the tumor bulk. Areas with visible fluorescence demonstrate generally a homogenous character
Visual fluorescence assessment, while intuitive and more easily implemented, also has limitations. It can misrepresent the levels of actual (i.e., absolute) PpIX biomarker in tissue. This is because what the surgeon “sees”, without accounting for more complex issues of how light interacts with tissue, will not actually reflect the true amount of PpIX. The subjectivity of vFI assessments on tissue fluorescence can be understood if we understand the concept of attenuation of light in tissue [10–12, 21, 22]. The attenuation of light in tissue, i.e., the loss of light as it passes through tissue, is a well described concept in biomedical optics [10, 11, 23]. In the above case of PpIX, the excitation light illuminated on tissue passes through tissue components prior to interacting (i.e., exciting) with PpIX molecules. As the excitation light travels through tissue, it will be differentially “lost” (e.g., undergo scattering and absorption) before interacting with PpIX. This loss of light through tissue will be a function of the specific differences in tissue biology (e.g., cellularity, vascularity, blood) at each spatial location in tissue. Similarly, when PpIX emits light (i.e., fluorescent light), this light will also be differentially “lost” before reaching the surgeon’s eyes, or collected unto a digital camera. Therefore, two tissues with the same absolute levels of PpIX but different spatial microscopic biological composition, will display different degrees of visual fluorescence (e.g., one tissue may display bright red-pink fluorescence while the other displays no fluorescence) [11, 12, 21–23].
Recent advances in biomedical optics with applications in neurosurgery provide a means to account for this differential loss of light in tissue. Technologies that provide means of measuring the microscopic properties of tissue can be used to correct for this differential loss of both excitation and emission light during PpIX fluorescence imaging, and thus enable the user (i.e., neurosurgeon) to accurately measure the absolute levels of PpIX found in tissue. This quantitative, absolute assessment of the levels of PpIX in tissue we call quantitative fluorescence imaging (qFI) [3, 11, 12, 20–24].
The most common clinical system for vFI used in meningiomas is the surgical microscope adapted for fluorescence imaging (e.g., Zeiss, Leica). These systems are equipped with a filter system that enables illumination with excitation light at 405 nm as well as illumination light at approximately 450–470 nm to provide additional illumination of tissue background, i.e., provide additional anatomical detail otherwise lost from filtration of excitation light. This system is then equipped with a long pass filter that enables passage of light greater than 450 nm, effectively blocking the 405 nm excitation light but allowing passage of the blue illumination light and the emitted PpIX red-pink fluorescence. This light is then detected by the surgeon via the surgical oculars or by a digital camera integrated into the microscope for digital display [9, 14, 15, 20, 23].
The clinical system described in the literature for qFI in meningiomas has been a handheld quantitative spectroscopic probe that is placed in contact with tissue to interrogate a 1.1 mm diameter (~ 1 mm2 area) region of tissue [3, 6, 12]. The qFI probe is a fiber optic bundle connected to a 405 nm light module (i.e., light emitting diode-LED), two white light modules and one spectrometer for collection of light. The tissue is sequentially illuminated with white light and 405 nm light. The reflected white light and fluorescence emissions are collected via the fiber optics and the spectrometer. This spectroscopic data is then processed using a model of how light interacts with tissue to extract the intrinsic optical properties of tissue (e.g., scattering, absorption). Estimated tissue optical properties are used to account for light loss to effectively correct the fluorescence loss, and to derive the absolute concentration of PpIX in tissues [12, 25, 26]. Concentration values can be used to determine whether the tissue is likely to be tumor or normal. As such, these assessments are quantitative and based on the absolute levels of tumor biomarker, unlike the subjective vFI scores which are a function of tumor biomarker levels and differences in biological composition of tissue. Quantitative fluorescence can also be assessed using wide-field imaging systems [24, 27], although these are a newer development and experience with these in meningioma is more limited [27].
The vFI and qFI systems present with different advantages and disadvantages (Table 1). Visual fluorescence is implemented in a manner more similar to current surgical practice, providing the surgeon immediate feedback through visual assessments, and thus is faster and less disruptive to the surgical workflow. Meanwhile, qFI requires the surgeon to place a probe in contact with tissue and then read off a concentration value to make a clinical decision [2, 9, 13–15, 20, 23]; wide-field qFI superposes quantitative information on the surgical field using either a heads-up display or external monitor and has greater ease of use. Visual fluorescence is prone to subjective, potentially inaccurate assessments of PpIX, which qFI significantly corrects for by accounting for tissue heterogeneity and its influence on how light travels in tissue. As such, qFI has also been reported to improve sensitivity for detecting levels of diagnostically significant PpIX in tissue that would have otherwise gone undetected and “missed” using vFI alone [12, 24, 26]. In the following sections, we present a more detailed description of the specific applications and use of both vFI and qFI and how they have been shown to be a useful adjunct during 5-ALA-PpIX fluorescence guided meningioma surgery.
Table 1.
Visual and Quantitative Fluorescence
| Advantages | Disadvantages | |
|---|---|---|
| Visual fluorescence | Less disruptive | Subjective, non quantitative |
| More similar to current surgical microscopy practice | Misestimates of fluorescence levels | |
| Immediate, visual feedback | Less sensitive | |
| Quantitative fluorescence | Objective, quantitative | More disruptive |
| Highly accurate estimates of fluorescence levels | Requires contact with tissue | |
| High sensitivity | Feedback less intuitive, ~ 1 s delay |
Visible fluorescence in meningiomas
Fluorescence in intracranial meningiomas
Case reports and first patient series constitute the current studies on intraoperative use of 5-ALA-PpIX fluorescence in intracranial meningiomas [1–3, 7–9, 12, 28–37] (Table 2) (Fig. 2). The first patient series by Kajimoto et al. included 24 patients with visible fluorescence observed in 83% of meningiomas [1]. Similarly, Coluccia et al. found visible fluorescence in 94% of patients (31/33) [8]. In a smaller, more recent series, Valdes et al. reported visible fluorescence in 80% of cases (12/15) [3]. In a study by Marbacher et al. visible fluorescence was observed in 77% of cases (85/110) [36]. Cornelius et al. also observed visible fluorescence in 94% of meningiomas (29/31) [32]. Additionally, Potapov et al. reported visible fluorescence in the vast majority (96%) of meningiomas in a series of 28 patients [37]. In the largest series to date comprising 204 meningiomas by Millesi et al., visible fluorescence was reported in 185 cases (91%) [9]. These studies would inform the neurosurgeon that when using 5-ALA-PpIX fluorescence guidance, they can expect to observe visible intraoperative fluorescence in the majority of intracranial meningiomas (range: 77–96%).
Table 2.
Literature overview of studies describing the application of 5-ALA in intracranial meningiomas
| Article | N | WHO | Fluorescence status | Fluorescence quality | Fluorescence homogeneity | Visible fluorescence at specific sites | |||
|---|---|---|---|---|---|---|---|---|---|
| Dural tail | Inflitrated Bone Flap | Satellite lesions | Adjacent cortex | ||||||
| Kajimoto et al. [1] | 24 | 18 °I | 20 positive (83%) | – | – | 6/8 (75%) | 1/1 (100%) | – | – |
| 4 °II | 4 negative (17%) | ||||||||
| 2 °III | |||||||||
| Morofuji et al. [28] | 1 | 1 °II | 1 positive (100%) | – | – | 0/1 (0%) | 1/1 (100%) | – | – |
| Eljamel et al [35] | 2 | – | 2 positive (100%) | – | – | – | – | – | – |
| Coluccia et al. [8] | 33 | 26 °I | 31 positive (94%) | – | – | – | 1/1 (100%) | – | – |
| 6 °II | |||||||||
| 1 °III | 2 negative (6%) | ||||||||
| Bekelis et al. [6] | 1 | 1 °I | 1 positive (100%) | – | – | – | – | – | – |
| Whitson et al [34] | 1 | 1 °II | 1 positive (100%) | – | – | 0/1 (0%) | – | – | – |
| Chae et al. [7] | 1 | 1 °I | 1 positive (100%) | – | – | – | – | – | – |
| Della Puppa et al. [38] | 3 | – | 2 positive (67%) | – | – | – | 2/3 (67%) | – | – |
| 1 negative (33%) | |||||||||
| Cornelius et al. [29] | 1 | 1 °I | 1 positive (100%) | – | – | – | – | – | – |
| Marbacher et al. [36] | 110 | 94 °I | 85 positive (77%) | – | – | – | – | – | – |
| 14 °II | 25 negative (23%) | ||||||||
| 2 °III | |||||||||
| Valdes et al. [3] | 15 | 11 °I | 12 positive (80%) | – | – | – | – | – | – |
| 4 °II | 3 negative (20%) | ||||||||
| Della Puppa et al. [38] | 12 | 10 °I | 12 positive (100%) | – | – | – | 12/12 (100%) | – | – |
| 2 °II | |||||||||
| Wilbers et al. [31] | 1 | 1 °II | 1 positive (100%) | – | – | – | – | – | 1/1 (100%) |
| Cornelius et al. [32] | 31 | 19 °I | 29 positive (94%) | 12 strong (41%) | – | – | – | 8/12 (67%) | |
| 8 °II | |||||||||
| 4 °III | 2 negative (6%) | 17 vague (59%) | |||||||
| Motekallemi et al. [2] | 2 | 2 °III | 2 positive (100%) | – | – | ||||
| Millesi et al. [9] | 204 | 155 °I | 185 positive (91%) | 168 strong (91%) | 113 homogeneous | 0/89 (0%) | 13/13 (100%) | 7/7 (100%) | 20/80 (25%) |
| 33 °II | 19 negative (9%) | 17 vague (9%) | (75%) | ||||||
| 16 °III | 37 inhomogeneous (25%) | ||||||||
| Potapov et al. [37] | 28 | 23 °I | 27 positive (96%) | 7 very intense (26%) | 8/8 (100%) | ||||
| 5 °II | 1 negative (4%) | 6 bright (22%) | |||||||
| 14 poor (52%) | |||||||||
| Scheichel et al. [30] | 1 | 1 °II | 1 positive (100%) | 1 strong (100%) | 1/1 (100%) | ||||
WHO world health organization
Fig. 2.
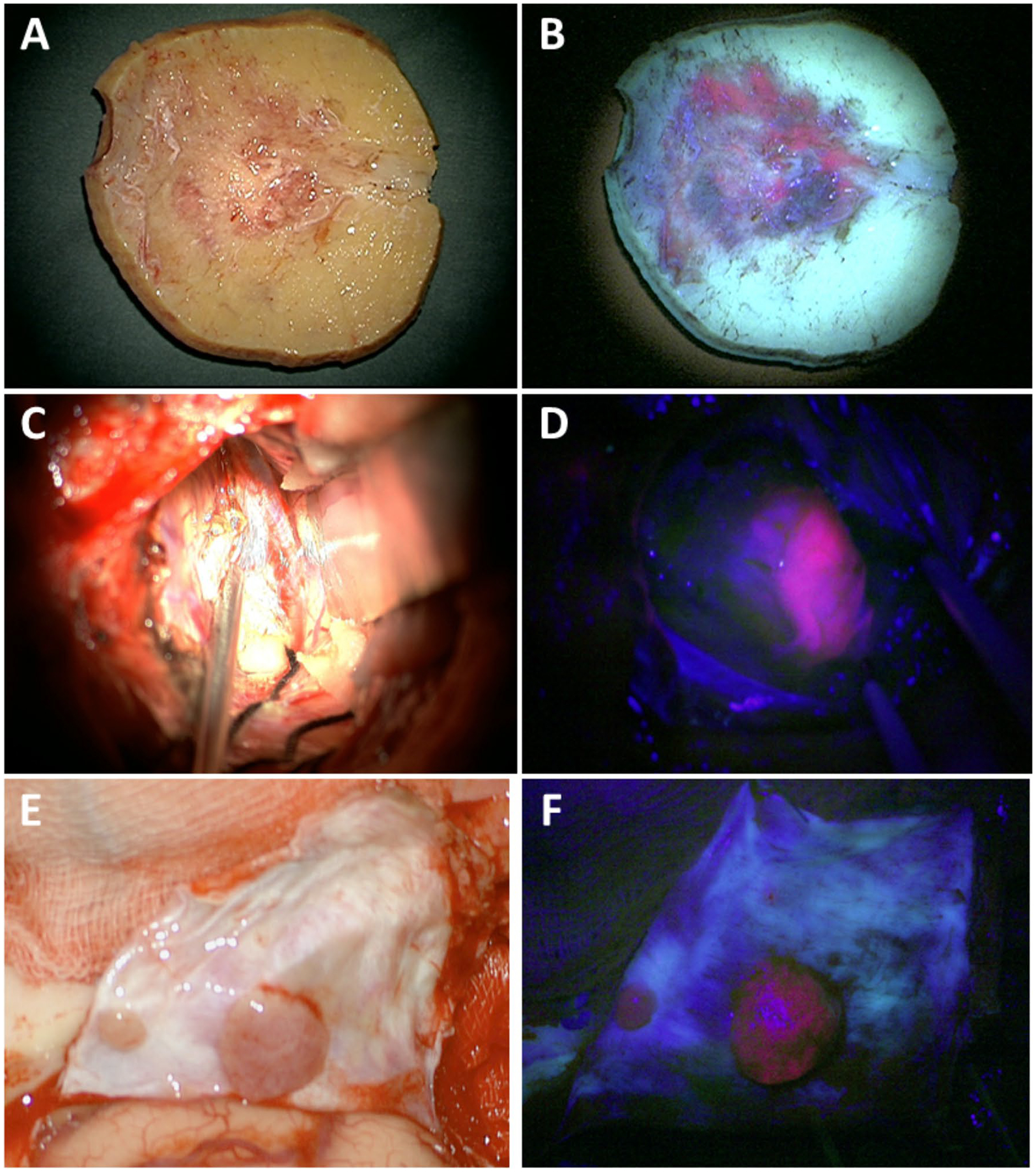
5-ALA-PpIX fluorescence for infiltrated bone, cortex and satellite lesions. Corresponding intraoperative white and violet-blue light images for a, b infiltrated bone flap with clear area of positive visible fluorescence corresponding to area of infiltrated bone not clearly visualized using white light; c, d adjacent cortex with high visible fluorescence corresponding to potentially tumor infiltrated cortex not appreciable with white light alone; and e, f multiple satellite lesions distant from tumor bulk with high visible fluorescence enables localization of distant spread
Fluorescence score and fluorescence homogeneity
The subjective visible fluorescence intensity score and degree of visible fluorescence homogeneity have been reported in a limited number of studies. Cornelius et al. scored intraoperative fluorescence as “no”, “low” or “high” fluorescence in 31 cases. Six percent of cases (2/31) were scored as “no” fluorescence; 55% were scored as “low” (17/31); and 39% of cases were scored as “high” (12/31) [32]. Millesi et al. observed strong visible fluorescence in a significantly larger proportion of cases as compared to vague fluorescence (91% vs 9%) [9]. Valdes et al. noted that when fluorescence was present in 80% of cases (12/15), fluorescence was “strong” or “high” and homogenous in character [3]. This was noted to be in contrast to the authors’ experience with gliomas in which visible fluorescence can be highly heterogenous and “patchy” in character [3]. Potapov et al. described using a four-tier classification system: poor rose-coloured fluorescence in 52% of cases; bright red fluorescence in 22% of cases; and very intense scarlet fluorescence in 26% of cases [37].
The study of Millesi et al. also described the fluorescence homogeneity in a large patient cohort [9]. Of the analyzed cases, fluorescence when present was homogeneous in 75% of cases (113/150) [9]. These studies further inform the neurosurgeon regarding the intensity and character of visible fluorescence to expect when operating on meningiomas using 5-ALA-PpIX fluorescence guidance. Neurosurgeons can expect that visible fluorescence, when present, will usually demonstrate “strong” or “high” intensity (range: 39–100% of cases), and that most meningiomas will demonstrate an intratumoral homogenous fluorescence pattern (75–100%).
Fluorescence and histopathology
The majority of studies have not shown a significant correlation between histopathological findings (e.g., WHO grade, mitotic index, MIB index) and visible intraoperative fluorescence in meningiomas [1, 3, 8]. Also, the largest study to date by Millesi et al. did not find a correlation between histology and intraoperative fluorescence [9]. However, Cornelius et al. did report a significant correlation between fluorescence intensity and WHO grade in a smaller series comprising 31 meningiomas (WHO I, 19; II, 8, III, 4) (ρ = 0.557, p-value = 0.001) [32]. Although the majority of studies to date have not shown a significant correlation between visible fluorescence and tumor histopathological findings, these have been mostly smaller studies using characteristics such as WHO grade, MIB index or mitotic index. Future work on larger series should help inform the community regarding additional molecular markers and their relationship (e.g., BRAF) to visible fluorescence.
Fluorescence in the dural tail
According to the Simpson classification, tumor removal including the surrounding dural tail is fundamental to minimize the risk of local tumor recurrence [4]. 5-ALA-PpIX fluorescence might be able to help the neurosurgeon to visualize the tumor infiltrated dural tail. In this sense, Kajimoto et al. reported visible fluorescence in the dural tail in 75% of cases(6/8), whereas tumor infiltration was verified by histopathology in 5 of these 6 fluorescing cases [1]. In contrast, Millesi et al. did not observe visible fluorescence in any of the analyzed dural tails (89 cases with dural tails on MRI) [9]. However, histopathological analyses revealed tumor cells in only 5 (31%) of the histopathologically investigated 16 dural tails [9]. Two additional case reports did not observe any visible fluorescence in the dural tail [28, 34]. Thus, the largest series in the literature to date suggests that intraoperative visible fluorescence tools using 5-ALA fluorescence are not likely to be helpful in visualizing the infiltrating dural tail.
Fluorescence in adjacent cortex and/or arachnoid
Millesi et al. reported findings of residual fluorescence in adjacent cortex following resection in a subgroup of patients [9]. The authors found residual fluorescence in the adjacent cortex in 25% of evaluated cases (20/80), with a significant association found in cases with a disrupted arachnoid (41% vs 11%, p-value = 0.002) [9]. They note no significant correlation between WHO grade and residual fluorescence in adjacent cortex [9]. The authors, however, did not provide histopathologic confirmation regarding the degree of tumor infiltration in areas of cortex and/or arachnoid with positive visible fluorescence [9]. In this sense, Cornelius et al. also observed cortical fluorescence at the brain-tumor interface in 8 high-grade meningioma patients. Biopsies taken from these areas revealed vital tumor cells in all cases [32]. Furthermore, histopathologically confirmed tumor tissue within residual fluorescence was also noted in adjacent dura, arachnoid, and cortex in a case report of an atypical WHO grade II meningioma [31]. These residual tumor cells would have otherwise gone unidentified and unresected, providing a nidus of potential recurrence despite achieving a Simpson grade I resection by standard gross intraoperative assessment. Thus, some data in the literature suggest that 5-ALA-PpIX fluorescence might be a useful surgical adjunct to help identify macroscopic residual tumor in adjacent cortex and arachnoid.
Fluorescence for detection of bone infiltration
Accurate assessment of bone infiltration is a significant factor in minimizing the degree of tumor recurrence in meningiomas. Multiple studies have shown the presence of visible fluorescence in tumor infiltrated bone [8, 9, 32, 37, 38]. Also, Scheichel et al. reported visible fluorescence in the bone flap continuous to the periosteal layer, temporalis muscle fascia and muscle [30]. Furthermore, Della Puppa et al. reported their experience in 12 patients with histopathologically confirmed tumor-infiltrated bone and visible fluorescence [38]. They note a specificity of 100%, sensitivity of 89%, PPV of 100%, and NPV of 83% for 5-ALA-PpIX for detecting bone invasion [38]. According to Della Puppa, tumor infiltration was histopathologically confirmed in all specimens (57/57) of fluorescing bone (hyperostotic and non-hyperostotic). Histopathological analysis confirmed that non-fluorescing bone (n = 23 hyperostotic and n = 18 non-hyperostotic) demonstrated tumor infiltration only in 30% of hyperostotic samples (7/23) [38]. The study by Millesi et al. on 204 meningiomas reported visible fluorescence in all histopathologically confirmed cases (13/13) of bone flaps suspected for bone invasion on preoperative imaging [9]. These limited results suggest that 5-ALA-PpIX may serve a useful role in identifying bone invasion during meningioma resection.
Fluorescence for detection of satellite lesions
Satellite lesions can be found up to 3 cm from the main meningioma tumor bulk [39]. Such satellite lesions are likely to go undetected, resulting in significant tumor recurrence and associated morbidity. Millesi et al. were able to identify seven small satellite lesions in the proximity of the main meningioma bulk that would have gone undetected with conventional white light microscopy. These satellite lesions were not noted on preoperative MRI but were only identified intraoperatively by inspection of surrounding dura [9]. Although limited, these results further suggest the role of 5-ALA-PpIX fluorescence might prove a powerful intraoperative tool to help detect and visualize both smaller satellite lesions as well as larger areas of tumor infiltration when craniotomy exposure is sufficient.
Fluorescence in spinal meningiomas
The use of 5-ALA has been also described for spinal meningiomas. Muroi et al. first described the presence of visible fluorescence in a spinal meningioma, noting the utility of visible fluorescence to identify residual tumor which would have otherwise gone unresected using macroscopic white light inspection alone [40]. In the first small patient series, Eicker et al. found visible fluorescence in 88% (7/8) of spinal meningiomas [41]. Moreover, Millesi et al. observed visible fluorescence in all 12 analyzed spinal meningiomas. Visible fluorescence can be found not only in intracranial meningiomas, but in most spinal meningiomas as well and might serve a similar adjunct role for spinal surgery as it has for cranial cases.
Quantitative fluorescence in meningiomas
The previous section highlighted the value of 5-ALA-PpIX as a surgical adjunct for the resection of meningiomas, and specifically, in terms of technologies, the role of vFI as a surgical adjunct. As was previously noted, vFI is vulnerable to significant error, either because of under or over estimating the levels of PpIX in tissue. What is of more relevance in meningiomas would be the degree to which it can underestimate residual, invasive tumor tissue, and thus lead to high rates of false negatives. Here we will highlight the reported clinical experience using qFI as a means to increase the dynamic range of detectable levels of PpIX fluorescence, i.e., increase our sensitivity for 5-ALA-PpIX fluorescence, and encourage the development of technological innovations to help advance the field and improve patient outcomes [3, 22] (Fig. 3).
Fig. 3.
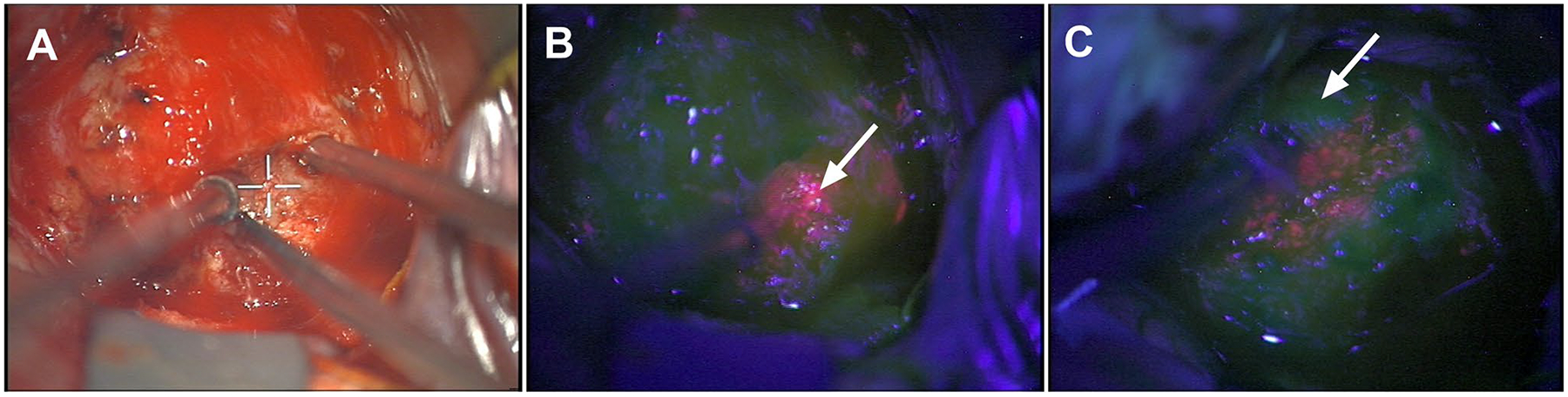
Quantitative fluorescence in intracranial meningioma. Corresponding a white and b, c blue light images at two distinct time points during surgery. b Region with strong visible fluorescence (arrow) and quantitative levels of PpIX = 2.9 μg/ml and c adjacent region with no visible fluorescence (arrow) but high, diagnostic levels of PpIX = 0.5 μg/ml
The major clinical study using quantitative fluorescence (qFI) for meningioma surgery described its use on 10 patients [3]. The authors used a quantitative intraoperative handheld spectroscopy probe, which has been previously described in detail and applied to multiple intracranial lesions (e.g., gliomas, metastases, meningiomas) [3]. In this study, all 10 patients harbored WHO grade I tumors and were interrogated at a total of 49 sites; 16 sites were control dura and 33 histopathologically confirmed tumor tissue [3]. The reported PpIX levels in normal dura were 0.006 ± 0.003 μg/ml (median < 0.001 μg/ml, i.q.r 0.000–0.000 μg/ml) and tumor levels were 1.694 ± 0.440 μg/ml (median 0.705 μg/ml, i.q.r 0.066–2.804 μg/ml) with tumor tissue accumulating significantly greater levels of PpIX compared to control tissue (p-value = 0.002) [3].
The correlation between qFI and vFI significantly differed in terms of areas with diagnostically significant qFI levels (> 0.010 μg/ml) and no visible fluorescence [3]. All visibly fluorescent tissue was histopathologically confirmed tumor tissue [3]. In this study, 13/33 (39%) of histopathologically confirmed tumor specimens did not display visible fluorescence. Sixty-nine percent of these (9/13) specimens demonstrated high levels of PpIX (> 0.010 μg/ml), i.e., they were false negatives for vFI but true positives with qFI [3]. Quantitative fluorescence was able to detect lower levels of PpIX that correlated with histopathological confirmed tumor tissue than vFI, improving detection of tumor tissue that would have otherwise been missed with vFI alone, i.e., false negatives [3].
A receiver operating characteristic (ROC) diagnostic performance analysis in this proof of concept study on 10 patients demonstrated improved sensitivity and negative predictive value for meningiomas using qFI compared to vFI [3]. Visible fluorescence imaging had an accuracy of 71% (area under the curve = 0.77, PPV = 0.95, NPV = 0.55, specificity = 0.94, and sensitivity = 0.61), meanwhile qFI had > 10% improvement in accuracy compared to vFI with an accuracy of 84% (area under the curve = 0.93, PPV = 1.00, NPV = 0.67, specificity = 1.00, and sensitivity = 0.76) [3]. These results highlight the ability of qFI to provide the surgeon with improved sensitivity with fewer false negatives and improved overall diagnostic performance (p = 0.007) [3]. Nevertheless, the surgeon should balance the need for improved specificity over sensitivity (e.g., areas near critical eloquent structures with residual fluorescence in adjacent arachnoid and/or cortex) and that of sensitivity over specificity (e.g., near end of resection to safely resect away from eloquent or high risks regions).
The utility of qFI can be further highlighted in two specific clinical instances of a skull base tumor and the thick fibrous tumor capsule found in some meningiomas [3, 6]. The first is the case of a skull base meningioma WHO grade I, specifically a sphenoid wing meningioma with intraorbital extension into the anterior clinoid process [6]. This particular case helps highlight the need for identification of small regions of invasion in hard to visualize areas. These areas can be of particular challenge in skull base meningiomas with involvement of cranial nerves and blood vessels, where rates of GTR decrease and recurrence as well as surgical morbidity increase. In this case report, the authors noted 80% sensitivity with vFI compared to 100% with qFI (PpIX tumor levels, mean 4.81 ug/ml, range 0.11–19.15 ug/ml) [6]. This study highlights the utility of qFI to delineate areas of invasion with improved sensitivity compared to vFI in a challenging case of a skull base meningioma.
In the previous proof of concept study [3], the authors described 13% (2/15) of cases not demonstrating visible fluorescence in the tumor capsule. Meanwhile, qFI showed a wide range of values (e.g., below diagnostically significant levels (0.001 and 0.002 ug/ml) as well as high levels (0.152 ug/ml)), and in both cases tissue was confirmed as tumor [3]. This specific case demonstrates the ability of qFI to identify large areas of tumor with no visible fluorescence that require identification and resection by the surgeon to provide patients with the best opportunity to decrease recurrence rates.
In summary, qFI is a novel technology that helps the surgeon further maximize the biological targeting potential and utility of 5-ALA-PpIX as a tumor biomarker. Quantitative fluorescence enables measurement of absolute PpIX levels not prone to the subjectivity of vFI. The major benefit of qFI as currently implemented in meningiomas is its ability to detect lower yet diagnostically significant concentrations of PpIX. This technology provides the neurosurgeon a more sensitive tool, improving accuracy for detecting tumor tissue, and increasing the surgeon’s confidence of minimizing the degree of residual tumor. Ultimately, this tool is a promising adjunct with the potential to help improve the rate of Simpson grade 1 resections, providing patients with the best chance for cure and minimizing the rate of disease recurrence.
Conclusion
5-ALA-PpIX has emerged as a novel, exciting surgical adjunct to help neurosurgeons maximize the extent of resection in gliomas. The more limited experience in meningiomas suggests that 5-ALA-PpIX can also provide the neurosurgeon with improved visualization of tumor including the tumor bulk, bone infiltration, and satellite lesions. The studies to date suggest that fluorescence guidance can help neurosurgeons in their goal of achieving a Simpson grade I resection. Furthermore, the experience using both qualitative and quantitative fluorescence guidance technologies provides the neurosurgical community with complementary technologies to help further improve the biological targeting capabilities of 5-ALA-PpIX, specifically, improving sensitivity and accuracy for tumor detection. The work to date in meningiomas should encourage neurosurgeons to further investigate the utility of this technology in larger cohorts and elaborate on additional benefits and limitations of 5-ALA-PpIX in meningiomas not presented here. Implementations to date of 5-ALA-PpIX in meningiomas hold promising potential so neurosurgeons can improve surgical outcomes for patients with meningiomas as well as be pioneers in developing improved fluorescence imaging technologies to improve surgical outcomes.
Funding
This study was funded by National Institutes of Health (US) Grant No. R01 NS052274 (D.W. Roberts).
Footnotes
Conflict of interest D.W. Roberts and P.A. Valdes have multiple patents for intraoperative fluorescence imaging devices noted in this manuscript. D.W. Roberts has equity in InSight Surgical Technologies LLC. M. Millesi and G. Widhalm report no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Kajimoto Y, Kuroiwa T, Miyatake S, Ichioka T, Miyashita M, Tanaka H, Tsuji M (2007) Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J Neurosurg 106(6):1070–1074. 10.3171/jns.2007.106.6.1070 [DOI] [PubMed] [Google Scholar]
- 2.Motekallemi A, Jeltema HR, Metzemaekers JD, van Dam GM, Crane LM, Groen RJ (2015) The current status of 5-ALA fluorescence-guided resection of intracranial meningiomas-a critical review. Neurosurg Rev 38(4):619–628. 10.1007/s10143-015-0615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdes PA, Bekelis K, Harris BT, Wilson BC, Leblond F, Kim A, Simmons NE, Erkmen K, Paulsen KD, Roberts DW (2014) 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurg 10 Suppl 1:74–82; discussion 82 – 73. 10.1227/NEU.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20(1):22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401. 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 6.Bekelis K, Valdes PA, Erkmen K, Leblond F, Kim A, Wilson BC, Harris BT, Paulsen KD, Roberts DW (2011) Quantitative and qualitative 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in skull base meningiomas. Neurosurg Focus 30(5):E8 10.3171/2011.2.FOCUS1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae MP, Song SW, Park SH, Park CK (2012) Experience with 5-aminolevulinic Acid in fluorescence-guided resection of a deep sylvian meningioma. J Korean Neurosurg Soc 52(6):558–560. 10.3340/jkns.2012.52.6.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H (2010) Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir 152(10):1711–1719. 10.1007/s00701-010-0708-4 [DOI] [PubMed] [Google Scholar]
- 9.Millesi M, Kiesel B, Mischkulnig M, Martinez-Moreno M, Wohrer A, Wolfsberger S, Knosp E, Widhalm G (2016) Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J Neurosurg 125(6):1408–1419. 10.3171/2015.12.JNS151513 [DOI] [PubMed] [Google Scholar]
- 10.Bradley RS, Thorniley MS (2006) A review of attenuation correction techniques for tissue fluorescence. J R Soc Interface 3(6):1–13. 10.1098/rsif.2005.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdes PA, Jacobs VL, Paulsen KD, Roberts DW, Leblond F (2011) In vivo fluorescence detection in surgery: a review of principles, methods, and applications. Curr Med Imaging Rev 8(3):211–232 [Google Scholar]
- 12.Valdes PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, Simmons NE, Paulsen KD, Roberts DW (2011) Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg 115(1):11–17. 10.3171/2011.2.JNS101451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 77(5):663–673. 10.1227/NEU.0000000000000929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogue BW, Gibbs-Strauss S, Valdes PA, Samkoe K, Roberts DW, Paulsen KD (2010) Review of neurosurgical fluorescence imaging methodologies. IEEE J Sel Top Quantum Electron 16(3):493–505. 10.1109/JSTQE.2009.2034541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ (1998) Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir 140(10):995–1000 [DOI] [PubMed] [Google Scholar]
- 16.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93(6):1003–1013. 10.3171/jns.2000.93.6.1003 [DOI] [PubMed] [Google Scholar]
- 17.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401. 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 18.Stummer W, Reulen HJ, Novotny A, Stepp H, Tonn JC (2003) Fluorescence-guided resections of malignant gliomas—an overview. Acta Neurochir Suppl 88:9–12 [DOI] [PubMed] [Google Scholar]
- 19.Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42(3):518–525 (discussion 525–516) [DOI] [PubMed] [Google Scholar]
- 20.Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 114(3):595–603. 10.3171/2010.2.JNS091322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdes PA, Angelo JP, Choi HS, Gioux S (2017) qF-SSOP: real-time optical property corrected fluroescence imaging. Biomed Opt Express 8(8):3597–3605. 10.1364/BOE.8.003597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdes PA, Jacobs V, Harris BT, Wilson BC, Leblond F, Paulsen KD, Roberts DW (2015) Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg 123(3):771–780. 10.3171/2014.12.JNS14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdes PA, Roberts DW, Lu FK, Golby A (2016) Optical technologies for intraoperative neurosurgical guidance. Neurosurg Focus 40(3):E8 10.3171/2015.12.FOCUS15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdes PA, Leblond F, Jacobs VL, Wilson BC, Paulsen KD, Roberts DW (2012) Quantitative, spectrally-resolved intraoperative fluorescence imaging. Sci Rep 2:798 10.1038/srep00798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim A, Khurana M, Moriyama Y, Wilson BC (2010) Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J Biomed Opt 15(6):067006 10.1117/1.3523616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdes PA, Kim A, Leblond F, Conde OM, Harris BT, Paulsen KD, Wilson BC, Roberts DW (2011) Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer bio-markers in low- and high-grade glioma surgery. J Biomed Opt 16(11):116007 10.1117/1.3646916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bravo JJ, Olson JD, Davis SC, Roberts DW, Paulsen KD, Kanick SC (2017) Hyperspectral data processing improves PpIX contrast during fluorescence guided surgery of human brain tumors. Sci Rep 7(1):9455 10.1038/s41598-017-09727-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I (2008) Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery 62 (3 Suppl 1):102–103 (discussion 103–104). 10.1227/01.neu.0000317378.22820.4600006123-200803001-00012 [DOI] [PubMed] [Google Scholar]
- 29.Cornelius JF, Slotty PJ, Stoffels G, Galldiks N, Langen KJ, Steiger HJ (2013) 5-Aminolevulinic acid and (18)F-FET-PET as metabolic imaging tools for surgery of a recurrent skull base meningioma. J Neurol Surg Part B Skull Base 74(4):211–216. 10.1055/s-0033-1342918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheichel F, Ungersboeck K, Kitzwoegerer M, Marhold F (2017) Fluorescence-guided resection of extracranial soft tissue tumour infiltration in atypical meningioma. Acta Neurochir 159(6):1027–1031. 10.1007/s00701-017-3166-4 [DOI] [PubMed] [Google Scholar]
- 31.Wilbers E, Hargus G, Wolfer J, Stummer W (2014) Usefulness of 5-ALA (Gliolan(R))-derived PPX fluorescence for demonstrating the extent of infiltration in atypical meningiomas. Acta Neurochir 156(10):1853–1854. 10.1007/s00701-014-2148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelius JF, Slotty PJ, Kamp MA, Schneiderhan TM, Steiger HJ, El-Khatib M (2014) Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas–with special regard to high-grade tumors. Photodiagn Photodyn Ther 11(4):481–490. 10.1016/j.pdpdt.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 33.Bekelis K, Valdés PA, Erkmen K, Leblond F, Kim A, Wilson BC, Harris BT, Paulsen KD, Roberts DW (2011) Quantitative and qualitative ALA-induced PpIX fluorescence in skull base meningiomas. Neurosurg Focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitson WJ, Valdes PA, Harris BT, Paulsen KD, Roberts DW (2011) Confocal microscopy for the histological fluorescence pattern of a recurrent atypical meningioma: case report. Neurosurgery 68(6):E1768–E1772. 10.1227/NEU.0b013e318217163c (discussion E1772–E1763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eljamel MS (2009) Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. Clin Neurosurg 56:93–97 [PubMed] [Google Scholar]
- 36.Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi AR, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36(2):E10 10.3171/2013.12.FOCUS13464 [DOI] [PubMed] [Google Scholar]
- 37.Potapov AA, Goryaynov SA, Okhlopkov VA, Shishkina LV, Loschenov VB, Savelieva TA, Golbin DA, Chumakova AP, Goldberg MF, Varyukhina MD, Spallone A (2016) Laser bio-spectroscopy and 5-ALA fluorescence navigation as a helpful tool in the meningioma resection. Neurosurg Rev 39(3):437–447. 10.1007/s10143-015-0697-0 [DOI] [PubMed] [Google Scholar]
- 38.Della Puppa A, Rustemi O, Gioffre G, Troncon I, Lombardi G, Rolma G, Sergi M, Munari M, Cecchin D, Gardiman MP, Scienza R (2014) Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J Neurosurg 120(4):840–845. 10.3171/2013.12.JNS131642 [DOI] [PubMed] [Google Scholar]
- 39.Borovich B, Doron Y (1986) Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg 64(1):58–63 [DOI] [PubMed] [Google Scholar]
- 40.Muroi C, Fandino J, Coluccia D, Berkmann S, Fathi AR, Landolt H (2013) 5-Aminolevulinic acid fluorescence-guided surgery for spinal meningioma. World neurosurgery 80(1–2):223–e1. 10.1016/j.wneu.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 41.Eicker SO, Floeth FW, Kamp M, Steiger HJ, Hanggi D (2013) The impact of fluorescence guidance on spinal intradural tumour surgery. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical. Spine Res Soc 22(6):1394–1401. 10.1007/s00586-013-2657-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


