Abstract
The pathogenic success of Mycobacterium tuberculosis (Mtb) is tightly linked to its ability to recalibrate host metabolic processes in infected host macrophages. Since changes in cellular metabolic intermediates or pathways also affect macrophage function in response to pathogens, we sought to analyse specific metabolic alterations induced by Mtb infection. Stimulation of macrophages with Mtb lysate or lipopolysaccharide (LPS) induced a relative increase in glycolysis versus oxidative phosphorylation. Cellular metabolomics revealed that Mtb infection induced a distinct metabolic profile compared to LPS in both M1 and M2 macrophages. Specifically, Mtb infection resulted in elevated intracellular levels of nicotinamide adenine dinucleotide (NAD+), creatine, creatine phosphate and glutathione compared to uninfected control macrophages. Correspondingly, RNA-sequencing datasets showed altered gene expression of key metabolic enzymes involved in NAD+, creatine, glucose and glutamine metabolism (e.g NAMPT, SLC6A8, HK2) in Mtb-infected M2 macrophages. These findings demonstrate clear modulation of host macrophage metabolic pathways by Mtb infection.
Subject terms: Preclinical research, Infection, Inflammation, Innate immune cells, Pathogens, Infection, Inflammation
Introduction
Mycobacterium tuberculosis (Mtb) is the causative pathogen of tuberculosis (TB) and responsible for over a million deaths annually1. Mtb is transmitted through inhalation of aerosol particles and transported to the lungs, where it infects alveolar macrophages and avoids eradication through interfering with innate antimicrobial mechanisms. Infected cells are sequestered at the core of the TB granuloma as part of the host immune response, a confined niche where Mtb can reside in a dormant state for decades before potential disease reactivation2,3. However, in order to persist Mtb must overcome the limitations set by the anti-mycobacterial microenvironment of the granuloma, which include hypoxia4 and nutrient scarcity5. These conditions compel Mtb to switch from using carbohydrates to lipids and cholesterol as primary carbon source during later stages of infection, as part of its transition to a dormant state6–9. Several studies have demonstrated that Mtb is able to reprogram macrophage metabolism, and these adaptations are thought to be essential for its pathogenic success10–12.
Besides providing the necessary nutrients, metabolic changes induced by Mtb could also rewire the activation state and anti-microbial effector functions of infected macrophages. Over recent years many studies in the emerging field of immunometabolism have attempted to define the associations between macrophage metabolic states and their immunological responses13. The outcome of macrophage immunometabolism is largely determined by the balance between glycolysis and mitochondrial metabolism through oxidative phosphorylation (OXPHOS) of tricarboxylic acid cycle (TCA) intermediates14,15. Glycolysis is associated with classical pro-inflammatory macrophages activated with IFNγ and/or the Toll-like receptor (TLR) 4 ligand lipopolysaccharide (LPS)16, and OXPHOS with the alternatively activated anti-inflammatory phenotype induced by the TH2 cytokines interleukin-(IL)-4 and IL-1317. Activation of myeloid cells and T cells has been demonstrated to enhance aerobic glycolysis18,19, resembling a process first observed in cancer cells by Otto Warburg and therefore known as the Warburg effect20. The Warburg effect supports pro-inflammatory effector functions through rapid production of ATP and other necessary metabolic intermediates. Several studies reported increased lactate production or glycolytic enzyme expression in human and murine macrophages or lung tissue after Mtb infection21–24, implying that glycolysis is induced as part of the host anti-mycobacterial response. However, stimulation with different pathogens or TLR ligands has since been shown to lead to more complex metabolic phenotypes in myeloid cells than what simply can be explained by the Warburg effect, including changes in lipid, cholesterol and amino acid metabolism25. Importantly, Mtb and other mycobacteria have been shown to manipulate macrophage lipid metabolism, leading to the formation of lipid-loaded foam cells which constitute a preferred niche for mycobacterial persistence8,11,26–28.
Considering the importance of metabolic adaptations for Mtb killing and survival, several studies aimed to dissect the precise impact of the bacterium on macrophage metabolism by cellular metabolomics29–31. However, these relied on phorbol 12-myristate 13-acetate (PMA)-activated macrophage-like THP-1 cells as a model for macrophage infection, which significantly differ from primary macrophages in terms of polarization and response to stimuli32,33. To address this critical gap in knowledge, we have here studied the effect of Mtb infection on primary human macrophage metabolism using not only untargeted liquid chromatography-mass spectrometry (LC-MS) metabolomics but also targeted 1H-nuclear magnetic resonance (NMR) spectroscopy34.
Results
Mtb lysate and LPS induced glycolytic metabolism in human macrophages
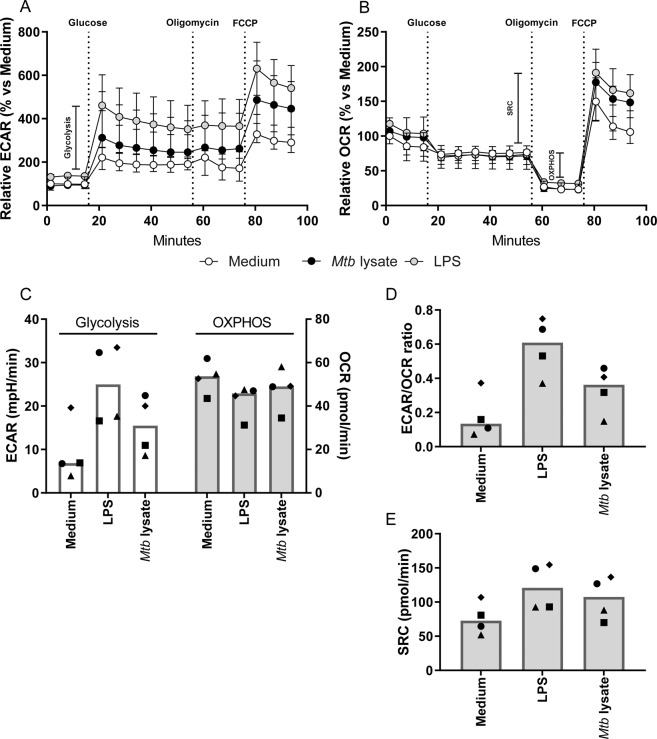
In vitro stimulation with TLR ligands or whole pathogen lysates is commonly used to model immune cell activation in response to bacterial infection, and has previously been demonstrated to modulate myeloid cell metabolism18,25. To validate whether primary human macrophage metabolism was truly affected by Mtb stimulation, macrophage colony-stimulating factor (M-CSF)-derived primary human macrophages (M2) were stimulated with Mtb lysate (10 µg/ml) as a model for Mtb infection and their metabolic activity was analyzed using a Seahorse XF Analyzer. LPS (100 ng/ml), a TLR4 ligand which is known to induce glycolysis in macrophages, and culture medium were used as a positive and negative control for metabolic skewing, respectively. Cellular glycolysis (Fig. 1A), OXPHOS and spare respiratory capacity (SRC) (Fig. 1B) were determined after a series of injections with D-glucose, ATP synthase inhibitor oligomycin and mitochondrial uncoupling agent FCCP. As expected, LPS stimulation showed a trend towards increased glycolysis-related acidification, while simultaneously decreasing macrophage mitochondrial respiration compared to medium control (Fig. 1C,D), albeit with a greater SRC (Fig. 1E). Mtb lysate induced similar tendencies for both extracellular acidification rate (ECAR)/oxygen consumption rate (OCR) ratio (Fig. 1C,D) and SRC (Fig. 1E), although the magnitude of this effect was less pronounced compared to LPS. Taken together, both stimulation with Mtb and LPS seem to result in metabolic skewing towards increased glycolysis while simultaneously decreasing OXPHOS in primary human macrophages.
Figure 1.
Stimulation with LPS or Mtb lysate induced a glycolytic shift in primary human macrophages. M2 macrophages were stimulated with medium (white circles), Mtb lysate (10 µg/ml; black circles) or LPS (100 ng/ml; grey circles) for 24 h. (A) Macrophage extracellular acidification rate (ECAR) and (B) oxygen-consumption rate (OCR) were measured during sequential injections of D-glucose (10 mM), oligomycin (1 µM) and FCCP (2 µM). (C) Glycolysis (white bars) as determined by the difference in ECAR pre- and post-glucose injection, and OXPHOS (grey bars) as the difference in OCR pre- and post-oligomycin injection. (D) ECAR/OCR ratio. (E) Spare respiratory capacity as determined by the difference in OCR pre-oligomycin and post-FCCP injection. Each symbol represents an individual donor, and bars represent group medians (n = 4). Data is depicted as medians with ranges (n = 4).
Exploratory metabolomics of Mtb-infected macrophages
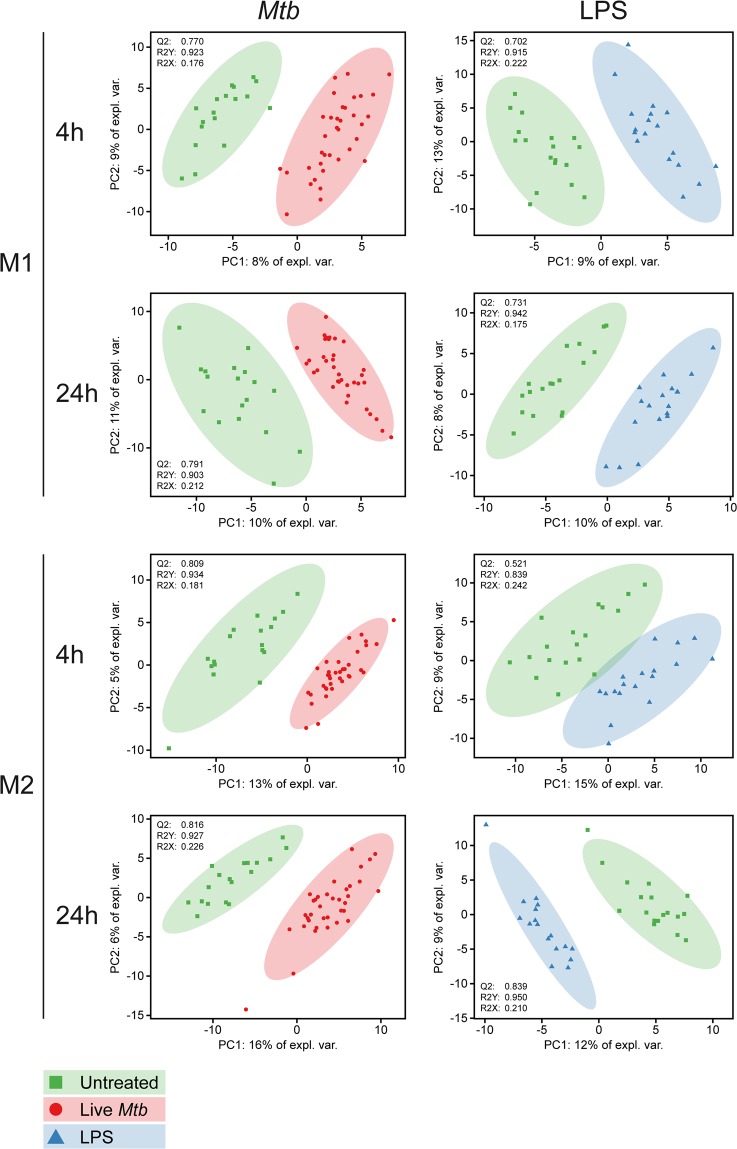
Since mycobacterial products are able to redirect macrophage metabolism, we sought to further characterize the effects of live Mtb infection on macrophage metabolism using exploratory metabolomics. Therefore, we generated a set consisting of granulocyte-macrophage colony-stimulating factor (GM-CSF) (M1) and M-CSF (M2) differentiated macrophages from six healthy blood bank donors which were either infected with Mtb-H37Rv, stimulated with LPS (100 ng/ml) or left untreated and subsequently harvested at either 4 or 24 h post-infection for metabolite extraction. Supplementary Fig. 1 (Fig. S1A–D) shows an exploratory analysis of the resulting dataset (peak intensities of 270 masses) by Principal Component Analysis (PCA); the resulting score plot is colored according to main possible sources of variance in the LC-MS data. A dichotomy in cell type indicating metabolic differences between M1 and M2 macrophages was observed in the first two principal components, explaining 24% of total variance (Fig. S1B). However, high inter-individual heterogeneity also strongly contributed to the variance explained by the first two components. Therefore, a multilevel PCA model was fitted on the dataset to separate the between-donor and within-donor data variation35 (Fig. S1E–G). This model successfully reduced the donor related variability (Fig. S1E), while retaining the metabolic effects of cell type (Fig. S1F) within the first two components, explaining 19% of total variance. To compensate for this difference in metabolic profile at baseline between M1 and M2 macrophages, distinct multilevel PCA models were fitted for both cell types (Fig. S2) to visualize potential effects of Mtb infection or LPS stimulation. The resulting score plots showed improved separation based on infection/treatment status in M2 macrophages (Fig. S2F), however this was not clearly observable in M1 macrophages (Fig. S2C).
For a more focused analysis of the effect of Mtb infection or LPS stimulation on macrophage metabolism, we performed group separation at specific time points per stimulation using Partial Least Squares Discrimination Analysis (PLS-DA) and extracted Variable Importance in Projection (VIP) scores from the resulting models to identify which masses carried the highest classification weight for each individual comparison. Separate multilevel PLS-DA models were built for each combination of cell type and time point using treatment group status as class variable. Resulting score plots and cross-validated model quality characteristics are displayed in Fig. 2. All models showed good predictive capabilities as evidenced by high Q2 and R2Y scores (>0.5 and >0.8 respectively), signifying clear metabolic effects of both Mtb infection and LPS stimulation for each cell type and time point compared to untreated control. Next, VIP scores were extracted from the first component of each PLS-DA model to examine which measured variables explained the largest proportion of data variance in each model. Volcano plots of metabolite VIP scores versus their respective regression coefficients for each individual PLS-DA model are displayed in Supplementary Fig. S3. In total, 46 masses reached a combined high VIP score of ≥2 and associated regression coefficient of ≥0.1 or ≤−0.1 in at least one model.
Figure 2.
PLS-DA models of M1 and M2 macrophages after 4 h or 24 h of Mtb infection or LPS stimulation. M1 and M2 macrophages were either infected with Mtb at a MOI of 10:1, stimulated with LPS (100 ng/ml) or left untreated. Cells were lysed at 4 h and 24 h post-infection/stimulation and intracellular metabolites were subsequently extracted and measured by LC-MS. Multilevel PLS-DA models were fitted for each group/time point comparison in M1 and M2 macrophages and score plots of the resulting eight models with associated quality metrics (Q2/R2Y/R2X) are displayed. Each point represents one technical replicate derived from six biological blood bank donors. Untreated samples are depicted as green squares, LPS stimulated samples as blue triangles and Mtb infected samples as red dots.
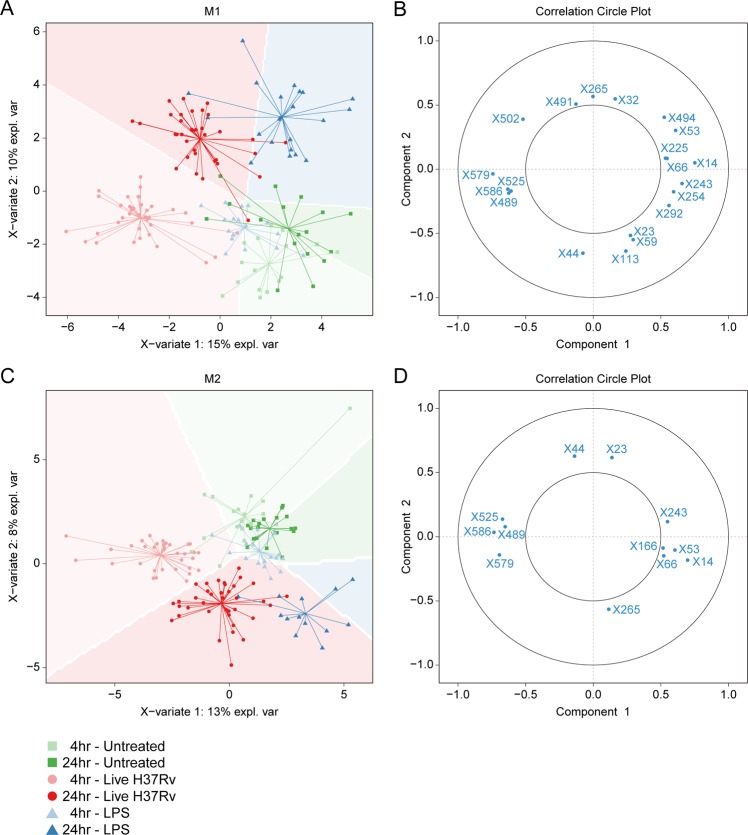
To verify the discriminatory capacity of this selection of masses, separate PLS-DA models were fitted on all combined samples derived from either M1 or M2 macrophages using only these 46 variables (Fig. 3A,C). Both cell type models still showed good class separation of Mtb-infected cells at 4 and 24 h and LPS-treated cells at 24 h compared to untreated controls samples based on the first two components. Circle correlation plots visualizing the correlation between individual variables and the first two components revealed 20 and 12 metabolites in M1 (Fig. 3B) and M2 (Fig. 3D) macrophages, respectively, with relatively good correlation scores (≥0.5), which combined constituted a total of 21 unique masses. Tentative annotations of these masses with degree of certainty are shown in Table 1, and boxplots of metabolite peak areas are displayed in Supplementary Fig. S4. In both cell types, LPS treatment was associated with increased levels of masses annotated as adenosine (X265, m/z = 269.104), nicotinamide (X32, m/z = 123.055) and propionylcarnitine (X166, m/z = 218.138), while Mtb infection showed high correlation with relatively large masses (X489, m/z = 382.189; X579, m/z = 458.249; X586, m/z = 470.241) which could not be annotated based on database searches. These unknown structures are fragments of either proteins or lipids for which the number of possible assignments cannot be reduced to a minimum required for tentative annotation.
Figure 3.
Group separation by selected metabolites with high Variable Importance for Projection (VIP) scores. Multilevel PLS-DA models were fitted on all samples using a selection of 46 metabolites which reached VIP scores of ≥2 and associated regression coefficients of ≤−0.1 or ≥0.1 in any pairwise PLS-DA model described in Fig. 2. Score plots of the resulting model for M1 (A) and M2 (C) macrophages (M2) are displayed. Untreated samples are depicted as green squares, LPS stimulated samples as blue triangles and Mtb infected samples as red dots. Shade of symbol color reflects time point (4 h = light, 24 h = dark). Strongly correlated metabolites (threshold: ≥0.5) are displayed in circle plots for both the M1 (B) and the M2 (C) PLS-DA model.
Table 1.
Tentative annotation of selected masses.
| Nr. | m/z | ID | Adduct | Error (ppm) | ID level |
|---|---|---|---|---|---|
| X14 | 104.107 | Choline | H+ | 6 | 3 |
| X23 | 116.071 | Proline | H+ | 1 | 2 |
| X32 | 123.055 | Nicotinamide | H+ | 1 | 2 |
| X44 | 133.061 | L-Asparagine | H+ | 2 | 2 |
| X53 | 142.026 | Dimethylglycine | K+ | 3 | 2 |
| X59 | 146.996 | NA | — | — | 4 |
| X66 | 149.063 | Glutamate | H+ | 1 | 2 |
| X113 | 184.073 | 3-Dehydroxycarnitine | K+ | 1 | 3 |
| X166 | 218.138 | Propionylcarnitine | H+ | 1 | 3 |
| X225 | 249.045 | Unknown peptide | H+ | — | 4 |
| X243 | 258.110 | Glycerophosphocholine | H+ | 1 | 3 |
| X254 | 263.086 | Hydroxysebacate | 2Na-H | 3 | 3 |
| X265 | 268.104 | Adenosine | H+ | 1 | 3 |
| X292 | 280.092 | Glycerophosphocholine | Na+ | 1 | 3 |
| X489 | 382.189 | Unknown peptide | — | — | 4 |
| X491 | 383.101 | NA | — | — | — |
| X494 | 385.175 | NA | — | — | — |
| X502 | 388.269 | NA | — | — | — |
| X525 | 415.224 | NA | — | — | — |
| X579 | 459.249 | NA | — | — | — |
| X586 | 470.241 | NA | — | — | — |
Targeted metabolomics of Mtb-infected macrophages by 1H-NMR spectroscopy
While the LC-MS approach clearly demonstrated that Mtb infection greatly impacts the metabolome of infected macrophages beyond an increase in glycolysis, the untargeted metabolic profiling often enables only tentative structural annotation. Therefore, to complement our untargeted dataset, we employed 1H-nuclear magnetic resonance (NMR) spectroscopy to further dissect the metabolic effects of Mtb infection in M2 macrophages compared to uninfected control samples at 4 h and 24 h post-infection. While not as sensitive as mass spectrometry, 1H-NMR spectroscopy is nonetheless quantitative and known for its robustness34.
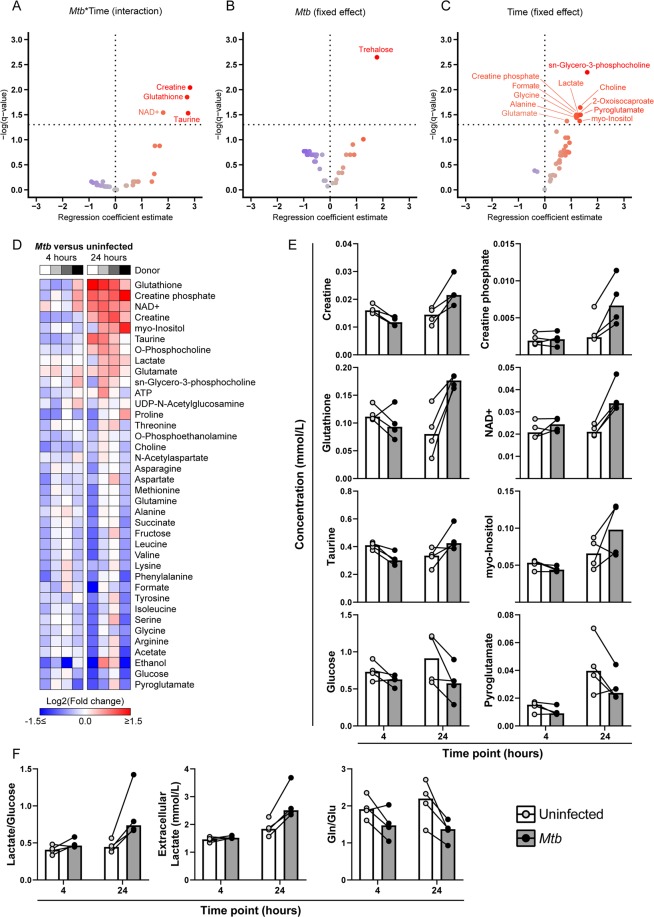
Separate linear random intercept models were fitted for each individual metabolite to model the interaction between infection status (Mtb vs uninfected) and time (24 h vs 4 h) (Fig. 4A). Significant interactions were detected for four metabolites, creatine (q = 9.09E−3), glutathione (q = 0.014), nicotinamide-adenine-dinucleotide (NAD+) (q = 0.029) and taurine (q = 0.029), all of which increased between 4 and 24 h in infected macrophages but either decreased or did not change in uninfected controls (Fig. 4E). Together with creatine-phosphate and myo-inositol, these metabolites constituted the top six most elevated factors in Mtb-infected versus uninfected macrophages according to median log2-transformed fold changes (Fig. 4D,E). Next, we fitted linear mixed models without interaction term to analyze the fixed effects of infection and time. The disaccharide trehalose, an important component of mycobacterial cell-wall glycolipids35, was only detected in infected macrophages and therefore significantly associated with Mtb infection (q = 2.26E−3) (Fig. 4B). Various metabolites showed a significant positive association with time (Fig. 4C), including sn-glycero-3-phosphocholine (q = 4.50E−3), choline (q = 0.023), creatine phosphate (q = 0.032), pyroglutamate (q = 0.032) and lactate (q = 0.033), although the magnitude of this increase could vary based on infection status (Fig. 4E).
Figure 4.
Targeted 1H-NMR spectroscopy analysis of Mtb-infected M2 macrophages. M2 macrophages were infected with Mtb at a MOI of 10:1 or left uninfected. Cells were lysed at 4 h and 24 h post-infection and intracellular metabolites were subsequently extracted and measured by 1H-NMR spectroscopy. Linear random intercept models for fitted for each metabolite to investigate: (A) the interaction between infection and time (Mtb:Time), or the separate fixed effects of (B) Mtb infection or (C) time. Resulting -log-transformed FDR-corrected p-values (q-values) are plotted against the regression coefficient estimate for each metabolite in volcano plots. (D) Heatmap of log2-transformed metabolite fold changes (Mtb/uninfected). Metabolites are sorted by average median fold change at 24 h. Individual donors are presented in greyscale. (E) Absolute levels of creatine, creatine phosphate, glutathione, NAD+, taurine, myo-inositol, glucose and pyroglutamate (mmol/L) in Mtb-infected macrophages (grey bars) or uninfected controls (white bars) at 4 h and 24 h. (F) Extracellular lactate concentrations and relative ratios of lactate/glucose and glutamate/glutamine (Glu/Gln). Each dot represents an individual donor, and measurements from matching donors are connected by black lines. Bars represent group medians.
As macrophage activation with Mtb lysate induced a relative increase in extracellular acidification indicative of anaerobic glycolysis, we wondered whether live Mtb infection would also trigger similar changes in glycolytic intermediates. While glucose levels were lower in Mtb-infected macrophages in 3/4 donors at both 4 h and 24 h, the lactate/glucose ratio was increased in all donors at 24 h (median increase of 81%) (Fig. 4F), indicative of increased glucose utilization for ATP production by anaerobic glycolysis36, which is congruent with the results from Fig. 1. Additionally, extracellular lactate concentrations were elevated in all donors at 24 h post-infection (Fig. 4F). As only one intermediate of the TCA cycle was detected (succinate) which in itself did not show obvious modulation by Mtb infection, the relative mitochondrial activity could not be assessed in a similar fashion. However, we did observe a decreased glutamine (Gln) to glutamate (Glu) ratio (Fig. 4F), reflecting increased glutamine catabolism.
Mtb-induced metabolic changes are reflected by the macrophage transcriptome
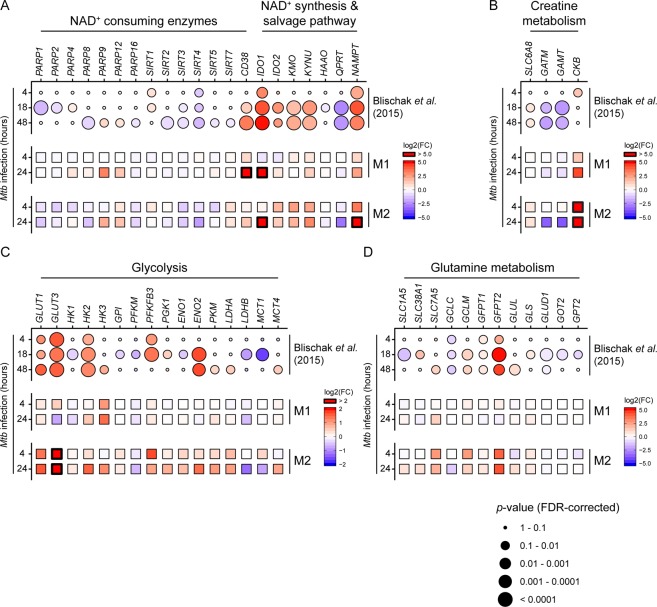
Finally, we wondered whether the observed metabolic changes induced by Mtb infection were reflected by alterations in macrophage gene expression levels. To study this, we analysed the results of previously published expression profiling of M2 macrophages infected with Mtb-H37Rv (n = 6) performed by Blischak et al.37 for differentially expressed genes involved in glycolysis, NAD+, creatine and glutamine metabolism, and compared their expression profiles to an exploratory RNA-seq dataset of Mtb-infected M1 and M2 macrophages acquired using our own specific Mtb infection model. Gene expression profiles showed significant linear correlation between both datasets, especially for M2 macrophages at 24 h (Fig. S5). In both sets, Mtb infection was associated with differential expression of NAD+-consuming enzymes, such as cyclic ADP ribose hydrolase (CD38) and various members of the poly (ADP-ribose) polymerase (PARP) and sirtuin (SIRT) protein families (Fig. 5A). Congruent with the observed increase in intracellular NAD+ levels, genes involved in NAD+ biosynthesis were strongly upregulated during Mtb infection, including nicotinamide phosphoribosyltransferase (NAMPT) and indoleamine 2,3-dioxygenase 1 (IDO1), rate-limiting enzymes of the NAD+ salvage and kynurenine pathway respectively. In contrast, expression of quinolate phosphoribosyltransferase (QPRT), which catalyzes quinolinic acid conversion downstream of IDO1, was decreased. With regard to creatine metabolism, expression of creatine synthesis enzyme genes GATM and GAMT decreased as a result of Mtb infection, while the creatine transporter SLC6A8 and creatine kinase (brain-type, CKB) were increased, most notably in our M2 infection model (Fig. 5B). Furthermore, Mtb infection induced expression of genes known to control glycolytic flux38, namely glucose transporter 1 (GLUT1) and 3 (GLUT3), hexokinase 2 (HK2), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and monocarboxylate transporter 4 (MCT4) (Fig. 5C), which is again in agreement with the NMR metabolic data. Finally, Mtb modulated expression of various genes involved in glutamine metabolism in both datasets, including members of the hexosamine (GFPT1 and GPFT2) and glutathione synthesis (GCLC and GCLM) pathways (Fig. 5D), corresponding with the increased levels of intracellular glutathione after Mtb infection. Taken together, we find that Mtb infection results in clear metabolic changes in macrophages, including alterations in NAD+, creatine, glucose and glutamine metabolism, which can be connected to corresponding changes in metabolic gene expression patterns.
Figure 5.
Mtb modulates gene expression of key metabolic enzymes in infected macrophages. Expression profiles of genes involved in (A) NAD+ consumption and synthesis, (B) creatine metabolism, (C) glycolysis and (D) glutamine metabolism from a previously published RNA-seq dataset (Blischak et al.)37 of M2 macrophages at 4, 18 and 48 h post-Mtb infection and an exploratory RNA-seq dataset derived from our M1/M2 Mtb infection model. Expression data is displayed as log2-transformed fold changes of Mtb infected vs non-infected macrophages. Up- and downregulation of genes is displayed by a color gradient (up = red, down = blue). Differential expression results (FDR-corrected p-values) from Blischak et al. are reflected by circle size.
Discussion
Since the emergence of the multidisciplinary field of immunometabolism, a growing body of evidence has accumulated connecting specific aspects of cellular metabolism to macrophage activation and function in response to danger signals or microbes. While we were able to validate that activation with LPS and Mtb lysate resulted in a relative shift toward increased glycolysis in primary macrophages, many of the published findings in the literature have yet to be translated to infections of primary human cells with live pathogens. Here, we contribute to this knowledge gap by employing both untargeted LC-MS metabolomics and targeted 1H-NMR spectroscopy to investigate the effect of live Mtb infection on macrophage metabolism. PLS-DA modeling of untargeted metabolomics data demonstrated that both Mtb infection and LPS stimulation were associated with marked changes in the cellular metabolome in both M1 and M2 macrophages. Tentative annotation of changed metabolites indicate increased levels of adenosine, nicotinamide and propionylcarnitine in LPS-stimulated M1 and M2 macrophages. Mtb infection was strongly associated with increased levels of relatively large masses, however these could not be annotated based on current metabolite databases and potentially constitute Mtb-derived protein fragments or lipids, one of which was identified as trehalose using quantitative 1H-NMR measurements. The latter also revealed various changes in specific metabolites in Mtb-infected versus uninfected macrophages, including NAD+, creatine and glutathione, which could be linked to changes in gene expression as determined by analysis of RNA-seq datasets.
Levels of NAD+, an important cofactor in many cellular pathways, were elevated in Mtb-infected macrophages. Mtb has been demonstrated to secrete toxins which can induce macrophage necrosis through NAD+-depletion39,40. In addition, recent studies have highlighted the importance of sustaining adequate NAD+ levels for pro-inflammatory macrophage function. Reactive oxygen species (ROS) produced during inflammatory activation were shown to induce DNA damage in macrophages, leading to PARP activation and depletion of NAD+ pools41. NAD+ salvage through activation of NAMPT was required for maintaining glycolytic flux and inflammatory functions. Furthermore, NAD+ synthetic capacity was reduced in aged mice compared to young controls, indicating that changes in NAD+ metabolism could play a role during aging-associated immune dysregulation42. Our results imply increased activity of NAD+ biosynthesis pathways as a result of Mtb infection. This result is corroborated by analysis of independent RNA-seq datasets, which showed increased expression of genes involved in NAD+ synthesis and salvage, including IDO1 and NAMPT. Interestingly, expression of QPRT was decreased in M2 macrophages, an effect which was found to limit de novo NAD+ synthesis through the kynurenine pathway in LPS-stimulated macrophages42. As the general interest in the therapeutic potential of NAD+-boosting drugs is on the rise43, these results call for further studies on the importance of macrophage NAD+ metabolism during Mtb infection, also in view of potential application in host-directed therapeutic strategies.
Besides NAD+, intracellular levels of the anti-oxidant glutathione (GSH; reduced form) also increased during macrophage Mtb infection. Glutathione is produced as part of the cellular redox response to increased levels of ROS and regulates macrophage anti-mycobacterial functions both through direct anti-microbial effects and by serving as a carrier of nitric oxide (NO)44. Furthermore, Mtb-infected cells showed an increase in glutamine catabolism via its conversion to glutamate. The latter is a precursor for the de novo synthesis of glutathione and together with the increased intracellular GSH, point to a metabolic switch caused by Mtb infection towards the synthesis of glutathione45. As glutamine has been reported to regulate macrophage cytokine production46 and support M2 polarization47, increased glutamine catabolism could potentially modulate macrophage cytokine responses to Mtb, although the resulting net effect on mycobacterial bacterial survival is unclear. Intracellular levels of creatine and creatine phosphate were also increased after 24 h of Mtb infection. The creatine phosphate system constitutes a spatiotemporal buffer for intracellular ATP concentrations, mostly in tissues with high energy consumption such as skeletal muscle and brain48. Creatine phosphate was also shown to be involved in cytoskeletal dynamics49,50 and macrophage phagocytosis51, possibly through increased localization of CKB to nascent phagosomes52. The observed increase in creatine phosphate and CKB expression could therefore be a reflection of macrophage phagocytic activity. Additionally, creatine is synthesized from arginine, and increased creatine production could reduce intracellular availability of arginine for anti-bacterial NO production53. However, as creatine synthesis in vivo occurs through respective actions of GATM of GAMT in the kidneys and liver, the elevated creatine levels could also be the result of increased expression of the creatine transporter SLC6A8, as was observed in the RNA-seq analysis. Finally, the intracellular lactate/glucose ratio was elevated during Mtb infection, further evidencing an increased glycolytic flux from glucose to lactate. These findings are corroborated by a recent study which also reported increased levels of lactate and glutamine-derived metabolites in Mtb lysate-stimulated peripheral blood mononuclear cells54. Interestingly, while increased glycolysis has been linked to improved Mtb clearance21, a recent paper demonstrated that Mtb can utilize lactate as a source of carbon for intracellular replication in macrophages55, calling for more extensive studies on the role of lactate production during macrophage Mtb infection.
Our study has several limitations that need to be discussed. Firstly, it was not possible to differentiate whether metabolites were of mycobacterial or human host origin in our experimental setup, with the exception of metabolites such as trehalose that can only be of mycobacterial origin, and were only detected in Mtb infected macrophages. However, we consider major mycobacterial contributions to metabolite levels unlikely due to the large difference in cell volume between macrophages and infecting Mtb. Furthermore, the majority of our observed changes (e.g. elevated creatine, glutathione, NAD+) manifested predominantly with prolonged infection time, which could be considered an argument against being mycobacterial metabolites as these were also present and likely measurable at 4 h, in a similar fashion to trehalose. Secondly, we were unable to perform extracellular flux analysis during live Mtb infection due to biosafety restrictions and therefore used Mtb lysate as a model for infection. While the observed increase in ECAR/OCR ratio is corroborated by other studies using γ-irradiated Mtb21,56, this result was contradicted by a recent paper which reported an overall decrease in macrophage bioenergetic profile during live Mtb infection57. Although we find that a potential role of toxicity on these results is not conclusively excluded by the authors, it would still be important to compare the effects of Mtb lysate stimulation to those of live Mtb infection in future experiments. Lastly, it is unclear how accurately the observed metabolic responses of human monocyte-derived macrophages reflect those of alveolar macrophages in vivo. Results from mouse studies are suggestive of a model in which the metabolic response of macrophages to Mtb infection is largely dependent on their ontology58. Therefore, it would be of great interest to study whether our results are reproducible using primary tissue-resident macrophage populations.
In conclusion, live Mtb infection induces pronounced metabolic changes in primary human macrophages, including activation of NAD+ and glutathione synthesis, glycolysis, glutaminolysis and the creatine phosphate pathway. Whether these changes benefit the host or the bacterium could not directly be inferred from these experiments and needs to be studied further: follow-up experiments using small-molecule inhibitors or small interfering RNAs (siRNAs) which target key enzymes of these pathways will be necessary to gauge their exact involvement in the macrophage anti-mycobacterial immune response.
Materials and Methods
Study design and sample collection
All experimental procedures were performed according to local and national guidelines on the work with pathogenic mycobacteria. Infection experiments with Mycobacterium tuberculosis H37Rv were performed as approved by the local biosafety officer and following the BSLIII permit granted to LUMC by the Dutch government.
Monocytes were isolated from buffy coats obtained from Sanquin blood products (Amsterdam, The Netherlands). The medical ethical review board of LUMC has approved use of buffy coats, remaining after blood donation, for scientific purposes. Healthy donors donating their blood have provided written informed consent for scientific use of their blood products.
Monocyte isolation and differentiation
CD14+ monocytes were isolated from buffy coats of healthy blood bank donors by positive selection using an autoMACS Pro Separator (Miltenyi Biotec BV, Leiden, The Netherlands). Monocytes were differentiated into macrophages using 50 ng/ml M-CSF (Miltenyi Biotec) or 5 ng/ml GM-CSF (Miltenyi Biotec) for six days at 37 °C/5%CO2. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin and 100 µg/ml streptomycin and GlutaMAX (Gibco, Thermo Fisher, Merelbeke, Belgium). After differentiation macrophages were harvested by trypsinization and seeded in multiwell plates. As a quality control, macrophages were stained for surface expression of CD14 and CD163 and acquired on a BD LSRFortessa flow cytometer (BD Biosciences, Erembodegem, Belgium).
Seahorse extracellular flux (XF) analysis
For cellular metabolic flux analysis, macrophages were stimulated overnight with either medium, 100 ng/ml LPS or H37Rv Mtb lysate (10 µg/ml) and measured on a Seahorse XF96 Analyzer (Seahorse Bioscience, North Billerica, MA, USA). Cell culture medium was replaced with RPMI without buffer and glucose supplemented with 5% FCS and L-glutamine and macrophages were incubated in a 37 °C dry incubator for one hour before start of measurements. Macrophage oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were determined in real-time throughout consecutive injections of D-glucose (10 mM), oligomycin (1 µM) and FCCP (2 µM). Acidification due to glycolysis was calculated as the difference in highest ECAR measurements pre- and post-glucose injection. Oxygen consumed for ATP production by oxidative phosphorylation was calculated as the difference between highest OCR measurements pre- and post-oligomycin injection. In case of obvious injection errors the affected measurements were excluded from the analysis.
Mtb H37Rv culture and infection
Mtb H37Rv cultures were grown to mid-log phase in Middlebrook 7H9 liquid medium (Difco, BD Biosciences) supplemented with albumin/dextrose/catalase (ADC) (BBL, BD Biosciences). Bacterial concentrations were determined by measuring the culture optical density at 600 nm59. Macrophages were infected with H37Rv at a multiplicity of infection (MOI) of 10:1 for 1 hour at 37 °C, after which the cells were washed twice with medium containing 30 µg/ml gentamicin and further cultured for 4 or 24 h in fresh medium containing 5 µg/ml gentamicin. MOI was confirmed by plating a dilution series of the inoculum on 7H10 square agar plates supplemented with oleate/albumin/dextrose/catalase (OADC) (BBL, BD Biosciences)59.
Sample preparation for LC-MS
Macrophages were seeded in 24-well plates at a density of 300,000 cells/well and either infected with Mtb, stimulated with LPS (100 ng/ml; Thermo Fisher) or left untreated. At 4 and 24 h post-infection/stimulation, macrophages were washed with ice-cold 1% NaCl and subsequently lysed in water by osmotic pressure for 15 minutes at 4 °C. Lysates were thoroughly resuspended and mixed with pre-heated 80% ethanol at a 1:3 ratio (end concentration: 60% ethanol) in polypropylene screwcap tubes and subsequently heated for 10 min at 90 °C. Samples were chilled for 10 minutes on ice before centrifugation at 13.2 × 1000 rpm for 10 minutes at 4 °C, after which the supernatants were harvested and stored at −80 °C for subsequent LC-MS analysis.
LC-MS/MS measurements and metabolite annotation
Samples were randomized before the analysis. The acquisition sequence was designed using a standard block structure: the material was injected by the blocks of five samples flanked by the QC pool samples. Fifty µL of each sample or QC pool were injected into the RPLCQ-TOF system (Ultimate 3000RS tandem Ultra High Performance Chromatography system, Thermo Scientific/Dionex, Amsterdam, Netherlands; Electrospray Ionization – Ultra High Resolution - Time of Flight mass spectromter maXis, Bruker Daltonics, Bremen, Germany). The details of the RPLC-Q-TOF method have previously been reported60. Pre-processing of the raw data (alignment of retention time, peak picking, filtering and normalization) was performed on the files converted into mzxml format. Retention time was aligned using the msalign package61 keeping the mass error parameter at 5 ppm. Peak picking and grouping were done within the XCMS package using centWave function62. A final data matrix included only the features with a relative standard deviation ≤0.3 within the QC pool. Finally, we manually removed the signals corresponding to the HEPES clusters and anticipating the potential difficulties with structural annotation, which are known for the of the untargeted profiling data, we filtered out the last quarter of the chromatogram.
Metabolite annotation was carried out according to the minimal reporting standards63. The Smart Formula tool within the Data Analysis software (version 4.1) as used for the initial ion annotation based on accurate mass (mass error < 5 ppm) and isotopic distribution (sigma value < 20). The results were matched against online metabolomics databases (METLIN, Human Metabolome Database, MassBank). When possible the hits were confirmed with MS–MS experiments of the sample with the highest intensity for each of the putative metabolites and the reference standards. MS–MS experiments were performed on the same RPLC-Q-TOF instrument in auto MS–MS mode.
Sample preparation for 1H-NMR spectroscopy
Macrophages were seeded in 24-well plates with 300,000 cells/well and either infected with H37Rv or left untreated. At 4 and 24 h post-infection, supernatants were harvested and the cells were washed once quickly with ice-cold PBS. Macrophages were rapidly quenched with liquid nitrogen and the plates were stored at −80 °C. Supernatants were filter-sterilized using 2 µM filter plates, mixed with methanol chilled at −80 °C at a ratio of 1:3 and subsequently stored at −80 °C. On the day of metabolite extraction, plates were put on ice and 300 µl 90% methanol/chloroform 9:1 was added to each well and cells were scraped thoroughly with a pipette tip before transferring of samples to eppendorf tubes. Samples were chilled for 10 minutes on ice before centrifugation at 13.2 × 1000 rpm for 15 minutes at 4 °C, after which the supernatants were harvested and put on ice. Dry protein pellets were stored at −20 °C and protein concentrations were determined by bicinchoninic acid assay (BCA) (Pierce, Thermo Fisher) according to manufacturer’s instructions. Supernatant/methanol samples from the −80 °C were centrifuged at 13.2 × 1000 rpm for 30 minutes at 4 °C, after which supernatants were collected and also put on ice. Both cellular- and supernatant- extracts were then dried by nitrogen stream and stored at −80 °C until day of measurement.
1H-NMR spectroscopy
NMR analysis of the intracellular metabolites was carried out as described previously34. Briefly, the dried extracts were reconstituted in 250 µl of 0.15 M K2HPO4/KH2PO4 buffer (pH = 7.4) in 99.9% deuterated water (D2O), including 0.2 mM NaN3 and 0.4 mM trimethylsilylpropionic acid sodium salt (TSP-d4), and transferred to 3-mm NMR tubes. NMR data were recorded on a 14.1 T NMR spectrometer (600 MHz for 1H; Bruker Avance II) under standardized conditions for all samples. All spectra were processed for phase and baseline correction and referenced to TSP-d4. One-dimensional (1D) spectra were imported into Chenomx NMR suit 8 (Chenomx Edmonton, Canada) for quantification. Metabolites were identified based on the Bbiorefcode (Bruker Biospin) and the Chenomx databases as well as in-house reference spectra. The concentrations of the quantified metabolites (mM) were normalized to the protein mass per sample.
RNA-sequencing
Total RNA was extracted from of M1 and M2 macrophages either uninfected or infected with Mtb in triplicate from a single blood bank donor using TRIzol Reagent (Thermo Fisher) at 4 and 24 h post-infection and purified using RNeasy MinElute Cleanup Kit (Qiagen, The Netherlands). The concentration and purity of RNA was evaluated by NanoDrop 2000 (Thermo Fisher). RNA-seq was performed using an Illumina Hi-Seq. 2500 as previously described64. The RNA-seq data were mapped versus the human genome (version GRCH38) and tag counts were performed by Bowtie 2 using GeneTiles software (http://www.genetiles.com)65. Normalization and gene expression analysis was performed using the R package DESeq266. The RNA-seq dataset has been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE148731. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE148731.
Statistical analysis
All statistical methods were performed in R (version 3.5.0) or GraphPad software (version 7.02, Prism, La Jolla, CA, USA). Morpheus (https://software.broadinstitute.org/morpheus) was used to generate the NMR metabolite heatmap. The following R packages were used: (multilevel) PCA and PLS-DA modeling was performed using mixOmics version 6.3.267, linear mixed models were fitted using lme4 version 1.1.1768 and lmerTest69 version 3.0.1, and graphical output was constructed using ggplot2 version 3.1.070.
Supplementary information
Acknowledgements
This study was supported by the TANDEM (Tuberculosis and Diabetes Mellitus) Grant of the ECFP7 (European Union’s Seventh Framework Programme) under Grant Agreement No. 305279 and by TBVAC2020 Grant of EC HOR2020 (Grant Agreement No. 643381) for data analysis and interpretation.
Author contributions
F.V., O.A.M., T.H.M.O. & S.A.J. conceived the study and designed experiments. F.V., S.K., H.P.S., M.C.H. & O.A.M. performed experiments, analysed data and drafted figures. T.H.M.O., M.C.H. & S.A.J. acquired funding. F.V., T.H.M.O. & S.A.J. drafted the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62911-1.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; (2018).
- 2.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nature reviews Immunology. 2012;12(5):352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 3.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature Immunology. 2009;10(9):943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belton M, et al. Hypoxia and tissue destruction in pulmonary TB. Thorax. 2016;71(12):1145–53.. doi: 10.1136/thoraxjnl-2015-207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berney M. & Berney-Meyer L. Mycobacterium tuberculosis in the Face of Host-Imposed Nutrient Limitation. Microbiol Spectr., 5(3) (2017). [DOI] [PMC free article] [PubMed]
- 6.Shi L, et al. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol Microbiol. 2010;78(5):1199–215. doi: 10.1111/j.1365-2958.2010.07399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. The Journal of Biological Chemistry. 2013;288(10):6788–800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyron P, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathogens. 2008;4(11):e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deb C, et al. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PloS One. 2009;4(6):e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V, et al. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host & Microbe. 2012;12(5):669–81. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Ouimet M, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nature Immunology. 2016;17(6):677–86. doi: 10.1038/ni.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan S, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. JImmunol. 2012;188(11):5593–603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bossche J, O’Neill LA, Menon D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017;38(6):395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–43. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. The Journal of Experimental Medicine. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan Z, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. Journal of Immunology. 2015;194(12):6082–9. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachmandas E, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nature Microbiology. 2016;2:16246. doi: 10.1038/nmicrobiol.2016.246. [DOI] [PubMed] [Google Scholar]
- 19.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–51. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson LE, et al. Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. Journal of Immunology. 2016;196(6):2444–9. doi: 10.4049/jimmunol.1501612. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, et al. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci Rep. 2015;5:18176. doi: 10.1038/srep18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin JH, et al. (1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res. 2011;10(5):2238–47. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 24.Lachmandas E, et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 2016;46(11):2574–86.. doi: 10.1002/eji.201546259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017;26(1):142–56.. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Fineran P, et al. Pathogenic mycobacteria achieve cellular persistence by inhibiting the Niemann-Pick Type C disease cellular pathway. Wellcome Open Research. 2016;1:18. doi: 10.12688/wellcomeopenres.10036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dkhar HK, et al. Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granulomas: a case of a heterologous and noncanonical ligand-receptor pair. Journal of Immunology. 2014;193(1):295–305. doi: 10.4049/jimmunol.1400092. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen I, et al. Mycolates of Mycobacterium tuberculosis modulate the flow of cholesterol for bacillary proliferation in murine macrophages. Journal of Lipid Research. 2017;58(4):709–18.. doi: 10.1194/jlr.M073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J, et al. Extraction, derivatization, and determination of metabolome in human macrophages. J Sep Sci. 2013;36(8):1418–28. doi: 10.1002/jssc.201201158. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann, M. et al. Integration of Metabolomics and Transcriptomics Reveals a Complex Diet of Mycobacterium tuberculosis during Early Macrophage Infection. mSystems., 2(4) (2017). [DOI] [PMC free article] [PubMed]
- 31.Beste DJ, et al. 13C-flux spectral analysis of host-pathogen metabolism reveals a mixed diet for intracellular Mycobacterium tuberculosis. Chem Biol. 2013;20(8):1012–21. doi: 10.1016/j.chembiol.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosshart H, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4(21):438. doi: 10.21037/atm.2016.08.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiratori H, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol. 2017;88:58–68. doi: 10.1016/j.molimm.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Kostidis S, Addie RD, Morreau H, Mayboroda OA, Giera M. Quantitative NMR analysis of intra- and extracellular metabolism of mammalian cells: A tutorial. Anal Chim Acta. 2017;980:1–24. doi: 10.1016/j.aca.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Kalscheuer R. & Koliwer-Brandl H. Genetics of Mycobacterial Trehalose Metabolism. Microbiol Spectr., 2(3) (2014). [DOI] [PubMed]
- 36.Meiser J, et al. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. The Journal of Biological Chemistry. 2016;291(8):3932–46. doi: 10.1074/jbc.M115.676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blischak JD, Tailleux L, Mitrano A, Barreiro LB, Gilad Y. Mycobacterial infection induces a specific human innate immune response. Sci Rep. 2015;5:16882. doi: 10.1038/srep16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner LB, et al. Four Key Steps Control Glycolytic Flux in Mammalian Cells. Cell Syst. 2018;7(1):49–62 e8. doi: 10.1016/j.cels.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pajuelo D, et al. NAD(+) Depletion Triggers Macrophage Necroptosis, a Cell Death Pathway Exploited by Mycobacterium tuberculosis. Cell Reports. 2018;24(2):429–40. doi: 10.1016/j.celrep.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freire DM, et al. An NAD(+) Phosphorylase Toxin Triggers Mycobacterium tuberculosis Cell Death. Mol Cell. 2019;73(6):1282–91 e8. doi: 10.1016/j.molcel.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron AM, et al. Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nature Immunology. 2019;20(4):420–32.. doi: 10.1038/s41590-019-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minhas PS, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nature Immunology. 2019;20(1):50–63. doi: 10.1038/s41590-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529–47.. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris D, et al. Glutathione and infection. Biochimica Et Biophysica Acta. 2013;1830(5):3329–49. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–8. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace C, Keast D. Glutamine and macrophage function. Metabolism. 1992;41(9):1016–20. doi: 10.1016/0026-0495(92)90130-3. [DOI] [PubMed] [Google Scholar]
- 47.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Greenhaff PL. The creatine-phosphocreatine system: there’s more than one song in its repertoire. J Physiol. 2001;537(Pt 3):657. doi: 10.1113/jphysiol.2001.013478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Connor RS, Steeds CM, Wiseman RW, Pavlath GK. Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion. J Physiol. 2008;586(12):2841–53. doi: 10.1113/jphysiol.2008.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuiper JW, et al. Local ATP generation by brain-type creatine kinase (CK-B) facilitates cell motility. PloS One. 2009;4(3):e5030. doi: 10.1371/journal.pone.0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loike JD, Kozler VF, Silverstein SC. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. The Journal of Biological Chemistry. 1979;254(19):9558–64. [PubMed] [Google Scholar]
- 52.Kuiper JW, et al. Creatine kinase-mediated ATP supply fuels actin-based events in phagocytosis. PLoS Biol. 2008;6(3):e51. doi: 10.1371/journal.pbio.0060051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karamat FA, van Montfrans GA, Brewster LM. Creatine synthesis demands the majority of the bioavailable L-arginine. J Hypertens. 2015;33(11):2368. doi: 10.1097/HJH.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 54.Koeken V, et al. Role of Glutamine Metabolism in Host Defense Against Mycobacterium tuberculosis Infection. J Infect Dis. 2019;219(10):1662–70.. doi: 10.1093/infdis/jiy709. [DOI] [PubMed] [Google Scholar]
- 55.Billig S, et al. Lactate oxidation facilitates growth of Mycobacterium tuberculosis in human macrophages. Sci Rep. 2017;7(1):6484. doi: 10.1038/s41598-017-05916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hackett EE, et al. Mycobacterium tuberculosis Limits Host Glycolysis and IL-1beta by Restriction of PFK-M via MicroRNA-21. Cell Reports. 2020;30(1):124–36 e4. doi: 10.1016/j.celrep.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cumming B. M., Addicott K. W., Adamson J. H. & Steyn A. J. Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. Elife., 7 (2018). [DOI] [PMC free article] [PubMed]
- 58.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. The Journal of Experimental Medicine. 2018;215(4):1135–52.. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vrieling F, et al. Oxidized low-density lipoprotein (oxLDL) supports Mycobacterium tuberculosis survival in macrophages by inducing lysosomal dysfunction. PLoS Pathogens. 2019;15(4):e1007724. doi: 10.1371/journal.ppat.1007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nevedomskaya E, Mayboroda OA, Deelder AM. Cross-platform analysis of longitudinal data in metabolomics. Mol Biosyst. 2011;7(12):3214–22. doi: 10.1039/c1mb05280b. [DOI] [PubMed] [Google Scholar]
- 61.Nevedomskaya E, Derks R, Deelder AM, Mayboroda OA, Palmblad M. Alignment of capillary electrophoresis-mass spectrometry datasets using accurate mass information. Anal Bioanal Chem. 2009;395(8):2527–33. doi: 10.1007/s00216-009-3166-1. [DOI] [PubMed] [Google Scholar]
- 62.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–87. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 63.Sumner LW, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) Metabolomics. 2007;3(3):211–21.. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marin-Juez R, Jong-Raadsen S, Yang S, Spaink HP. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. The Journal of Endocrinology. 2014;222(2):229–41. doi: 10.1530/JOE-14-0178. [DOI] [PubMed] [Google Scholar]
- 65.Veneman WJ, et al. Analysis of RNAseq datasets from a comparative infectious disease zebrafish model using GeneTiles bioinformatics. Immunogenetics. 2015;67(3):135–47. doi: 10.1007/s00251-014-0820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohart F, Gautier B, Singh A, Le Cao KA. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. 2015. 2015;67(1):48. [Google Scholar]
- 69.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. 2017. 2017;82(13):26. [Google Scholar]
- 70.Wickham H. ggplot2: elegant graphics for data analysis: Springer; (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.