Abstract
The aim of this study was to investigate the long-term trends of human immunodeficiency virus (HIV) mortality in China and its associations with age, period and birth cohort. We used HIV mortality data obtained from the Global Burden of Disease Study (GBD) 2016 and analysed the data with an age-period-cohort framework. Age effects indicate different risks of different outcomes at specific periods in life; period effects reflect population- wide exposure at a circumscribed point in time; and cohort effects generally reflect differences in risk across birth cohorts.Our results showed that the overall annual percentage change (net drift) of HIV mortality was 11.3% (95% CI: 11.0% to 11.6%) for males and 7.2% (95% CI: 7.0% to 7.5%) for females, and the annual percentage changes in each age group (local drift) were greater than 5% (p < 0.01 for all) in both sexes. In the same birth cohort, the risk of death from HIV increased with age in both sexes after controlling for period effects, and the risk for each five-year period was 1.98 for males and 1.57 for females compared to their previous life stage. Compared to the period of 2002–2006, the relative risk (RR) of HIV mortality in 2012–2016 increased by 56.1% in males and 3.7% in females, and compared to the 1955–1959 birth cohort, the cohort RRs increased markedly, by 82.9 times in males and 34.8 times in females. Considering the rapidly increasing risk of HIV mortality, Chinese policymakers should take immediate measures to target the key age group of 15–44 years in both sexes.
Subject terms: HIV infections, Epidemiology
Introduction
Human immunodeficiency virus (HIV) and the consequent acquired immune deficiency syndrome (AIDS) have caused one of the worst epidemics affecting humanity since the late 20th century, leading to a substantial number of deaths1,2. In 2018, 770,000 people died from AIDS-related diseases; the Eastern and southern Africa regions had the highest numbers of AIDS-related deaths, accounting for a combined 40.3%, followed by Asia and the Pacific region, accounting for 25.0%3. From 2010 to 2018, the number of global AIDS-related deaths decreased by 33%; however, the HIV mortality ratio increased from 1.54 per 100,000 to 1.71 per 100,000 individuals3. HIV/AIDS has also become the leading infectious cause of death in many countries4. In South Africa, from 1992 to 2013, more than 60% of deaths were attributed to HIV5. In Russia, HIV/AIDS has been the fastest increasing cause of premature death in the past decade, with an 86.5% increase6.
HIV became the leading infectious cause of death in China in 2009, and the number of deaths due to HIV/AIDS increased from 5,544 in 2007 to 21,234 in 20117,8. In addition, HIV mortality in males is much higher than that in females in China9. An existing study showed that the HIV prevalence in China increased exponentially over the past 16 years, and HIV mortality increased from 0.0002 per 100,000 individuals in 1992 to 0.9362 per 100,000 individuals in 201610. However, previous studies mainly focused on the age distribution of the incidence or mortality, and period and cohort effects were not taken into account. The trends of HIV mortality in different age groups remain unclear, and the relative risk (RR) due to period and cohort effects remains unknown. Therefore, a comprehensive analysis to address these limitations and elucidate answers to these questions was necessary.
In this study, we used data from the Global Burden of Disease Study (GBD) 2016 to investigate the long-term trends in HIV mortality in China and to examine the contributions of age, period, and cohort effects from 1992 and 2016. The findings from our study may provide guidance for resource allocation to prevent HIV-related deaths in vulnerable target populations.
Data sources and methods
Data sources
The data used in this study were extracted from the GBD 2016, a large international cooperative project that globally, regionally, and nationally assessed age-sex mortality for 264 causes of death, including HIV, from 1980 to 201611. Original data, which the GBD adapted to estimate HIV mortality, were mainly obtained from the Disease Surveillance Point (DSP) and the Notifiable Infectious Disease Reporting (NIDR) systems. Both systems are administered by the Chinese Center for Disease Control11. We also used data from the GBD 2013 global age-standardized population to standardize the HIV mortality rate for both males and females12.
Statistical analyses
Age-period-cohort (APC) analysis was used to evaluate the effects of age, period and cohort on the disease rate outcomes. Age effects indicate the different risks of different outcomes during different periods of life; period effects reflect population-wide exposure at a circumscribed point in time; and cohort effects generally represent the differences in risk across birth cohorts13,14.By decomposing the age and cycling a queue into their linear and nonlinear parts15, we can not only avoid directly separating the contributions of age, period and cohort15 but also estimate many useful functions, such as net drift; longitudinal age trend; and age, period and cohort deviations14,16. The net drift indicates the overall annual percentage change adjusted for age group over time, and local drifts indicate annual percentage changes for each age group. The longitudinal age curve, which is adjusted for period deviations, indicates the fitted longitudinal age-specific rates in the reference cohort. The period RR indicates the period RR adjusted for age and nonlinear cohort effects in each period relative to the reference period, and the cohort RR indicates the cohort RR adjusted for age and nonlinear period effects in each cohort relative to the reference period. Although the APC model has advantages in analysis, it also has unique and unfortunate limitations, including identifiability problems and uncertainty principles. The identifiability problem refers to the fact that the three time scales of age, period and cohort are collinear (cohort equals period minus age); therefore, the log-linear trends in rates cannot uniquely be attributed to the influences of age, period, or cohort17. The uncertainty principle refers to the measurement of absolute rates in cohorts, which is rarely considered in the context of most epidemiological cohort and case-control studies18.
To conduct the APC analysis, the mortality and population data were arranged into consecutive 5-year periods from 1992–1996 (median 1994) to 2012–2016 (median 2014) (data from 1990 to 1992 were not considered because they were not sufficient for a 5-year period); successive 5-year age groups from 15–19 years to 75–79 years (individuals younger than 15 years and older than 80 years were excluded); and 17 consecutive cohorts, including those born from 1915–1919 (median 1917) to 1990–1994 (median 1992). We used the APC Web Tool (Biostatistics Branch, National Cancer Institute, Bethesda, MD, USA) to obtain these estimable parameters. In all APC analyses, the central age group, period, and birth cohort were defined as the reference. The reference value was set as the lower of the 2 central values in the event of an even number of categories. We used Wald chi-square tests to calculate the significance of the estimable parameters and functions. A general linear model was used to compare the significance of the slope of the period RR and cohort RR by checking the interaction effect between sex and calendar year/birth cohort19. All statistical tests were 2-sided, and p-values less than 0.05 were considered statistically significant.
Results
Figure 1 shows the trends in crude mortality rates (CMRs) and the age-standardized mortality rates (ASMR) for HIV by sex for the period of 1992 to 2016. The HIV CMRs showed generally increasing trends in both sexes, from 0.58 to 2.77 per 100,000 individuals for males and 0.28 to 0.99 per 100,000 individuals for females, and there was a slight decrease from 2010 to 2013. The ASMRs in both sexes were similar to the trends in the CMRs, the ASMR increased 6.4-fold for males and 4.4-fold for females over the past 25 years. Our results also indicated that the ASMR of HIV of in males increased much faster than that in females, and in 2016, the male ASMR was nearly three times that of females.
Figure 1.
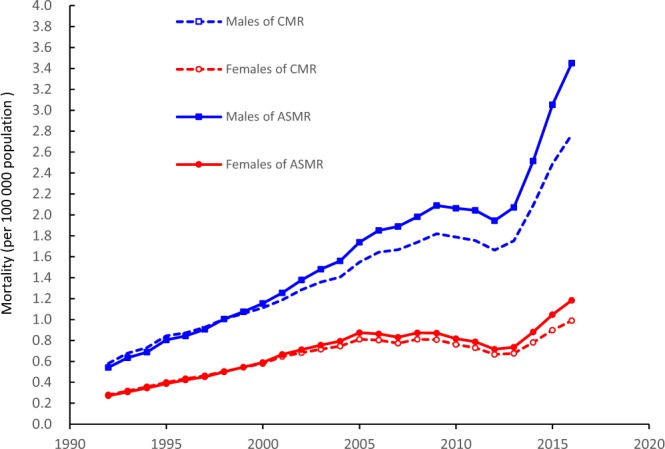
Trends in the HIV CMRs and the ASMRs per 100,000 population by sex from 1992 to 2016 using the GBD 2013 global age-standardized population.
The net drift and local drifts are displayed in Figure 2. From 1990 to 2016, the net drifts were 11.3% (95% CI, 11.0% to 11.6%) per year for males and 7.2% (95% CI, 7.0% to 7.5%) per year for females, indicating that the overall annual percentage increase in HIV mortality in males was nearly 1.6 times that in females. The local drift values were above 0 for all age groups in both sexes and in each age group. Rapidly increasing trends in HIV mortality were observed in males aged 15–44 years and 65–74 years and in females aged 15–44 years. The mortality rate increased from 10.9% to 12.0% in the 15–44 years age group and from 12.2% to 13.4% in the 65–74 years age group in males and from 6.9% to 10.3% in the 15–44 years age group females aged.
Figure 2.
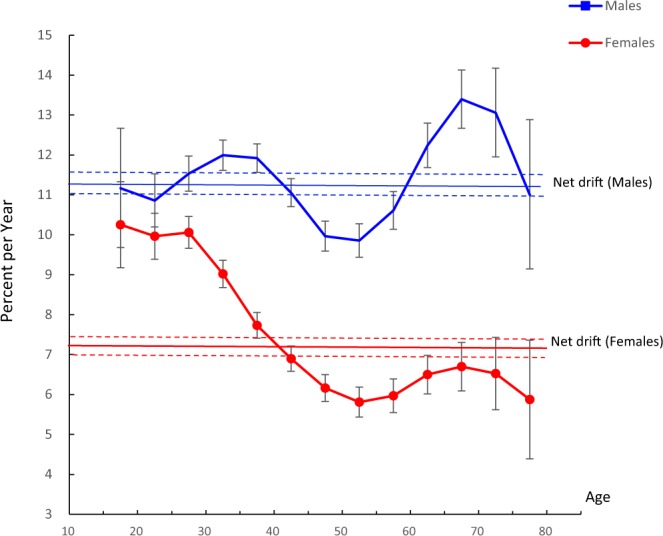
Local drift and net drift values for HIV mortality in China. Age group-specific annual percentage change (local drift) with the overall annual percentage change (net drift) in the HIV mortality rate and the corresponding 95% confidence intervals (some of them were too narrow to show in the figure).
Table S1 shows the trends in HIV/AIDS mortality in China from 1992 to 2016, and Table S2 shows the numbers of HIV/AIDS deaths across each age period. From 1992 to 2016, the HIV/AIDS mortality rate in males experienced a significant growth, especially in the 15–44 years age group and 65–74 years age group. For example, males aged 65–69 (median 67) had HIV/AIDS mortality rates of 0.38, 0.55, 1.12, 1.22 and 1.25 per 100,000 individuals during the periods of 1994, 1999, 2004, 2009 and 2014, respectively. The HIV/AIDS mortality rate in females also increased between 1992 and 2016, and the growth of the HIV/AIDS mortality rate for females was highest in the 15–19 year age group (median 17), from 0.02 per 100,000 individuals in 1992–1996 (median 1994) to 0.15 per 100,000 individuals in 2012–2016 (median 2014).
Figure 3 illustrates the longitudinal age curve of HIV mortality by sex adjusted for period. In the same birth cohort, the mortality risk in both males and females increased with age, and for males, the mortality risk showed an accelerated increase. There was a striking difference between males and females, and the risk of death from HIV in males was much higher than that in females, especially for people older than 40 years. The mortality risk for males in this age group was 6–32 times that for females in this group. We also estimated the longitudinal age curves and found that the male curve followed an exponential distribution and the female curve followed a linear distribution. The curves can be expressed as rate=0.0017*e0.1363* mean_age for men (R-square=0.918) and rate=0.0064*e0.0901* mean_age for females (R-square=0.849), indicating that the RR for HIV mortality in each life stage from the 20–24 years of age to 75–79 years of age was 1.98 for males and 1.57 for females compared to their previous life stage.
Figure 3.
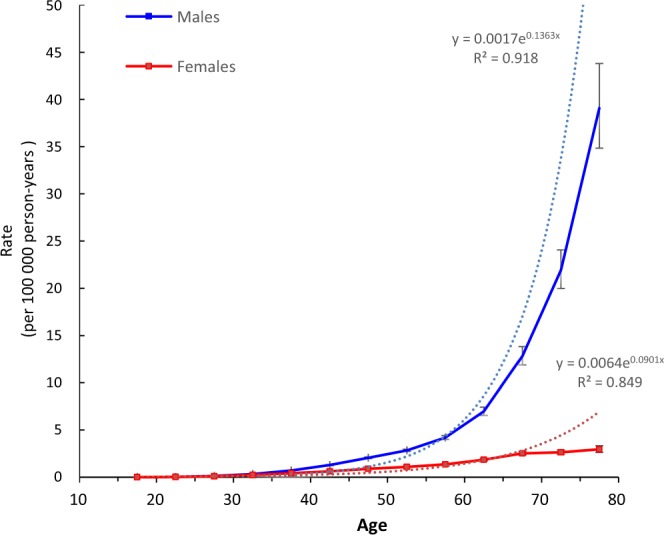
Longitudinal age curves of HIV mortality in China. Fitted longitudinal age-specific rates of HIV mortality (per 100 000 person-years) and the corresponding 95% confidence intervals (some of them were too narrow to show in the figure).
Figures 4 and 5 show the estimated period and cohort RRs by sex. The period RRs were found to have similar monotonically increased patterns in both sexes, with more rapid increases in males than in females after 2005. The cohort RRs showed similar monotonic increasing patterns in both sexes, and the increasing trend in males was accelerated in the cohort born in the 1980s. Compared to the period of 2002–2006, the period RRs of HIV mortality in 2012–2016 increased by 56.1% in males and 3.7% in females, and compared to the 1955–1959 birth cohort, the cohort RRs markedly increased by 82.9 times for males and by 34.8-fold in females. In addition, based on the specific results of the Wald tests, there were statistically significant cohort and period RRs for both sexes (p < 0.01 for all), and the net drift and local drifts were also statistically significant (p < 0.01 for all).
Figure 4.
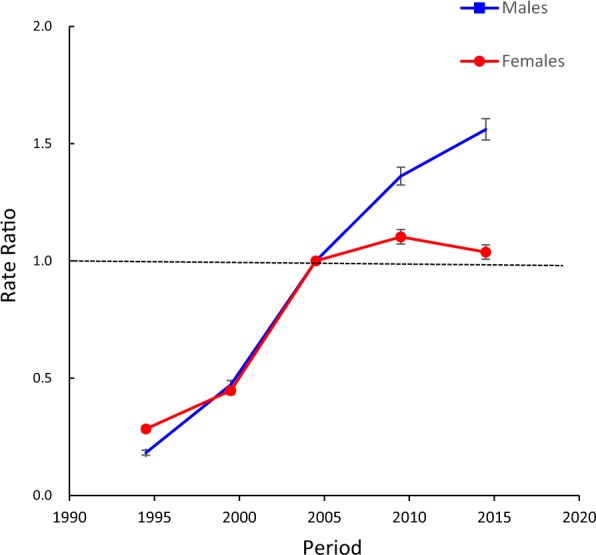
Period RRs of HIV mortality by sex in China. Period effects obtained from APC analyses for HIV mortality rates and the corresponding 95% confidence intervals (some of them were too narrow to show in the figure) by sex in China.
Figure 5.
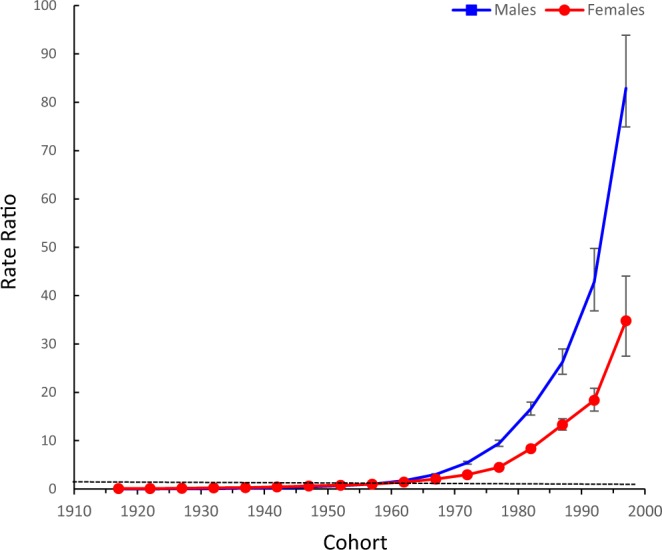
Cohort relative risks (RRs) of HIV mortality rates by sex in China. Cohort effects obtained from APC analyses for HIV mortality rates and the corresponding 95% confidence intervals (some of them were too narrow to show in the figure) by sex in China.
Discussion
Our study explored the long-term trends in HIV mortality in China between 1992 and 2016 with the aid of an APC framework; to our knowledge, this is the first study to investigate APC-specific effects of HIV mortality by sex. Our results indicated that HIV mortality in China increased rapidly, especially in males. In addition, the percentage change in males aged 15–44 years and aged 65–74 years and in females aged 15–44 years also increased dramatically during the period from 1992 to 2016.
In theory, with the development of antiretroviral treatment (ART), the HIV mortality rate should have decreased; however, the present study showed that HIV mortality in China continued to rise from 1992 to 2016. In China, the low perception of risk20 and low rates of HIV testing (especially among key populations, such as men who have sex with men, MSM)21 are likely to have contributed to this increasing trend, which was similar to the general trend for all of Asia22. The net drift of HIV mortality in both sexes also indicated that the risk of HIV mortality increased in all age groups, and the reasons why HIV mortality among males was higher than that among females may be as follows. First, MSM are at a much higher risk of HIV infection than females23–26. Second, females benefit more than males from HIV treatment, which helps to reduce the HIV death rate27–29. Third, the sex distribution in China is unbalanced; from 1990 to 2014, the sex ratio (male vs female ratio) at birth (SRB) was greater than 11030.
The local drift results also indicated that HIV mortality in those aged 15–44 years in both sexes and in males aged 65–74 years showed a significant increase over the past 25 years; these groups have been identified as target groups for HIV prevention and treatment. These results were similar to those reported in some provinces in China31–33. The reasons for the increase in HIV/AIDS mortality among females aged 15–44 years may be as follows. First, with the continuous spread of HIV in China10, the mortality rate of HIV/AIDS has also increased rapidly, including in females aged 15–44 years. Second, the average age of first intercourse in Chinese adolescents is 18–20 years34, and considering the characteristics of adolescents and their frequency of sexual behaviour, they usually pursue high-intensity stimulation without condom use, which increases the risk of HIV infection35. The survival period (from diagnosis to death) of HIV patients in China is 5 to 20 years;36–39 therefore, when young females are infected with HIV at a young age, they will likely die within the following 20 years. Third, late HIV diagnosis is a severe public health problem in China40,41, suggesting that as epidemic HIV/AIDS expands in China, an increasing number of HIV-infected people are not diagnosed early. Chinese studies have found that more than 1/3 of HIV-infected patients had CD4 counts of<200 cells/µL at diagnosis;41–43 another Chinese study found that more than 2/3 of patients died within one year after diagnosis44. HIV infection via heterosexual behaviour has a high rate of late diagnosis, and most females are infected during heterosexual encounters; thus, their rate of late HIV diagnosis is high45. Therefore, the increase in HIV mortality in females aged 15–44 years in this study is likely due to the increase in late HIV diagnosis, females diagnosed at later stages of infection missed the optimal time for ART and often due of HIV/AIDS-related diseases at a very young age.
Age is one of the most important demographic factors that affects HIV mortality, and many surveys40,46 have shown that age over 40 years is strongly associated with AIDS-related disease mortality. Our research also showed that in the same birth cohort, the risk of HIV mortality increased rapidly with age after adjusting for period deviations, especially in males; however, for females, the RR of HIV mortality in the older age group remained relatively low. The reasons for these findings may be as follows. First, the number of infected females was much lower than the number of infected males47. Second, among individuals who receive HIV treatment, females have a longer life expectancy than males28.
In this study, we separately estimated the period effect and cohort effect in the APC model; however, interpreting these models separately in the real world is difficult. On the one hand, the period effect may influence certain age groups differently and can lead to cohort effects. On the other hand, individuals from different cohorts were born in different periods, which inevitably has a confounding impact on period effects to some extent. Therefore, we systematically analysed the possible reasons for the increasing trends in the period and cohort effects. The most probable reasons that explain the increase in HIV mortality and HIV mortality RRs in China over the last 25 years are as follows. First, when HIV was identified in China in 198548, China was in a period of sexual liberation49. Chinese people gradually abandoned the traditional concept of sex and recognized premarital sex. However, at that time, sex education was not popularized in China (even now it still imperfect), and the majority of HIV transmission was through sexual intercourse; therefore, many Chinese people were infected with HIV during this time. Second, although the introduction of ART has helped many HIV-infected people in China50, highly active antiretroviral therapy (HAART), late diagnosis, poverty, low education status and other reasons have been demonstrated to be strongly related to AIDS-associated deaths46. Therefore, to prevent the risk of death caused by HIV/AIDS, HIV prevention should be a priority.
HIV preexposure prophylaxis (PrEP) has been proven to be an effective tool to reduce HIV transmission, especially transmission via sexual practices among MSM51–53, but it is often underutilized54,55. The reasons for the underutilization of PrEP include lack of knowledge of the drug or concern about its side effects56,57. Medical providers play an important role in advising high-risk HIV/AIDS groups about PrEP, and this education should lead to improved utilization rates51.Therefore, it is necessary to emphasize the role of medical staff in patient education. Post-exposure prophylaxis (PEP) is another successful method to prevent HIV infection after exposure58, but it’s utilization also faces many challenges, such as failure to access the agent in time or false-negative results59,60. It has been proven that simple collaborative interventions and improved communication and coordination among different departments (including the pharmacy department and infectious disease department) can effectively promote PEP to reduce critical delays in HIV prevention in vulnerable populations59. As PrEP and PEP play a significant role in the prevention of HIV transmission, the Chinese government should consider both methods together with ART when developing strategies to prevent the spread of HIV and reduce HIV/AIDS-related deaths.
Our study has some limitations. First, this study did not include individuals under 15 years and over 80 years old in the analysis because HIV mortality in those under 15 years old is very rare, and individuals over 80 years old were recorded as only one group (an all-ages group) in the GBD 2016 database. Although these age groups made up only a small proportion and did not influence our conclusion, we still cannot neglect them, and according to our prediction, the HIV mortality rate among elderly individuals over 80 years old is still increasing. Second, our study has an ecological fallacy and unique limitations associated with the APC model (including the identifiability problem and uncertainty principle); these limitations were inevitable because the interpretations of results at the population levels do not necessarily hold true at the individual level. Many other studies focused on the trends of cardiovascular disease mortality or cancer mortality using the same web tool for the APC model, similar to our study13,61,62. Therefore, future large-scale cohort studies are needed to confirm the related hypotheses in this study.
Conclusions
The ASMR of HIV in China increased dramatically from 1992 to 2016, especially in males. Considering the rapidly increasing risk of HIV mortality in young people, Chinese policymakers should take immediate measures, such as HIV promotion and education about PrEP and PEP, increasing HIV testing rate and surveillance and urging infected persons to receive ART as soon as possible to prevent HIV infection and reduce the mortality rate, to target the key age group of 15 to 44 years.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81673245 to YM, No. 81773454 to ZZ) and the China Scholarship Council (No. 201806015008 to ZZ). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Z. Zou and Y. Ma conceived and designed the project; Z. Zou collected the data; D. Gao and Z. Zou analysed the data and prepared the manuscript; and W. Zhang, T. Chen and W. Cui were involved in writing the article and had final approval of the submitted and published versions.
Data availability
The data set supporting the conclusions of this article are available in the GBD Data Tool repository (http://ghdx.healthdata.org/gbd-2016).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Disi Gao and Zhiyong Zou.
Supplementary information
is available for this paper at 10.1038/s41598-020-63141-1.
References
- 1.Streatfield PK, et al. HIV/AIDS-related mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action. 2014;7:25370. doi: 10.3402/gha.v7.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oti SO, et al. HIV mortality in urban slums of Nairobi, Kenya 2003–2010: a period effect analysis. Bmc Public Health. 2013;13:588,. doi: 10.1186/1471-2458-13-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. AIDS-related death. http://aidsinfo.unaids.org/ (2020).
- 4.Bezerra LMD. Global Report: UNAIDS Report on the Global AIDS Epidemic: 2010[J] Geneva Switzerland Unaids. 2012;27(7):553–556. [Google Scholar]
- 5.Mee P, et al. The development of a localised HIV epidemic and the associated excess mortality burden in a rural area of South Africa. Glob Health Epidemiol Genom. 2016;1:e7. doi: 10.1017/gheg.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute for Health Metrics and Evaluation. http://www.healthdata.org/russia/ (2020)
- 7.Jiang ZS, Jiang JN. Research progress in death risk factors of HIV infector and AIDS patients. Inter J Epidemiol Infect Dis. 2012;39:63–67. [Google Scholar]
- 8.Ministry of Health of the People’s Republic of China, China 2010 UNGASS Country Progress Report (2008–2009). http://data.unaids.org/pub/Report/2010/china_2010_country_progress_report_en.pdf. (2010).
- 9.Dou Z, et al. Gender difference in 2-year mortality and immunological response to ART in an HIV-infected Chinese population, 2006-2008. Plos One. 2011;6:e22707. doi: 10.1371/journal.pone.0022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y. & Huang K. S. Population size, spatial distribution and mortality characteristics of HIV/AIDS worldwide and China. Population and Society. 34, 10.14132/j.2095-79632018.04.008 (2018).
- 11.Amanuel AA, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M, et al. Cause-specific mortality for 240 causes in China during 1990–2013: A systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 13.Zou Z, et al. Time Trends in Cardiovascular Disease Mortality Across the BRICS: An Age-Period-Cohort Analysis of Key Nations with Emerging Economies Using the Global Burden of Disease Study 2017. Circulation. 2020;119:042864. doi: 10.1161/CIRCULATIONAHA.119.042864. [DOI] [PubMed] [Google Scholar]
- 14.Holford T. Age-period-cohort analysis.1-25 (2005).
- 15.Theodore R. Holford. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–324. doi: 10.2307/2531004. [DOI] [PubMed] [Google Scholar]
- 16.Robertson C, Gandini S, Boyle P. Age-period-cohort models: A comparative study of available methodologies. J Clin Epidemiol. 1999;52:569–583. doi: 10.1016/S0895-4356(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 17.Holford T. R. Age-Period-Cohort Analysis. 105–123 (2005).
- 18.Rosenberg PS, Anderson WF. Age-Period-Cohort Models in Cancer Surveillance Research: Ready for Prime Time? Cancer Epidemiology Biomarkers & Prevention. 2012;20:1263–1268. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg PS, Check DP, Anderson WF. A web tool for Age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomark. Prev. 2014;23:2296–2302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao DS, et al. Research of the HIV knowledge and high-risk sexual behaviors of Men who have sex with Men in college students four cities of China. Zhong Guo Xue Xiao Wei Sheng. 2019;40:359–363. [Google Scholar]
- 21.Guimaraes MDC, Carneiro M, Dmx A, Franca EB. HIV/AIDS Mortality in Brazil 2000-2015: Are there reasons for concern? Revista Brasileira de Epidemiologia. 2017;suppl 01:182–190. doi: 10.1590/1980-5497201700050015. [DOI] [PubMed] [Google Scholar]
- 22.Zayeri F, Talebi Ghane E, Borumandnia N. Assessing the trend of HIV/AIDS mortality rate in Asia and North Africa: an application of latent growth models. Epidemiology and Infection. 2016;144:548–555. doi: 10.1017/S0950268815001351. [DOI] [PubMed] [Google Scholar]
- 23.King R, et al. Men at Risk; a Qualitative Study on HIV Risk, Gender Identity and Violence among Men Who Have Sex with Men Who Report High Risk Behavior in Kampala, Uganda. Plos One. 2013;8:e82937. doi: 10.1371/journal.pone.0082937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drabkin AS, et al. Risk patterns preceding diagnosis among newly HIV-diagnosed men who have sex with men in New York City. Aids Patient Care Stds. 2013;27:333–341. doi: 10.1089/apc.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, et al. A Cross-Sectional Study of the Relationship between Sexual Compulsivity and Unprotected Anal Intercourse among Men Who Have Sex with Men in Shanghai, China. BMC Infectious Diseases. 2018;18:465. doi: 10.1186/s12879-018-3360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha GM, Kerr LR, de Brito AM, Dourado I, Guimarães MD. Unprotected receptive anal intercourse among men who have sex with men in Brazil. Aids & Behavior. 2013;17:1288–1295. doi: 10.1007/s10461-012-0398-4. [DOI] [PubMed] [Google Scholar]
- 27.Bor J, et al. Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa. Plos Medicine. 2015;12:e1001905. doi: 10.1371/journal.pmed.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takarinda KC, et al. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007-2010. Int J Infect Dis. 2015;30:98–105. doi: 10.1016/j.ijid.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang MY, Ni C. Recognition of the imbalance of sex ratio at birth in China. Zhong Guo Ren Li Zi Yuan Kai Fa. 2018;35:112–121. [Google Scholar]
- 31.Ailixiati N. Death Causes of AIDS and Its Impact on Life Expectancy in Yining City. Xinjiang Medical University. (2016)
- 32.Zhao HY, et al. Analysis of death data of HIV-infected and AIDS patients in Chuxiong Prefecture, Yunnan Province, 1996–2013. Pi Fu Bing Yu Xing Bing. 2016;38:118–121. [Google Scholar]
- 33.Jiang DD, et al. Retrospective analysis of AIDS deaths in Guangxi from 2004 to 2005. Ying Yong Yu Fang Yi Xue. 2014;19:340–343. [Google Scholar]
- 34.Zhang L, et al. Path Design of Supportive Environment Improvement for Sexual and Reproductive Health of Unmarried Migrant Youths in China. Population & Development. 2016;22:49–59. [Google Scholar]
- 35.Cai R, et al. Trends in high-risk sexual behaviors among general population groups in China: a systematic review. Plos One. 2013;8:e79320. doi: 10.1371/journal.pone.0079320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao D, et al. Secular trends in HIV/AIDS mortality in China from 1990 to 2016: Gender disparities. Plos one. 2019;14:e0219689. doi: 10.1371/journal.pone.0219689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang FY, et al. Survival time and related factors among MSM with HIV/AIDS in Jinhua city. Chin J Public Health. 2019;35:1633–1636. [Google Scholar]
- 38.Chen L, et al. Survival and its related factors among adolescent and adult HIV/AIDS patients in Fujian province, 1987-2017. Chin J Public Health. 2018;34:1603–1607. [Google Scholar]
- 39.Liu HX, et al. Survival time of HIV/AIDS cases and its related factors in Changping district of Beijing. Chin J Public Health. 2019;25:951–952+963. [Google Scholar]
- 40.Zhang F, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11:516–524. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 41.Shen Y, et al. Analysis of the immunologic status of a newly diagnosed HIV positive populaiotn in China. BMC Infect Dis. 2013;13:429. doi: 10.1186/1471-2334-13-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma JH, Chen WW, Cai L, Yang B, Zhao YY. Analysis on results of first CD4 + T lymphocytes detection in HIV /AIDS cases in Mile City of Yunnan Province from 2009-2013. Occup and Health. 2015;31:35–37+40. [Google Scholar]
- 43.Lin HJ, et al. Strategy of HIV/AIDS identification in a coastal prefecture in eastern China. Chin J Dis Control Prev. 2015;19:578–581. [Google Scholar]
- 44.Dai SY, et al. Prevalence and factors associated with late HIV diagnosis. J Med Virol. 2015;87:970–977. doi: 10.1002/jmv.24066. [DOI] [PubMed] [Google Scholar]
- 45.Jang H. B. Study on the impact of late HIV diagnosis on the progress of people living with HIV/AIDS. Huazhong University of Science and Technology (2016).
- 46.Chen L, et al. HIV cause-specific deaths, mortality, risk factors, and the combined influence of HAART and late diagnosis in Zhejiang, China, 2006–2013. Sci Rep. 2017;7:42366. doi: 10.1038/srep42366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z, et al. Evolution of China’s response to HIV/AIDS. Lancet. 2007;369:679–690. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng, Y. et al. Detection of antibody to LAV/HTLV-III in sera from hemophiliacs in China. AIDS Res. 2, S147–S149 (1986). [PubMed]
- 49.Lu H, et al. Effectiveness of HIV Risk Reduction Interventions among Men who have Sex with Men in China: A Systematic Review and Meta-Analysis. Plos One. 2013;8:e72747. doi: 10.1371/journal.pone.0072747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Z, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013;382:1195–1203. doi: 10.1016/S0140-6736(12)61898-4. [DOI] [PubMed] [Google Scholar]
- 51.Maude R, Volpe G, Stone D. Knowledge, Attitude and Practice of Pre-exposure prophylaxis (PrEP) against HIV infection of medical providers at an academic center. Open Forum Infectious Diseases. 2017;4:S437–S437. doi: 10.1093/ofid/ofx163.1106. [DOI] [Google Scholar]
- 52.Grant RM, et al. Pre-exposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krakower, et al. What Primary Care Providers Need to Know About Preexposure Prophylaxis for HIV Prevention. Annals of Internal Medicine. 2012;157:490. doi: 10.7326/0003-4819-157-7-201210020-00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auerbach, et al. Knowledge, Attitudes, and Likelihood of Pre-Exposure Prophylaxis (PrEP) Use Among US Women at Risk of Acquiring HIV. Aids Patient Care & Stds. 2014;29:102–110. doi: 10.1089/apc.2014.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park C, et al. HIV PrEP and PEP in Graduate Medical Education: A Novel Curriculum. Open Forum Infectious Diseases. 2017;4:S443–S443. doi: 10.1093/ofid/ofx163.1124. [DOI] [Google Scholar]
- 56.Santos RobertoP, et al. Adolescents’ Knowledge and Acceptance of Pre-exposure Prophylaxis (PrEP) in the Capital District Region of New York. Open Forum Infect Dis. 2018;5:S393–S394. doi: 10.1093/ofid/ofy210.1121. [DOI] [Google Scholar]
- 57.Elizabeth H, et al. HIV Pre-Exposure Prophylaxis (PrEP) Uptake, Initiation, and Persistence in the Detroit Public Health STD Clinic. Open Forum Infect Dis. 2017;4:S437. [Google Scholar]
- 58.Thomas R, et al. Adherence to Post-Exposure Prophylaxis (PEP) and Incidence of HIV Seroconversion in a Major North American Cohort. PLoS One. 2015;10:e0142534. doi: 10.1371/journal.pone.0142534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Epstein R, et al. Reducing Delays to Antiretroviral (ARV) Receipt in Children Prescribed Post-Exposure Prophylaxis (PEP) for HIV: Meds-in- Hand and a Multidisciplinary Team Approach. Open Forum Infectious Diseases. 2017;4:S664–S664. doi: 10.1093/ofid/ofx163.1771. [DOI] [Google Scholar]
- 60.Tamara E, et al. Challenges of HIV diagnosis and management in the context of pre‐exposure prophylaxis (PrEP), post‐exposure prophylaxis (PEP), test and start and acute HIV infection: a scoping review. J Int AIDS Soc. 2019;22:e25419. doi: 10.1002/jia2.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZK, et al. Age–Period–Cohort Analysis of Stroke Mortality in China Data From the Global Burden of Disease Study 2013. Stroke. 2017;48:271–275. doi: 10.1161/STROKEAHA.116.015031. [DOI] [PubMed] [Google Scholar]
- 62.Moon EK, et al. Trends and Age-Period-Cohort Effects on the Incidence and Mortality Rate of Cervical Cancer in Korea. Cancer Res Treat. 2017;49:526–533. doi: 10.4143/crt.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set supporting the conclusions of this article are available in the GBD Data Tool repository (http://ghdx.healthdata.org/gbd-2016).


