Abstract
BRCA1/BRCA2 genes play a central role in DNA repair and their mutations increase sensitivity to DNA-damaging agents. There are conflicting data regarding the prognostic value of BRCA germline mutations in breast cancer (BC) patients. We collected clinical, pathological and genetic data of a cohort 925 BC patients preselected for genetic screening and treated with neoadjuvant or adjuvant chemotherapy, of whom 266 were BRCA carriers. Overall, 171 women carried a BRCA1 mutation, 95 carried a BRCA2 mutation, and 659 were non-carriers. In the entire cohort, there was a prolonged disease-free survival (DFS) for BRCA carriers (hazard ratio (HR) = 0.63; 95% confidence interval (CI), 0.44–0.90 for BRCA1; HR = 0.72; 95%CI, 0.47–1.1 for BRCA2; p = 0.020) and a trend toward prolonged disease-specific survival (DSS; HR = 0.65; 95%CI, 0.40–1.1 for BRCA1; HR = 0.78; 95%CI, 0.44–1.38 for BRCA2; p = 0.19) though not statistically significant. In the TNBC group, BRCA carriers had prolonged DFS (adjusted HR = 0.50; 95%CI, 0.28–0.89 for BRCA1; adjusted HR = 0.37; 95%CI, 0.11–1.25, for BRCA2; p = 0.034) and DSS (adjusted HR = 0.42; 95%CI, 0.21–0.82 for BRCA1; adjusted HR = 0.45; 95%CI, 0.11–1.9 for BRCA2; p = 0.023). In the non-TNBC group, the BRCA1 or BRCA2 mutations did not have any impact on survival. These results suggest that BRCA1/BRCA2 germline mutations are associated with prolonged survival only if women were diagnosed with TNBC.
Subject terms: Prognostic markers, Breast cancer, Cancer genetics
Introduction
BRCA1/BRCA2 germline mutations account for approximately 5% of all breast cancers1. These tumor suppressor genes encode large, ubiquitous and multifunctional proteins that play a central role in DNA repair, cell-cycle control and chromosomal stability2. Cells with non-functional BRCA1/BRCA2 proteins are severely impaired in their ability to repair DNA double strand breaks (DSBs) through homologous recombination2. As a consequence, tumors harboring deleterious mutations of BRCA1/BRCA2 genes are highly sensitive to DNA-damaging agents, such as interstrand crosslinking agents (platinum or alkylating agents), topo-isomerase II inhibitors (anthracyclines) or PARP inhibitors2–4.
In breast cancer patients, the tumor phenotype differs according to the BRCA1 or BRCA2 germline mutation status. BRCA1 mutation carriers mainly develop triple negative breast cancers (TNBC), whereas BRCA2 carriers are more likely to develop estrogen receptor (ER) and/or progesterone receptor (PR) positive tumors5. Not all BRCA carriers who develop breast cancer receive adjuvant chemotherapy, depending on several factors, including tumor stage, grade and molecular subtype. Currently, there are conflicting data regarding the predictive and prognostic values of BRCA mutations on the survival of non-metastatic breast cancer patients6–8. BRCA carriers with TNBC have been shown to be more sensitive to DNA-damaging agents9–15 but this did not translate into a survival benefit6,9,12,16,17.
BRCA germline mutations account for approximately 10–15% of ovarian cancers18. The majority of ovarian cancers that develop in BRCA carriers (either BRCA1 or BRCA2) are high-grade serous ovarian carcinomas (HGSOC). Ovarian cancers are frequently diagnosed at advanced stages and receive platinum-based chemotherapy19. Several studies have shown that among ovarian cancer patients, BRCA1 and especially BRCA2 carriers respond better than non-carriers to platinum-based chemotherapy and have prolonged survival20–22. We hypothesized that BRCA germline mutations would lead to prolonged survival in breast cancer patients treated by DNA-damage agents such as alkylating agents and/or anthracylines23. We conducted a multicentric retrospective study with the primary objective of assessing the prognostic value of BRCA germline mutation on survival among stage I-III breast cancer patients treated with chemotherapy. Patients were included if they have been selected for genetic testing of BRCA germline mutation.
Results
Patient demographics and clinical characteristics
From the entire cohort, a total of 925 patients were identified (677 from the French cohort and 248 from the Swiss cohort)(supplementary Figure S1), of whom 659 were non-carriers, 171 were BRCA1 carriers, and 95 were BRCA2 carriers (supplementary Table S1). Patient demographics, tumor characteristics, and type of administered chemotherapy are summarized in Table 1. The median age at diagnosis (40 years) was similar between carriers and non-carriers. Most BRCA1 carriers developed TNBC (68%) compared to 19% among BRCA2 carriers and 24% among the non-carriers (p < 0.0001). BRCA1 carriers were more likely to develop high grade (p < 0.0001) and high mitotic index tumors (p < 0.0001). Axillary node involvement was more frequent in BRCA2 carriers (p = 0.016).
Table 1.
Patients characteristics of the entire cohort.
| Variable | All (n = 925) | BRCA status | p | ||
|---|---|---|---|---|---|
| Non-carriers (n = 659) | BRCA1 (n = 171) | BRCA2 (n = 95) | |||
| Age, years, median (25th–75th) NA = 0 | 40 (34–48) | 39 (34–48) | 40 (35–49) | 40 (35–47) | 0.73 |
| cT (%) | 0.20 | ||||
| cT0 | 47 (7%) | 35 (7%) | 8 (6%) | 4 (6%) | |
| cT1 | 254 (36%) | 172 (34%) | 59 (42%) | 23 (39%) | |
| cT2 | 293 (41%) | 211 (42%) | 53 (38%) | 29 (43%) | |
| cT3 | 86 (12%) | 66 (13%) | 13 (9%) | 7 (10%) | |
| cT4 NA = 216 | 29 (4%) | 17 (3%) | 7 (5%) | 5 (7%) | |
| cN (%) | 0.20 | ||||
| cN0 | 477 (68%) | 328 (66%) | 104 (76%) | 44 (66%) | |
| cN1 | 210 (30%) | 159 (32%) | 31 (23%) | 20 (29%) | |
| cN2 | 8 (1%) | 6 (12%) | 1 (1%) | 1 (1%) | |
| cN3 NA = 222 | 8 (1%) | 6 (12%) | 0 (0%) | 2 (3%) | |
| Positive nodes *** NA = 22 | 430 (48%) | 312 (48%) | 64 (39%) | 54 (57%) | 0.016 |
| Grade (%) | <0.0001 | ||||
| 1 | 45 (5%) | 40 (6%) | 1 (1%) | 4 (4%) | |
| 2 | 341 (38%) | 269 (42%) | 33 (20%) | 39 (43%) | |
| 3 NA = 25 | 514 (57%) | 335 (52%) | 132 (80%) | 47 (52%) | |
| Mitotic index (%) | <0.0001 | ||||
| 1 | 218 (27%) | 182 (31%) | 13 (9%) | 23 (29%) | |
| 2 | 247 (30%) | 182 (31%) | 41 (28%) | 24 (31%) | |
| 3 NA = 109 | 351 (43%) | 227 (38%) | 93 (63%) | 31 (40%) | |
| Positive ER (%) NA = 5 | 554 (60%) | 444 (68%) | 40 (23%) | 70 (76%) | <0.0001 |
| Positive PR (%) NA = 5 | 484 (53%) | 386 (59%) | 37 (22%) | 61 (66%) | <0.0001 |
| Positive HER-2 (%) NA = 67 | 173 (20%) | 153 (25%) | 7 (5%) | 13 (16%) | <0.0001 |
| TNBC (%) NA = 67 | 270 (31%) | 148 (24%) | 106 (68%) | 16 (19%) | <0.0001 |
| Chemotherapy (%) | 0.67 | ||||
| Neoadjuvant | 254 (27%) | 184 (28%) | 41 (24%) | 29 (31%) | |
| Adjuvant | 662 (72%) | 467 (71%) | 129 (76%) | 66 (69%) | |
| Both NA = 0 | 9 (1%) | 8 (1%) | 1 (1%) | 0 (0%) | |
| Anthracyclines (%) NA = 4 | 751 (82%) | 529 (80%) | 143 (85%) | 79 (84%) | 0.39 |
| Taxanes (%) NA = 4 | 717 (78%) | 526 (80%) | 121 (72%) | 70 (75%) | 0.046 |
| Alkylating agent (%) NA = 4 | 874 (95%) | 620 (94%) | 162 (96%) | 92 (98%) | 0.33 |
| Platinum (%) NA = 4 | 31 (3%) | 24 (4%) | 5 (3%) | 2 (2%) | 0.87 |
| Trastuzumab (%) NA = 4 | 143 (16%) | 131 (20%) | 3 (2%) | 9 (10%) | <0.0001 |
NA: not available. ER: estrogen receptors, PR: progesterone receptors, HER-2: human epidermal growth factor receptor-2, TNBC: triple negative breast cancers. ***Positive nodes: pN if pre-chemotherapy biopsy positive; yN or nodal scar in the removed lymph node if neoadjuvant chemotherapy.
ER, PR, and HER-2 status were available for 858 patients. Among the 270 who developed TNBC, 106 were BRCA1 carriers, 16 were BRCA2 and 148 were non-carriers. Patients and tumor characteristics were comparable between BRCA carriers and non-carriers (supplementary Table S2). Among the 588 women who developed non-TNBC, BRCA1 carriers were older than BRCA2 and non-carriers (p = 0.014; supplementary Table S3). BRCA1 carriers developed tumors displaying higher grade (p = 0.056), and a higher mitotic index (p = 0.047) and were less frequently expressing ER (p = 0.0053) than BRCA2 carriers or non-carriers. HER-2 was less frequently overexpressed/amplified in tumors from BRCA carriers compared to non-carriers (p = 0.004).
Chemotherapy
The majority of patients received adjuvant chemotherapy (72% for the entire cohort, 66% for TNBC, and 73% for non-TNBC). Most of the patients received two DNA damaging-agents: an alkylating agent (95%) and an anthracycline (82%; Table 1). Non-carriers were more likely to receive taxanes (p = 0.046; Table 1), in particular among those who developed TNBC (p = 0.0088; supplementary Table S2). Non-carriers more frequently received trastuzumab (p < 0.0001; Table 1). Very few patients received platinum derivatives (3%; Table 1).
Survival estimates
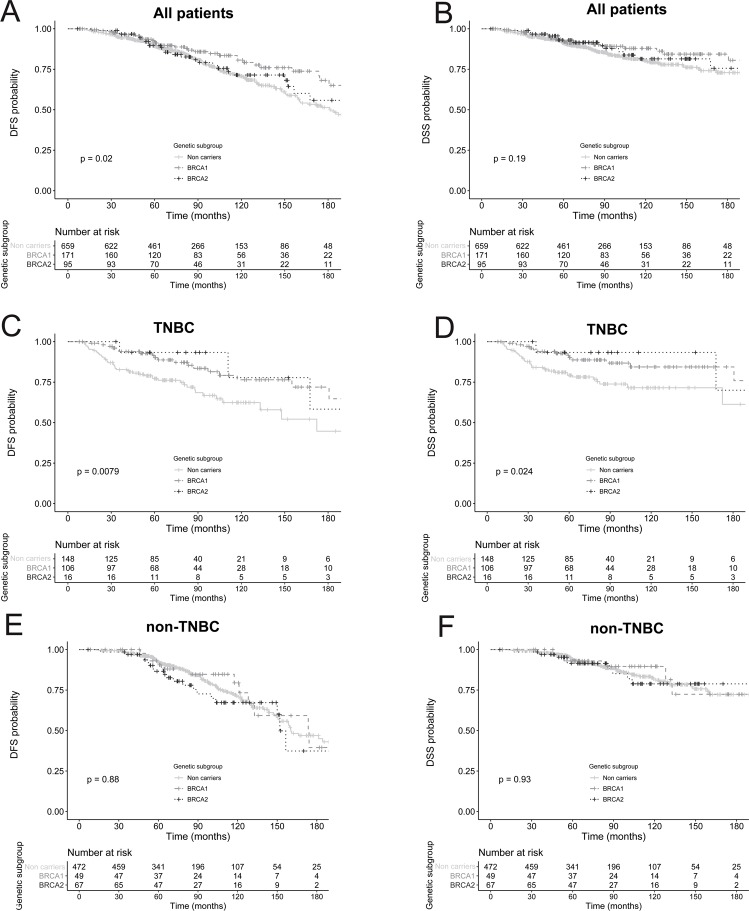
The median follow-up for the entire cohort was 7.3 years (7–7.8). Overall, 237 patients relapsed during the follow-up: 178 non-carriers, 35 BRCA1, and 24 BRCA2 carriers. There were 133 deaths related to breast cancer: 101 non-carriers, 19 BRCA1 carriers, and 13 BRCA2 carriers. In the entire cohort (n = 925), there was a prolonged DFS for BRCA1 (5-year rate 92%; hazard ratio (HR) = 0.63; 95% confidence interval (CI), 0.44–0.90) as well as for BRCA2 (5-year rate 90%; HR = 0.72; 95%CI, 0.47–1.1; p = 0.020; Fig. 1A and Table 2) compared to non-carriers (5-year rate 89%). A trend toward prolonged DSS was observed in BRCA carriers (5-year rate 93%; HR = 0.65; 95%CI, 0.40–0.1.1 for BRCA1; 5-year rate 93%; HR = 0.78; 95%CI, 0.44–1.38 for BRCA2; p = 0.19 and a 5-year rate 91% for non-carriers; Fig. 1B and Table 2) though not statistically significant.
Figure 1.
DFS and DSS according to BRCA1/BRCA2 status and molecular phenotype.
Table 2.
Multivariate analysis of DFS and DSS in the entire cohort.
| Cox proportional hazards regression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Disease-free survival | Disease-specific survival | |||||||||
| Unadjusted analysis | Adjusted Analysis | 5-years DSS rate (95% CI) | Unadjusted analysis | Adjusted Analysis | |||||||
| 5-years DFS rate (95% CI) | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |||
| BRCA status | |||||||||||
| Non-carriers | 659 (71%) | 89 (87–92) | 1 | 1 | 91 (89–37) | 1 | 1 | ||||
| BRCA1 | 171 (18%) | 92 (88–96) | 0.63 (0.44–0.90) | 0.020 | 0.63 (0.43–0.92) | 0.018 | 93 (89–97) | 0.65 (0.40–1.1) | 0.19 | 0.66 (0.39–1.1) | 0.18 |
| BRCA2 | 95 (10%) | 90 (83–96) | 0.72 (0.47–1.1) | 0.70 (0.45–1.1) | 93 (88–99) | 0.78 (0.44–1.38) | 0.74 (0.42–1.3) | ||||
| Grade | |||||||||||
| 1 | 45 (5%) | 98 (93–100) | 1 | 0.30 | NI | NI | 95 (89–100) | 1 | 0.72 | NI | NI |
| 2 | 341 (37%) | 89 (86–93) | 1.4 (0.76–2.7) | 93 (90–96) | 1.3 (0.57–3.1) | ||||||
| 3 | 514 (56%) | 89 (86–92) | 1.2 (0.64–2.2) | 91 (88–93) | 1.2 (0.51–2.7) | ||||||
| Age | 0.16 | NI | NI | ||||||||
| >35 | 666 (72%) | 91 (88–93) | 1 | 92 (90–94) | 1 | 0.26 | NI | NI | |||
| ≤35 | 258 (28%) | 88 (84–92) | 1.2 (0.93–1.6) | 98 (86–94) | 1.2 (0.86–1.8) | ||||||
| Nodal status | |||||||||||
| Negative | 473 (52%) | 93 (90–95) | 1 | 0.0029 | 1 | 0.0032 | 95 (92–97) | 1 | <0.0001 | 1 | <0.0001 |
| Positive | 430 (48%) | 87 (84–91) | 1.5 (1.1–1.9) | 1.5 (1.1–1.9) | 89 (86–92) | 2.1 (1.5–3.1) | 2.1 (1.4–3.0) | ||||
NI: not-included.
Subgroup analysis by molecular subtype revealed that BRCA carriers had significantly prolonged DFS and DSS in the TNBC subgroup only (n = 270; Table 3). After adjustment for nodal status, BRCA1 (5-year rate 91%; HR = 0.50; 95%CI, 0.28–0.89) and BRCA2 carriers (5-year rate 93%; HR = 0.37; 95%CI, 0.11–1.25) had prolonged DFS compared to non-carriers (5-year rate 77%; p = 0.034; Table 3 and Fig. 1C). BRCA1 (5-year rate 91%; HR = 0.42; 95%CI, 0.21–0.82) and BRCA2 carriers (5-year rate 93%; HR = 0.45; 95%CI, 0.11–1.9) consistently had prolonged DSS compared to non-carriers (5-year rate 79%; p = 0.023; Table 3 and Fig. 1D). The landmark analysis at one year performed as a sensitivity analysis was consistent with this estimated impact of BRCA status on DFS and DSS in the TNBC” (supplementary Table 4). For women with non-TNBC, the BRCA1 (5-year rate 91%; HR = 0.91; 95%CI, 0.50–1.7) or BRCA2 (5-year rate 87%; HR = 1.1; 95%CI, 0.70–1.9) status did not have any impact on DFS (p = 0.88; supplementary Table S5 and Fig. 1E). Similarly, the BRCA1/BRCA2 status did not have any impact on the 5-year DSS (p = 0.93; supplementary Table S5 and Fig. 1F) in the multivariate analysis.
Table 3.
Multivariate analysis of DFS and DSS in TNBC.
| Cox proportional hazards regression | |||||||||||
| N | Disease-free survival | Disease-specific survival | |||||||||
| 5-years DFS rate (95% CI) | Unadjusted analysis | Adjusted Analysis | Unadjusted analysis | Adjusted Analysis | |||||||
| HR (95%CI) | p | HR (95%CI) | p | 5-years DSS rate (95% CI) | HR (95%CI) | p | HR (95%CI) | p | |||
| BRCA status | |||||||||||
| Non-carriers | 148 (55%) | 77 (70–84) | 1 | 1 | 79 (73–86) | 1 | 1 | 0.023 | |||
| BRCA1 | 106 (39%) | 91 (86–97) | 0.47 (0.28–0.81) | 0.0079 | 0.50 (0.28–0.89) | 0.034 | 91 (86–97) | 0.45 (0.24–0.85) | 0.024 | 0.42 (0.21–0.82) | |
| BRCA2 | 16 (6%) | 93 (82–100) | 0.34 (0.10–1.1) | 0.37 (0.11–1.25) | 93 (82–100) | 0.39 (0.09–1.6) | 0.45 (0.11–1.9) | ||||
| Grade | |||||||||||
| 1 | 3 (1%) | 100 (100–100) | 1 | 1 | 0.0023 | 100 (100–100) | 1 | 0.028 | NI* | NI | |
| 2 | 44 (16%) | 65 (51–82) | 0.69 (0.09–5.2) | 0.0 (0.0-NA) | 69 (56–86) | 0.79 (0.10–6.1) | |||||
| 3 | 220 (82%) | 87 (82–92) | 0.49 (0.28–0.85) | 0.040 | 0.40 (0.22–0.72) | 87 (83–92) | 0.44 (0.24–0.82) | ||||
| Age | |||||||||||
| >35 | 202 (75%) | 84 (78–89) | 1 | 0.81 | NI | NI | 85 (80–90) | 1 | 0.61 | NI | NI |
| ≤35 | 66 (25%) | 84 (75–94) | 0.93 (0.53–1.64) | 84 (75–94) | 0.83 (0.43–1.6) | ||||||
| Nodal status | |||||||||||
| Negative | 186 (72%) | 90 (86–95) | 1 | 0.00010 | 1 | <0.0001 | 91 (87–96) | 1 | <0.0001 | 1 | <0.0001 |
| Positive | 73 (28%) | 69 (59–81) | 2.7 (1.6–4.4) | 3.1 (1.9–5.1) | 71 (60–82) | 3.4 (1.9–6.0) | 3.3 (1.9–6.0) | ||||
NI: not included.
Response to neoadjuvant chemotherapy
Of the 263 (28%) patients who received neoadjuvant chemotherapy, the ER, PR and HER-2 status was available in 250 patients (95%). The pCR rate was significantly higher in BRCA1 (45%) compared to BRCA2 carriers (28%) and non-carriers (25%; p = 0.040; Table 4). Subgroup analysis by molecular subtype revealed that BRCA1 (54%) and BRCA2 carriers (57%) had significantly increased chemosensitivity compared to non-carriers (25%; p = 0.015) in the TNBC-subgroup only. In the HER-2 positive and the ER/PR positive/HER-2 negative subgroups, there was no difference between BRCA1/BRCA2 carriers and non-carriers regarding the pCR rate.
Table 4.
Pathologic complete response according to BRCA status and molecular subtype.
| pCR rate | ||||
|---|---|---|---|---|
| Non-carriers N (%) | BRCA1 N (%) | BRCA2 N (%) | p | |
| Entire cohort | 48/192 (25%) | 18/40 (45%) | 8/29 (28%) | 0.040 |
| TNBC | 13/53 (25%) | 15/28 (54%) | 4/7 (57%) | 0.015 |
| HER-2 positive * | 24/68 (35%) | 1/3 (33%) | 2/6 (33%) | 1.0 |
| ER/PR positive, HER-2 negative | 9/62 (15%) | 1/6 (17%) | 1/14 (7%) | 0.74 |
pCR: pathologic complete response. *HER-2 status missing in 12 cases.
Discussion
In the current study, we observed better disease-free survival of breast cancer patients who were selected for genetic screening, treated by chemotherapy and are BRCA carriers. Subgroup analysis revealed that the BRCA germline mutation is an independent prognostic factor associated with prolonged survival (both DFS and DSS) only for women with TNBC. For those who had ER/PR positive and/or HER-2 positive tumors (non-TNBC), BRCA mutations did not have any impact on outcome.
TNBC, mostly belonging to the basal-like subtype, share several molecular features of HGSOC including high levels of genomic instability and frequent TP53 mutations19,24,25. The majority of HGSOC patients are diagnosed at advanced stages and receive platinum-based chemotherapy19. BRCA carriers who developed HGSOC have increased survival compared to non-carriers19–21. This survival benefit has been linked to impaired DNA DSBs repair and consequently increased sensitivity to platinum26. For breast cancer patients, there are conflicting results regarding the prognosis and the predictive value of the BRCA germline status due to several issues: i) the phenotype of the tumor varies whether it is a BRCA1 (mainly TNBC) or a BRCA2 (mainly ER/PR positive) mutations; ii) adjuvant chemotherapy is not systematic and depends, among other characteristics, on tumor stage, grade, and molecular subtypes. Overall, it seems that BRCA1 carriers have poorer survival, probably due to the fact that they frequently develop TNBC, whereas BRCA2 germline mutation was not found to have a prognostic impact8,27. Whereas the prognostic value depends on tumor characteristics, the predictive value depends on the administered treatment.
DNA interstrand crosslinks (ICLs) are among the most lethal lesions to DNA. They are generated by several chemotherapeutic drugs such as platinum, mitomycine and alkylating agents. Although these drugs are backbone therapy of multiple cancers, it is well after their introduction to the clinics that it was discovered that they act by inducing ICLs28. Cells defective in BRCA genes are highly sensitive to drugs that generate ICLs such as bifunctional alkylating agents and platinum28–30. Another chemotherapeutic drugs that have biological background for efficacy in BRCA mutated tumors are topo-isomerase II inhibitors like anthracyclines31,32. Sensitivity to anthracylines and alkylating agents in BRCA carriers with breast cancer are emphasized by recent reports from INFORM and GeparOcto clinical trials15,23.
We hypothesized that among breast cancer patients who received DNA damage agents BRCA carriers will be more chemosensitive and this could translate into survival benefit. A quarter of the patients in our cohort received neoadjuvant chemotherapy. We observed that pCR rates significantly differ according to BRCA1/BRCA2 status and molecular subtype. For TNBC, our data are consistent with previous reports showing increased pCR rate in BRCA19–12,33 and/or BRCA2 carriers10,33,34. However, less than half of BRCA carriers would develop TNBC35, 45% in our cohort, and few are known on chemosensitivity of BRCA2 carriers. We did not observe any impact of BRCA mutations on pCR in HER-2 positive or ER/PR-positive HER-2 negative tumors. ER/PR-positive tumors in BRCA2 carriers seemed resistant to chemotherapy with a response rate estimated to 7% only. Our observations should be interpreted cautiously given the limited number of patients in each subgroup and the highly selected population. Nevertheless, it suggests that chemosensitivity in BRCA carriers may dramatically vary with the molecular phenotype of the tumor10,34,36.
We observed a survival benefit in BRCA1/BRCA2 carriers who developed TNBC. There are contradicting results regarding the survival benefit of BRCA mutations in TNBC6,9,12,16,17,33. Plausible explanations are: i) we did not exclude BRCA2 carriers and they are rare compared to BRCA1, ii) our cohort of BRCA1/BRCA2 carriers who developed TNBC included more than 100 BRCA carriers. We did not observe any survival benefit in BRCA1/BRCA2 carriers with HER-2 positive or ER/PR-positive HER-2 negative breast cancers (non-TNBC). This result was unexpected and mirrors the response rates to neoadjuvant chemotherapy in the different subgroups. It suggests the existence of different types of breast tumors arising in BRCA carriers with distinct responses to DNA-damaging agents. Investigating the molecular mechanisms underlying these differences, such as mutational signatures37, somatic loss of the wild-type allele38, BRCA genotype, the references doi: 10.1007/s10549-018-05127-2 and doi: 10.1158/1078-0432 recombination deficiency scores and/or infiltration by lymphocytes39,40 are important questions that need to be addressed in the future.
Our results are consistent with the recently published POSH study, a large prospective cohort (>2,700) that addressed the prognostic value of BRCA mutations in young women (<40 years). The majority of participants (89%) and virtually all cases of TNBC (98%) received chemotherapy. The POSH study showed survival benefit only in BRCA carriers who had developed TNBC and this benefit was observed in the first two years following diagnosis35. The POSH study brings new insights into the prognostic value of BRCA mutations in the context of breast cancer in young women treated by chemotherapy. The Geparquinto trial consistently showed survival benefit from BRCA germline mutations in TNBC33.
Our study had several limitations. It is a retrospective study that included patients screened for BRCA1/BRCA2 germline mutations. We recruited only women who were preselected based on their personal or family history that suggests a genetic predisposition. There might be a very specific additional risk factor profile for both environmental and genetic factors in these patients41,42. In the French cohort we included all BRCA carriers and a subgroup of non-carriers who were randomly selected. This lead to a substantial enrichment of BRCA carriers among women with TNBC (45%), much higher than expected for unselected TNBC10,33. These biases are reflected by the young age of our cohort that does not represent the general population of breast cancer patients. There is a survival bias related to the time from cancer diagnosis to genetic testing. We excluded women who did not receive adjuvant chemotherapy and thus could not address the prognostic value of the BRCA status among this population. We probably missed a substantial proportion of BRCA carriers who did not undergo genetic screening due to the absence of personal or family history35,37. Moreover, this study does not include a central review of pathology data. Nevertheless, BRCA carriers in our cohort had clinical and pathological characteristics consistent with previous reports5,7.
The strengths of our study are the following: we conducted a multicentric, international study with patients recruited in cancer comprehensive center, university hospitals, and private clinics. All patients underwent complete BRCA1 and BRCA2 gene sequencing, avoiding a selection bias in studies with founder mutations only6. We analyzed separately the impact of BRCA1 and BRCA2 mutations on survival and pCR and we did not focus on one molecular subtype or chemotherapy regimen or setting.
In summary, our study suggests that the prognostic value of BRCA1/BRCA2 germline mutations in breast cancer patients who were preselected for genetic screening and treated with neoadjuvant or adjuvant chemotherapy depends on the molecular subtype with a survival benefit only in women with TNBC.
Methods
Patient population
Women with non-metastatic invasive breast cancers who had been preselected for genetic screening for BRCA1/BRCA2 germline mutation and who received neoadjuvant or adjuvant chemotherapy were included in this study. BRCA status was determined at the Centre Léon Bérard, the Hospices Civils de Lyon, Lyon, France (1995–2014; French cohort) and the Hôpitaux Universitaires de Genève (1995–2016; Swiss cohort). From Geneva, all women (BRCA carriers and non-carriers) who met the inclusion criteria were included. In order to reduce the number of non-BRCA carriers in the study cohort, all BRCA carriers and a subgroup of non-carriers diagnosed in Lyon (randomly selected) were included. A protocol with a standardized case report form was used for all data collection and submitted to the Geneva Commission cantonale d’ethique de la recherche (CCER 15–158). The study protocol was approved by the Geneva Commission cantonale d’ethique de la recherche and the local institutional review boards in both hospitals in France. Informed written consent was obtained from all patients in the French cohort, and all living patients in the Swiss cohort. The research was performed in accordance with relevant guidelines/regulations. Exclusion criteria were the absence of neoadjuvant or adjuvant chemotherapy, no genetic screening, no follow-up or metastatic disease at diagnosis.
Data collection
Patient and treatment characteristics were collected from the medical records of patients treated at the Centre Leon Bérard, the Hospices Civils de Lyon, the Hôpitaux Universitaires de Genève and among 7 medical oncologists in private clinics in Geneva, Switzerland. We recorded date of birth, date of diagnosis, chemotherapy regimen, and timing (neoadjuvant or adjuvant). Chemotherapy agents were classified as anthracyclines, alkylating agents, taxanes, or platinum. Trastuzumab and hormonal therapy administration was recorded.
Tumor characteristics were collected from pathological reports. This included histological subtype, grade, estrogen and progesterone receptors status (positivity was defined as nuclear staining of >1% by immunohistochemistry (IHC)), HER-2 status (defined as either 3+ by IHC or as assessed by gene amplification through fluorescence or chromogenic in situ hybridization). TNBC were defined as ER, PR and HER-2 negative tumors. Non-TNBC were defined as ER/PR and/or HER-2 positive tumors.
TNM staging was evaluated according to the timing of chemotherapy. If the patient received adjuvant chemotherapy, the pTNM was recorded. If the patient received neoadjuvant chemotherapy, the cTNM and yTNM were recorded. Axillary lymph nodes were considered positive if a pre-chemotherapy biopsy was positive or if there was at least one yN+ or the presence of a histological scar in the removed lymph nodes after neoadjuvant chemotherapy.
Genetic analysis
Women were referred to the genetic unit for complete BRCA1 and BRCA2 germline screening based on the presence of personal history of breast cancer presented at a young age, or the display of a particular tumor phenotype (TNBC) or association with ovarian cancer, or in the context of a positive family history. Blood samples for germline DNA testing were obtained after a signed consent. All participants were screened for BRCA1 and BRCA2 mutations. BRCA1 and BRCA2 variants were classified as pathogenic according to the ENIGMA BRCA1/2 Gene Variant Classification Criteria (http://www.enigmaconsortium.org/). Women with variants of uncertain significance were considered as non-carriers.
Outcome measures
The primary objectives were to compare Disease-free survival (DFS) and Disease-specific survival (DSS) among breast cancer patients according to BRCA germline mutations. Secondary objectives were to compare i) DSS and DFS according to BRCA status in the TNBC and the non-TNBC population; ii) pCR according to molecular subtype (TNBC vs non-TNBC) and BRCA status in the subgroup of patients who received neoadjuvant chemotherapy.
Statistical analyses
Based on a sample size of 600 non-carriers, 150 BRCA1 carriers and 100 BRCA2 carriers, a 80% 5-year DFS among non-carriers and a median follow-up of 6 years, the study had a 93% power to show an improvement of the 5-year DFS from 80% among non-carriers to 89% among the BRCA carriers (translating in a hazard ratio of 0.5) at a 2-sided alpha risk of 5%.
DFS was calculated from the time of diagnosis until the date of first documented local, regional, or distal invasive recurrence or death from breast cancer, or to the time of last follow-up. DSS was defined as the time from diagnosis to death caused by breast cancer. Survival outcomes were estimated using the Kaplan–Meier product-limit method and compared by a long-rank test. Cox proportional-hazards (PH) models were fitted to determine the association of the BRCA germline status (with time to event outcomes before and after adjustment for significant patient and clinical characteristics. The proportional hazards hypothesis was assessed both graphically and statistically. Cox proportional-hazards models were used for all analyses given the absence of significant deviation from the PH hypothesis in all subgroups and for all reported outcome measures. The following prognostic variables were assessed in univariate analyses: BRCA status, age (≤ or> 35 years of age), lymph node status, SBR grade. Variables yielding p values less than 0.1 by univariate analysis were retained for the multivariate analysis. The proportional hazards assumption was assessed using scaled Schoenfeld residuals. Because of the high correlation between grade and lymph node involvement and in order to avoid colinearity, grade was not included in the multivariate model. P values of ≤ 0.05 were considered statistically significant. As a sensitivity analysis of the main outcomes, a landmark analysis was conducted to exclude patients with DFS or DSS of less than 12 months, in order to avoid any immortal time bias related to the time between the cancer diagnosis and the time of the genetic counseling/testing.
Pathological complete response (pCR) was defined as the absence of any invasive disease in the breast and in the ipsilateral axillary lymph nodes (ypT0/is ypN0) in accordance with the Union for International Cancer Control TNM system43. Patient or tumor characteristics and chemotherapy regimens were compared according to the BRCA germline status using χ2 tests or the Fisher’s exact test for categorical variables, and non-parametric Kruskall-Wallis tests for continuous variables. All statistical analyses were carried out using the R software version 3.3.1 (http://www.r-project.org/).
Supplementary information
Supplementary Tables S1-S5 and Supplementary Figure S1.
Acknowledgements
We thank all the patients who agreed to participate to this study. We thank Dr. A. Hugli, Dr M. Forni, Dr. B. Exquis, Dr. C. De Pree, Dr. C. Irle, Dr. L. Walechli and Pr A.-P. Sappino for providing clinical data. We thank Mrs L. Zulianello for the iconographic support.
Author contributions
Conceptualization: S.I.L.G. and O.T., methodology: J.P., data collection: S.D.T., A.V., A.A., I.T., J.C.T., N.L.; resources: S.G., A.F., V.V., V.B., P.O.C., C.L., A.B.; Writing: S.D.T., J.P., O.T. and S.I.L.G. All the authors reviewed and edited the manuscript.
Data availability
All data analyzed during the study has been included in the manuscript (and its supplementary information files).
Competing interests
Dr. I. Labidi-Galy, Dr. A.Bodmer and Dr. O. Tredan have received compensation as members of advisory boards of Astra-Zeneca.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63759-1.
References
- 1.Rebbeck TR, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313:1347–1361. doi: 10.1001/jama.2014.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat. Rev. Cancer. 2012;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 4.Robson M, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 5.Mavaddat N, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennert G, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin PJ, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:211–220. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung N, et al. Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Res Treat. 2014;146:175–182. doi: 10.1007/s10549-014-2995-6. [DOI] [PubMed] [Google Scholar]
- 10.Hahnen E, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017;3:1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappuis PO, et al. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. Journal of medical genetics. 2002;39:608–610. doi: 10.1136/jmg.39.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, et al. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:523–528. doi: 10.1093/annonc/mdu559. [DOI] [PubMed] [Google Scholar]
- 13.Isakoff SJ, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telli ML, et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib As Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:1895–1901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohl-Rescigno, E. et al. Association of Germline Variant Status With Therapy Response in High-risk Early-Stage Breast Cancer: A Secondary Analysis of the GeparOcto Randomized Clinical Trial. JAMA Oncol, 10.1001/jamaoncol.2020.0007 (2020). [DOI] [PMC free article] [PubMed]
- 16.Bayraktar S, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast cancer research and treatment. 2011;130:145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paluch-Shimon S, et al. Neo-adjuvant doxorubicin and cyclophosphamide followed by paclitaxel in triple-negative breast cancer among BRCA1 mutation carriers and non-carriers. Breast cancer research and treatment. 2016;157:157–165. doi: 10.1007/s10549-016-3800-5. [DOI] [PubMed] [Google Scholar]
- 18.Alsop K, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolton KL, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norquist BM, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA oncology. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung, N. et al. TBCRC 031: Randomized Phase II Study of Neoadjuvant Cisplatin Versus Doxorubicin-Cyclophosphamide in Germline BRCA Carriers With HER2-Negative Breast Cancer (the INFORM trial). J. Clin. Oncol., JCO1903292, 10.1200/JCO.19.03292 (2020). [DOI] [PMC free article] [PubMed]
- 24.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manie E, et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 2009;69:663–671. doi: 10.1158/0008-5472.CAN-08-1560. [DOI] [PubMed] [Google Scholar]
- 26.Lord, C. J. & Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 16, 110–120. [DOI] [PubMed]
- 27.Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine. 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner N, Tutt A, Ashworth A. Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol. 2005;5:388–393. doi: 10.1016/j.coph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Tutt AN, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol. 2005;70:139–148. doi: 10.1101/sqb.2005.70.012. [DOI] [PubMed] [Google Scholar]
- 31.Tan, D. S. & Kaye, S. B. Chemotherapy for Patients with BRCA1 and BRCA2-Mutated Ovarian Cancer: Same or Different? Am Soc Clin Oncol Educ Book, 114–121, 10.14694/EdBook_AM.2015.35.114 (2015). [DOI] [PubMed]
- 32.Kaye SB, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J. Clin. Oncol. 2012;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 33.Fasching PA, et al. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J. Clin. Oncol. 2018;36:2281–2287. doi: 10.1200/JCO.2017.77.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bignon, L. et al. Efficacy of anthracycline/taxane-based neo-adjuvant chemotherapy on triple-negative breast cancer in BRCA1/BRCA2 mutation carriers. The breast journal, 10.1111/tbj.12887 (2017). [DOI] [PubMed]
- 35.Copson ER, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arun B, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J. Clin. Oncol. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies H, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med. 2017;23:517–525. doi: 10.1038/nm.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maxwell KN, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8:319. doi: 10.1038/s41467-017-00388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smid M, et al. Breast cancer genome and transcriptome integration implicates specific mutational signatures with immune cell infiltration. Nat Commun. 2016;7:12910. doi: 10.1038/ncomms12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakhani SR, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 41.Couch FJ, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet. 2013;9:e1003212. doi: 10.1371/journal.pgen.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasching PA, et al. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet. 2012;21:3926–3939. doi: 10.1093/hmg/dds159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Control, T. U. f. I. C. AJCC cancer staging manual (Springer, 2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables S1-S5 and Supplementary Figure S1.
Data Availability Statement
All data analyzed during the study has been included in the manuscript (and its supplementary information files).