Abstract
Nowadays, increasing population, widespread urbanization, rise in living standards together with versatile use of polymers have caused non-biodegradable polymeric wastes affecting the environment a chronic global problem, simultaneously, the existing high energy demand in our society is a matter of great concern. Hence forth, this review article provides an insight into the technological approach of pyrolysis emphasizing catalytic pyrolysis for conversion of polymeric wastes into energy products and presents an alternative waste management technique which is a leap towards developing sustainable environment. Pyrolysis of waste non-biodegradable polymer materials involves controlled thermal decomposition in the absence of oxygen, cracking their macromolecules into lower molecular weight ones, resulting into the formation of a wide range of products from hydrogen, hydrocarbons to coke. Nanocatalyzed pyrolysis is a recommended solution to the low thermal conductivity of polymers, promoting faster reactions in breaking the C-C bonds at lower temperatures, denoting less energy consumption and enabling enhancement in the process selectivity, whereby higher value added products are generated with increased yield. Nanotechnology plays an indispensable role in academic research as well as in industrial applications. Existing reviews illustrate that one of the oldest application field of nanotechnology is in the arena of nanocatalysis. Nanocatalysis closes the gap between homo and heterogeneous catalyses while combines their advantageous characteristics and positive aspects, reducing the respective drawbacks. During the current nanohype, nanostructured catalysts are esteemed materials and their exploration provide promising solutions for challenges from the perspective of cost and factors influencing catalytic activity, due to their featured high surface area to volume ratio which render enhanced properties with respect to the bulk catalyst.
Keywords: Energy, Nanotechnology, Polymeric wastes, Catalytic pyrolysis, Nanocatalysts, Energy products, Environment
1. Introduction
Polymers are materials that are presently contributing fundamentally in our daily chores. As a result the global production and disposal of polymers have increased enormously over the years due to their vast applications in various sectors. However, the polymeric wastes are bulkier than the organic residues and a large part of these wastes do not degrade. Therefore their continuous demand has caused non-biodegradable polymeric waste accumulation in the landfill, consuming massive space and contributing to environmental hazards [1, 2, 3, 4, 5, 6, 7, 8]. The rising demand of polymers has also given rise to the depletion of petroleum products as a part of non-renewable fossil fuel since these are the petroleum-based materials [9, 10]. Waste polymeric materials are usually mixtures of different kinds of polymers, such as high density polyethylene (HDPE), low density polyethylene (LDPE), polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), polyamide(PA), polyvinyl-chloride(PVC), polyacrylate (PAC), etc. [11, 12, 13, 14]. Alternatives developed for managing polymeric wastes are incineration, recycling and energy recovery system [15, 16, 17, 18, 19, 20, 21, 22, 23]. However, incineration leads to unacceptable emission of harmful compounds, while recycling have high costs for the separation process and water contamination drawbacks [24], which reduce the process sustainability.
Henceforth, utilization of non-biodegradable polymeric wastes for energy recovery stands as a better option to solve the staggering environmental problem as well as compensates the prevalent high energy demand [25, 26, 27, 28]. Extensive research and development of technology for this waste conversion to energy holds great potential, since, petroleum is one of the main sources of non-biodegradable polymer manufacturing and their recovery to liquid oil through the process of pyrolysis produces components having high calorific value in comparable to the commercial fuel [29]. Pyrolysis is also one of the tertiary recycling methods for non-biodegradable polymeric materials like plastics, in accordance with ASTM D5033-00, which has divided such recycling methods into four types, based on the final result [30, 31]. In general [32, 33], the variety of products obtained through pyrolysis can be classified into the non-condensable gas fraction called syn-gas, the liquid fraction consisting possible recovery of gasoline range hydrocarbons (C4-C12), kerosene (C10-C18), diesel (C12-C23), motor oil (C23-C40), etc. categorized as bio-oil or tar and the third fraction of solids as char.
Viable technologies and investigations towards this direction, for conversion of waste non-biodegradable polymers to potent chemical feedstocks and ultimately fuels, are rapidly growing through pyrolysis [34, 35]. Pyrolysis being a simple thermal process, involves the controlled thermal degradation of materials in an oxygen starved chamber, heating and cracking the polymers to lower molecular weight, i.e. breaking down polymers to smaller molecules, also into oligomers and monomers, as mixed products of gaseous, liquid along with solid hydrocarbons [36]. However, pyrolysis suffers from few hindrances, requiring high temperatures typically above 500 °C because of low thermal conductivity of polymers and endothermic reaction of degradation, together with that the products obtained are in a broad range, also not as good fuels due to their poor quality [37, 38]. The main process parameters of pyrolysis for particular type of polymeric material that influence the final products include type of reactors, feedstock composition and feeding arrangement, type of fluidizing gas and its flow rate, residence/retention time, temperature, pressure, catalysts [39, 40, 41, 42, 43, 44, 45, 46, 47, 48], in addition there are several points to optimize the fuel production.
Catalytic pyrolysis provides a key to mitigate some of the factors affecting the pyrolysis process, such as the feedstock composition, retention time, temperature, etc. [49, 50, 51, 52, 53, 54]. Pyrolysis carried out via catalytic routes overcome such process issues, capable of converting ∼80% of the waste polymeric material into liquid oil characteristically similar to conventional diesel fuel, having higher heating value (HHV) of about 38–45.86 MJ/kg, density of 0.77–0.84 g/cm3, viscosity around 1.74–2.5 mm2/s, kinematic viscosity of 1.1–2.27 cSt, pour point of (−9) to (−67) °C, boiling point near 68–352 °C, and flash point of 26.1–48 °C [55, 56, 57]. This liquid fraction from catalytic pyrolysisis with improved quality can be used in various energy related applications, for e.g. as transport fuel, for electricity generation, as well as a heating source [58, 59]. Apart from it, the other by-products like char are potential adsorbent material for wastewater treatment and polluted air [60, 61], whereas, the produced gases are potentially good energy carriers [57]. Consequently, utilization of waste polymers to convert into carbon derived valuable light materials which are constituted of high quality components, through catalytic degradation seems interesting.
The application of degradation catalysts for pyrolysis processes, were initially carried out with clays and amorphous silica alumina, gradually with time evolved emphasize on zeolites which are chiefly acidic catalysts e.g. zeolite Y, ZSM-5 and Mordenite [62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77]. Mainly acidic catalysts promotes production of light hydrocarbons (C5-C12) in the range of gasoline, while pyrolysis using non-acidic catalysts produces compounds (C12-C22) in the range of kerosene and diesel [78, 79, 80, 81, 82]. Whereas, the metal base catalysts, yield 97% of the given mixed to the costly middle distillate products which include 84% of liquid hydrocarbon (C5-C10) with 13% gas (C1- C4) and minimal percent of residue. Also, large amounts of waste polymeric materials are efficiently processed with the combined method of pyrolysis and catalytic reforming. Investigation on catalysts discovered the concept that the presence of large pores in combination with acidic properties provides means to address vital issues [83, 84, 85] such as to estimate the quantity of polymeric degradation and predict the kinetic behavior in catalytic pyrolysis process.
Inspite of having enormous potential advantages, catalytic pyrolysis face limitations from the aspects of high parasitic energy, catalyst cost and lowered lifecycle due to less reuse [86, 87]. The dissolving solutions for these challenges are through exploration of cost-efficient catalysts, catalyst regeneration together with overall process optimization. Detail evaluation of the factors affecting catalytic pyrolysis process such as the feedstock composition, retention time, temperature and the use of catalysts, stresses the effect of different catalysts and its characteristics, having great role on the quantity as well as the quality of the evolved pyrolysis products [29, 47, 88]. As the focus has always been on the products evolved, finding an appropriate catalyst can help in achieving the desired outcomes. Therefore, reforming, regeneration and exploration of novel cheaper catalysts needs to be the centre of research in order to make catalytic pyrolysis a viable process without economical constraints. Scientists and engineers are trying since years to bring innovation in the catalytic pyrolysis process for generating alternative fuel like products and high quality chemicals from waste polymeric materials.
Finally, development of nano-engineered catalysts with superior characteristics regarding selectivity, activity, durability, as well as recoverability [89, 90, 91, 92], hold huge possibilities which may contribute to solve the current issues in catalytic pyrolysis process [93, 94]. Nanocatalysis is not new, though nanoscience and nanotechnology is considered the research area of the 21st century [95, 96]. The knowledge of nanocatalysis prevails from 1950s, even when the term nanotechnology did not exist [97]. The high surface area to volume ratio (S/V) attained by decreasing the size of the catalyst material specifically engineered at the nanoscale renders increased catalytically active site, thereby promoting enhanced interaction between the reactants and the catalyst [98]. Nanosized catalysts additionally display unique properties in comparison to the ones at macroscale and combine the positive aspects of conventional catalytic methods [99, 100, 101, 102], pursuing the key targets close to 100% of selective reactions with extremely high activity and excellent yield [103]. Nanocatalysis is expected to concomitantly lower pyrolysis process energy consumption, impart longer lifetime to the catalyst system with greater chances of isolation and reusability of the active nanostructured catalyst [104]. The objective of this review is to highlight the influence of nanocatalysts in the pyrolysis of non-biodegradable polymeric wastes that prominently illustrates efforts towards “green chemistry” and sustainable environment [105, 106, 107, 108] for which catalysis is considered as an important factor.
2. Main text
2.1. Overview of polymeric waste management towards sustainable environment
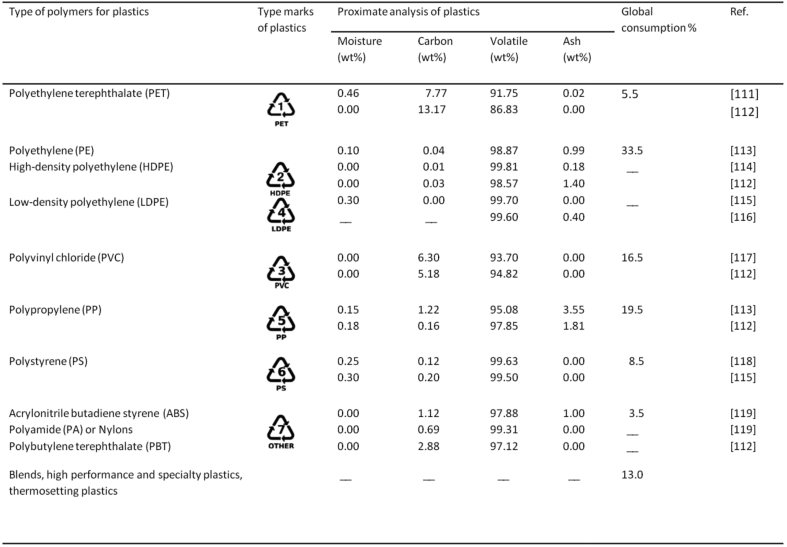
Materials consisting of a specific or a broad range of synthetic or semi-synthetic organic polymers of high molecular mass, usually derived from petrochemicals, such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinylchloride (PVC), polyethylene terephthalate (PET), etc., are typically referred as plastics due to their property of plasticity being malleable that can be molded or easily shaped when soft then set into rigid solid objects i.e. thermosets and slightly elastic forms i.e. thermoplastics [109, 110]. These polymeric plastic materials have been extensively used and are regarded among the greatest innovations of the millennium. Characteristics of lightweight, flexible, reusable, non-rust or rot and cheap have caused their production rise up by almost 10% each year since 1950, which accounts an increase from ∼1.3 million tones to >245 MT in recent years, on a global basis [29]. Global consumption of individual polymers along with the proximate analysis is accounted in Fig. 1.
Fig. 1.
Individual plastic types and global consumption [111, 112, 114, 115, 116, 117, 118, 119].
Wide application areas from automotive field to medical and healthcare, electrical and electronics to telecommunication, building-infrastructure to furniture signify plastic revolution taking place in various sectors. But at the same time, forecast days not far when our earth will get completely covered with waste plastics and we humans will have to live over it. Reasoning for this fact is that most plastic materials are non-biodegradable in nature and may remain as such for hundreds of years [120] therefore their disposal is turning out to be an environmental menace. Mostly 40% of plastics consumed have shelf-life duration lesser than a month and generally the service life of polymeric products ranges from 1-35 years depending on its area of application. Even biodegradable polymers, those that can be converted back to biomass, need a realistic time period and appropriate conditions which are necessary for the degradation process of such plastics, e.g. presence of light required for the photodegradable plastics, also greenhouse gases like methane releases when plastics degrade anaerobically.
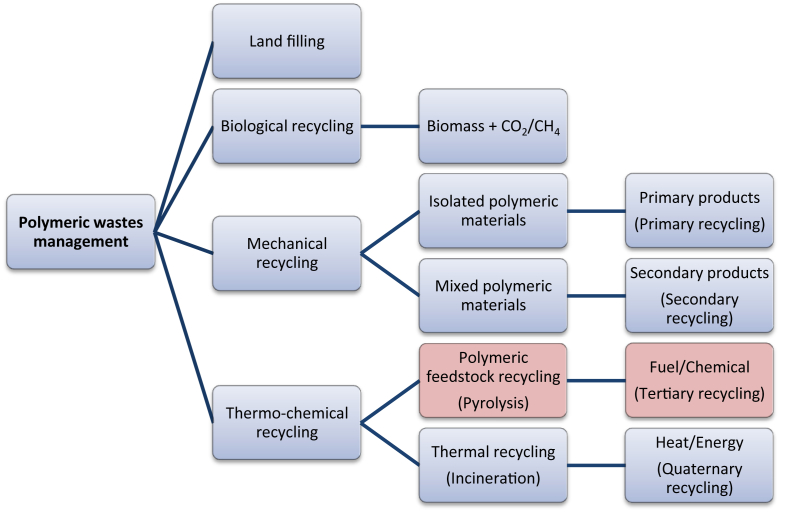
Disposal methods employed for polymeric wastes are land filling, mechanical, thermal, chemical and biological recycling. Suitable treatment of polymers is the primary factor in polymeric wastes management and considered important from the environmental, socio-economic and energetic, point of view. Though different techniques for managing the waste polymeric materials exist today [29], major portion of the polymeric wastes are being subjected to landfill. Scarcity of land along with high volume to weight ratio of polymeric materials, poor biodegradability of common polymers together with formation of methane like explosive greenhouse gases, make land-filling an unattractive option which should be strictly regulated. Certain regulations imposed are expected to attain a reduction of ∼35% in land filling [121] between the periods 1995 to 2020.
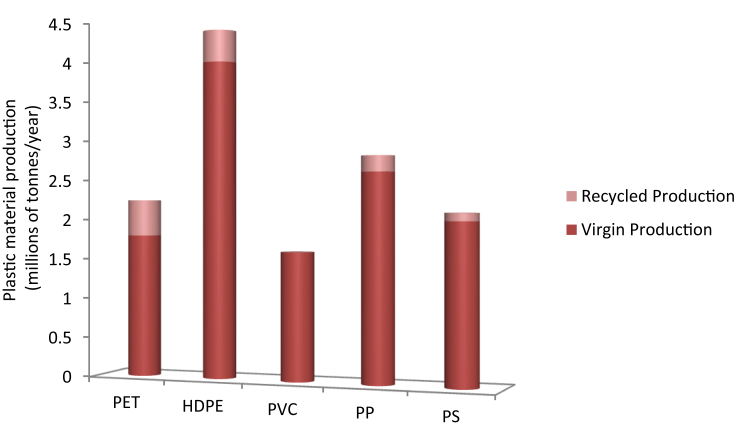
Reprocessing of the consumed plastics forming similarly new products is the technique of mechanical recycling. Even though this seems to be ‘green operation,’ it is not cost efficient, requiring high energy for transportation, sorting clean, and reprocessing in order to obtain a serviceable product but practically less performance level is achieved than the original product. Life-cycle analysis (LCA) which considers the energy embodied in plastic materials during production manufacturing to use and disposal, reflects that the embodied energy of the recycled plastics are about half than that of the virgin materials, summarized in Table 1, because of the degraded quality of recycled materials [122]. That is why the contribution to production and consumption of recycled plastic materials is small, illustrated in Fig. 2.
Table 1.
Energies of virgin and recycled plastics [122].
| Commodity polymeric materials | Applications | Embodied energy, virgin material (MJ/kg) | Embodied energy, recycled material (MJ/kg) |
|---|---|---|---|
| PET | Food packaging, printing sheets, magnetic tapes, electrical insulation, X-ray photographic film, etc. | ∼79–88 | ∼60–64 |
| HDPE | Toys, milk bottles, oil containers, detergent bottles, etc. | ∼77–85 | ∼35–45 |
| PVC | Electrical insulations, medical devices, blood bags, food foil, automotive interiors, packaging, credit cards, synthetic leather, etc. | ∼63–70 | ∼35–40 |
| PP | Flowerpots, car bumpers, carpets, furniture, office folders, storage boxes, etc. | ∼75–83 | ∼35–45 |
| PS | Electronics, medical, food packaging, construction, appliances, toys, etc. | ∼96–105 | ∼40–50 |
Fig. 2.
Contribution of recycling of polymers to consumption [122].
Incineration of polymeric wastes is another alternative method for recovering energy by which hydrocarbon polymers may replace fossil fuels as for e.g. polyethylene (PE) has calorific value similar to the fuel oil, also the energy produced by incineration of PE is having the same order as that required in its manufacturing, however, green house gases and obnoxious pollutants are produced making it disadvantageous [120, 121]. Thermochemical processes as a chief route applied for conversion of non-biodegradable polymeric wastes to more useful fuel as a valuable source of energy to satiate the world's ever increasing demand for energy may restrict the depletion and help in conserving the available limited fossil fuel. Pyrolysis is one of the energy recovery technologies and an alternative method of plastic waste recycling in an intelligent way to polymeric waste minimization while exploring their full utilization for generating potential energy resources. Alternative routes for polymeric waste management are depicted in Fig. 3.
Fig. 3.
Alternatives for polymeric wastes management [29].
2.2. Pyrolysis of polymeric wastes
The word ‘pyrolysis’ has been derived from the Greek words ‘pyro-’ meaning heat and ‘-lysis’ meaning releasing which refers to breaking down or decomposition. The process of breaking down long polymer chains with the application of heat is termed as pyrolysis. The reaction of pyrolysis is carried out in the absence of oxygen with or without the presence of a catalyst [123, 124]. Pyrolysis without catalyst, is defined as thermal pyrolysis, thermolysis or thermal cracking, while if it is in the presence of a catalyst, it is termed as catalytic pyrolysis or catalytic cracking. Whereas, catalytic hydrogenation pyrolysis [125, 126] is the process conducted using hydrogen atmosphere accompanied with catalytic conversion for increasing saturated compounds in the resulting fuel.
2.2.1. Thermal pyrolysis
In thermal pyrolysis organic part of the plastic polymer materials are decomposed by the application of heat in an inert atmosphere, at reaction temperatures ranging between 350 to 900 °C, generating gases and liquids that can be used as fuels along with solid by-products called char. Analysis of polymeric waste pyrolysis oil as fuel obtained from thermal process gives the general observation of: delayed ignition, higher heat release rate, increased exhaust temperature, higher unburnt hydrocarbons with increased smoke, NOx higher by 19–25% at peak loads and SOx production near to zero due to nil sulphur content in waste polymeric oil in comparison to heavy fuel oil used in diesel engines [127, 128, 129]. The physical properties of liquid oil yielded from various types of polymeric waste pyrolysis are tabulated in Table 2.
Table 2.
Properties of pyrolysis oil obtained from waste polymeric materials vs. conventional fuel [57].
| Material | Parameters |
Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| HHV (MJ/kg) | Density (g/cm3) | Viscosity (mm2/s) | Kinematic viscosity (cSt) | Pour point (°C) | Flash point (°C) | Boiling point (°C) | ||
| PET | 28.2 | 0.90 | -- | -- | -- | -- | -- | [130] |
| PE | 41.45 | 0.85 | 1.74 | -- | -- | -- | -- | [131] |
| HDPE | 45.86 | 0.79 | 2.1 | 1.63 | −15 | 48 | 82–352 | [132] |
| LDPE | 38–39 | 0.78 | 1.89 | -- | -- | 41 | -- | [29, 133] |
| PVC | 43.22 | 0.84 | 6.36 | -- | -- | 40 | -- | [134] |
| PP | 40.8 | 0.86 | 4.09 | 2.27 | <−45 | 30 | 68–346 | [29, 135] |
| PS | 43.0 | 0.85 | 1.4 | 1.10 | −67 | 26.1 | -- | [134] |
| Mixed Plastic | 44.40 | 0.84 | 2.52 | -- | -- | -- | -- | [136] |
| Diesel | 46.67 | 0.81–0.87 | 2.0–5.0 | 2.0–5.0 | 6 | 52 | 150–390 | [137] |
| Kerosene | 43.0–46.2 | 0.78–0.81 | -- | -- | −47 | 37.78 | 150–300 | [8] |
| Gasoline | 43.4–46.5 | 0.71–0.77 | 1.17 | -- | <−20 | −42.78 | 40–200 | [8] |
Myriads of researchers pursued studies to bring forth the potential applications of this liquid oil as transport fuel and blended it with conventional diesel in varying ratio of 10%, 20%, 30% and 40%, using in different types of diesel engines. Under intermediate load there were reports of decreased engine performance in many cases but no difference in performance was also noted by few reporters up to the blending ratio of 20%–80%. At 20% blended ratio, exhaust emissions of 0.26 g/km to 0.40 g/km and 0.52 g/km to 0.69 g/km for NOx at low and full load respectively, were observed. However, for conventional diesel NOx emissions were accounted to be 0.26–0.41 g/km and 0.51–0.71 g/km at low and full loads respectively. CO emissions increased with the blended oil in few experimental observations [138, 139, 140, 141], due to incomplete combustion.
Another by-product of pyrolysis is char, basically the unburnt leftover part of the waste polymeric material in the reactor, present in very minute quantity (∼1–1.3g from 1kg polymer). It can be potentially used in a diversity of environmental and energy related applications. In general the char is having a fixed carbon content of ∼46.03%, volatile matter of ∼51.40%, moisture content of ∼2.41% and a little amount of ash, with HHV of ∼(23.04–36.29) MJ/kg, a suitable energy source for boilers [142]. Moreover, the thermally activated char, with increased BET pore volume and surface area, applied in various environmental purposes like adsorption of toxic gases, heavy metals and removal of dyes from industrial and municipal waste water [143], showed significant results.
The chief components of the gases produced from pyrolysis of different polymeric materials are hydrogen, methane, ethane, propane, butane, ethene and propene, while hazardous chlorine is also present only in the pyrolysis of PVC [144, 145]. Increase in the reaction process temperature and the application of catalyst enhances the gas production due to increase in the process of cracking [146]. Researchers have reported production of ∼13–26.9% gases by weight from 1kg of polymeric feedstock [146]. The pyrolysis gases obtained from waste polymeric materials have high calorific values ranging from ∼42-50 MJ/kg [113, 138, 147]. These gases can be potentially used, without any flue gas treatment, in boilers for heating as well as in gas turbine for electricity generation [148]. Moreover, based on their composition [8, 138] ethane, propene, isoprene and 1-butene can be recovered through condensation and after separation can be used as chemical feedstocks for polyolefins or in tire production.
2.2.1.1. Parameters affecting the general pyrolysis process
Parameters play vital role in optimizing the resultant product and its composition in any process. In the pyrolysis of non-biodegradable waste polymeric materials, there exist key process parameters that influence the final yield of the gaseous syn-gas, liquid tar and solid char. Major parameters affecting the pyrolysis process may be mentioned as: (i) type of reactors, (ii) type of fluidizing gas and its rate, (iii) residence time and feedstock composition, (iv) temperature, (v) pressure, (vi) catalysts, etc. Achievement of the desired products can be possible by controlling some of these parameters at different settings. Their respective effects are discussed in brief in the following subsections and summarized in Table 3.
Table 3.
| Polymer | Thermal decomposition mode | Reactor type | Pyrolysis process parameters |
Product yield |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Heating rate (°C/min) | Pressure | Duration (min) | Gas (wt%) | Liquid (wt%) | Solid (wt%) | ||||
| PET | Fixed bed | 500 | 10 | -- | -- | 76.9 | 23.1 | 0 | ||
| -- | 500 | 6 | 1 atm | -- | 52.13 | 38.89 | 8.98 | |||
| PE | HDPE | Random chain lysis (fragmentation along polymer chain length resulting in monomers and oligomers) | Horizontal steel | 350 | 20 | -- | 30 | 17.24 | 80.88 | 1.88 |
| Batch | 450 | -- | -- | 60 | 5.8 | 74.5 | 19.7 | |||
| Semi-batch | 400 | 7 | 1 atm | -- | 16 | 82 | 2 | |||
| 450 | 25 | 1 atm | -- | 4.1 | 91.2 | 4.7 | ||||
| Fluidized bed | 500 | -- | -- | 60 | 10 | 85 | 5 | |||
| 650 | -- | -- | 20–25 | 31.5 | 68.5 | 0 | ||||
| LDPE | Batch | 430 | 3 | -- | -- | 8.2 | 75.6 | 7.5 | ||
| 500 | 6 | 1 atm | -- | 19.43 | 80.41 | 0.16 | ||||
| 550 | 5 | -- | -- | 14.6 | 93.1 | 0 | ||||
| Pressurized batch | 425 | 10 | 0.8–4.3 MPa | 60 | 10 | 89.5 | 0.5 | |||
| Fixed bed | 500 | 10 | -- | 20 | 5 | 95 | 0 | |||
| Fluidized bed | 600 | -- | 1 atm | -- | 24.2 | 51 | 0 | |||
| PVC | Chain-stripping (side chain reactions of substituents eliminating reactive substitutes (HCl), dehydrogenation and cyclization) | Vacuum batch | 520 | 10 | 2 kPa | -- | 0.34 | 12.79 | 28.13 | |
| Fixed bed | 500 | 10 | -- | -- | 87.7 | 12.3 | 0 | |||
| PP | Random chain fragmentation | Horizontal steel | 300 | 20 | -- | 30 | 28.84 | 69.82 | 1.34 | |
| Batch | 380 | 3 | 1 atm | -- | 6.6 | 80.1 | 13.3 | |||
| 740 | -- | -- | -- | 49.6 | 48.8 | 1.6 | ||||
| Semi-batch | 400 | 7 | 1 atm | -- | 13 | 85 | 2 | |||
| 450 | 25 | 1 atm | -- | 4.1 | 92.3 | 3.6 | ||||
| 500 | 6 | 1 atm | -- | 17.76 | 82.12 | 0.12 | ||||
| PS | Combination of chain rupture and unzipping, formation of oligomers | Batch | 500 | -- | -- | 150 | 3.27 | 96.73 | 0 | |
| 581 | -- | -- | -- | 9.9 | 89.5 | 0.6 | ||||
| Semi-batch | 400 | 7 | 1 atm | -- | 6 | 90 | 4 | |||
| Pressurized batch | 425 | 10 | 0.31–1.6 MPa | 60 | 2.50 | 97 | 0.5 | |||
2.2.1.1.1. Reactors
The type of reactor employed in pyrolysis process determines chiefly the quality of heat transfer, mixing of the polymeric feedstock and catalysts, residence time, and the efficacy of the reaction towards attaining the desired final products. A wide variety of reactors have been designed on the lab-scale as well on the industrial level for the pyrolysis of polymers having their respective advantages and downsides. The reactor set-ups arranged hitherto may be categorized into the following: (i) Batch reactor and semi-batch reactor (ii) continuous-flow reactors (CFR) such as fixed-bed reactor, fluidized bed reactor and conical spouted bed reactor (CSBR) (iii) microwave-assisted technology.
Laboratory scale performance of pyrolysis is suitable in batch or semi-batch reactors, which have simplest designs, enable control of the operating parameters and allow the reactant to remain in the reactor for an extended duration, even a catalyst can be mixed together with the polymeric sample inside the reactor, at temperatures ranging from 300–800 °C for thermal as well as catalytic pyrolysis. Batch reactor is primarily a closed system i.e. when the reaction is being carried out there is no possibility of inflow or outflow of the reactants and the products; whereas in semi-batch reactor reactant addition and product removal is possible simultaneously over time which is advantageous in terms of the reaction selectivity. The disadvantage of both batch and semi-batch reactor is in respect of high labor cost along with variable product yield from batch to batch, feasible for small-scale purpose on experimental basis.
In fixed bed reactor, the polymer and the catalysts samples are heated separately and made to react by vapor phase contact. The degraded fragments of polymer are carried by a carrier gas such as N2 to the catalyst bed/mesh. Usually the catalyst bed is maintained at a temperature higher to the polymer bed. In certain set-ups, the fixed-bed reactor is used as a secondary pyrolysis reactor where the products of primary pyrolysis consisting of liquid and gaseous matter are fed into it. While, in fluidized bed reactor the catalyst is placed on a distributer plate from where the fluidizing gas passes through it carrying the particles in a fluid state, providing better access to the catalyst with well-mixing to the fluid and larger surface area of contact for the occurring reaction. The flowing gas makes the catalyst particles behave just like a fluid, even upon addition of the polymer contacting the catalyst surface and drawn into the catalyst macropores through capillary action, the fluidized bed still remains fluidized [8]. Hence, fluidized bed reactor is considered best to perform catalytic polymeric pyrolysis for conventional design scale used in pilot plant because apart from having lower operating cost has the advantages of the catalyst being reused several times without discharging, also providing a constant temperature together with high heat and mass transfer, requiring shorter residence time with consequent uniformity in the spectrum of products. The conical spouted bed reactor (CSBR) facilitates good mixing and handling large particle size distribution and difference in particle densities.
The current investigation in microwave technology brings novelty in the techniques for polymeric waste recovery through pyrolysis. In this process, a high microwave-absorbent material e.g. particulate carbon is mixed with the polymer sample [149]. The microwave absorbent material absorbs the microwave energy creating adequate thermal energy for attaining the temperature required for undergoing pyrolysis.
2.2.1.1.2. Fluidizing gas and its rate
The fluidizing or carrier gas is an inert gas, has its role only in the transportation of the vaporized components without being involved in the actual pyrolysis. Different types of fluidizing gases are used for polymeric pyrolysis such as hydrogen, ethylene, propylene nitrogen, helium, and argon, each type having different reactivity and composition of the yielded product, depending on the molecular weight [8, 45, 46]. The lighter gases are capable of producing higher quantity of condensed product i.e. liquid oil. However, nitrogen (N2) is the easiest and safest to be handled as well as from the availability and cost factor to be used as a carrier gas. Besides, it has been also investigated that the fluidizing flow rate has an impact on the final yield, reporting drastic drop in the rate of degradation with high residue at the lowest fluidizing flow rate of ∼300 ml/min, having high contact time for primary products, while gasoline and hydrocarbon gas fraction maximized at the highest fluidizing flow rate of 900 ml/min.
2.2.1.1.3. Residence time and feedstock composition
Residence or retention time may be defined as the average time spent by the sample particles in the reactor having a temperature dependent influence upon the final product distribution. Longer residence time enhances the conversion of primary products, yielding more thermally stable products such as low molecular mass hydrocarbons and non-condensable gas fraction [150]. Higher liquid yield may be obtained at lengthy residence time but at temperatures below 685 °C. The feedstock composition [147] has a significant effect on the pyrolysis yield based on their varying parameter requirement for complete decomposition depending upon their complex molecular structures.
2.2.1.1.4. Temperature
Since temperature provides the enthalpy of the C–C bond, resulting into the breaking of the polymer chain, it is one of the most important operating parameter that controls the cracking reaction in pyrolysis. The thermal degradation behavior of polymeric materials can be measured through thermogravimetric analyzer which produces two different graphs of thermogravimetric (TG) curve and derivative thermogravimetric (DTG) curve. The TG curve is a measure of the mass transfer or the weight loss of the material as a function of temperature, while the DTG curve by the number of peaks indicates the degradation steps occurring during the process [150, 151]. Reports of pyrolysis study conducted by researchers indicate that the degradation temperature ranges from 200 °C to 600 °C or even above and the optimum temperature required to obtain a particular yield varies with the different type of waste polymeric material used as a feedstock. As, it has been proven that temperature has an impact on reaction rate and may influence the product composition of syngas, tar and char, the operating temperature can be maintained depending upon the product preference. For syngas or char as major product there is higher temperature requirement of above 500 °C and for tar as the preferred yield lower temperatures in the range of 300–500 °C is recommended [8], for all sorts of polymeric materials.
2.2.1.1.5. Pressure
Studies discovered that the increase in gaseous product was tremendous as the pressure was raised from 0.1 to 0.8 MPa at temperature range of 410–440 °C. The distribution of carbon number of the liquid fraction was also affected, by applying high pressure, which shifted towards lower molecular mass. Besides this, the double bond formation rate decreased with increased pressure suggesting that the C–C linked scission rate in polymers was affected by pressure [152]. However, pressure similar to residence time is temperature dependent factor while influencing the product distribution in polymer pyrolysis, therefore mostly pyrolysis of polymers is conducted at atmospheric pressure and the temperature factor is more focused.
2.2.1.2. Catalysts
The application of catalyst in the pyrolysis of polymers has an influence upon the mechanism and kinetics, hence, play a vital role in improving the quality of product distribution as well as optimizing the process parameters [77, 131]. Catalyst loading increases the conversion with increase in the rate of cracking reaction that leads to enhanced yield of gases with reduced yield of liquid fraction however the oil produced is of superior quality [131]. This may be due to the fact that many of the larger carbon chain compounds are adsorbed on the catalyst and broken down into smaller ones. Therefore, characteristics of catalysts such as the Brunauer Emmett Teller (BET) surface area, pore volume, pore size, acidity, etc. are some of the major factors affecting the catalytic activity in the pyrolysis process.
2.2.2. Catalytic pyrolysis
The low thermal conductivity of polymers with endothermic cracking demands the requirement of high temperatures due to which thermal pyrolysis becomes a huge energy intensive process along with varying reaction conditions which is not very selective therefore a possible solution for reducing such reaction conditions is catalyzed pyrolysis. Catalytic pyrolysis is a favorable alternative for the recycling of pure or mixed polymeric wastes. Polymeric wastes may contain heteroatoms like chlorine, sulphur and nitrogen due to additives, presence of surface contaminants and several kinds of pollutants which also raises the degradation temperatures upto 700 or 900 °C and compromises the quality of the liquid oil, even the gases are not suitable as fuel source without refining.
2.2.2.1. Applications of catalytic pyrolysis
Application of different catalysts adopted with ‘in situ’ methods in the pyrolysis process overcomes the challenges of feed-stock contamination to a great extent. Catalytic pyrolysis promotes: (i) decomposition reactions at lower temperatures denoting reduced energy consumption, (ii) increase in the product yield with higher added value, (iii) increase in the process selectivity, (iv) swift cracking reactions, reducing the residence time and volume of the reactors, (v) inhibition in the formation of undesirable by-products and (vi) attainment of liquid products having lower boiling point range.
Catalytic reactions of waste polymer cracking for the production of valuable hydrocarbons have been enthusiastically carried out using various types of catalysts as well as mixed catalysts putting emphasis on the effects of zeolitic or multi-zeolite catalysts [86, 153, 154]. References exist of temperatures ∼450 °C utilizing different reactors from batch to fluidized bed reactors. However, configuration of reactors associated with catalytic polymer recycling posses engineering and economic constraints since the problems arising out of limitations in the polymer-catalyst contact mode inside the reactor and blockage of the catalyst pores due to the sticky nature of the polymeric feedstock. Lack of good contact allows high tendency of residue and coke formation on the catalyst surface [155], thereby deactivating and decreasing catalyst efficiency and its life cycle along with posing challenge for separation of the catalyst from the residue at the termination of the process therefore restricting to permit the feasibility of industrial scale-up.
2.2.2.2. Catalyst contact modes
Investigations have been carried out on catalytic steps involved in the polymer degradation process based on the modes of catalyst introduction to polymer feedstock [49]. Two modes of catalyst contact were considered in batch pyrolysis with the use of various solid acid catalysts: (i) liquid phase or melt phase contact and (ii) vapor phase contact. Catalytic degradation carried in the liquid phase contact mode places the catalyst and the polymer feed together in the reactor and then applies heat to the operating temperature. While, the vapor phase contact mode first thermally degrades the sample polymer into hydrocarbon vapor then allows contact with the catalyst. In the liquid phase contact mode of catalytic pyrolysis the evolving products cannot escape easily, hence interaction is enhanced occurring further cracking of the hydrocarbon vapors even though the final product yield reported in the liquid phase contact mode do not have significant difference than that obtained through purely thermal pyrolysis, for example in the case of polypropylene [156].
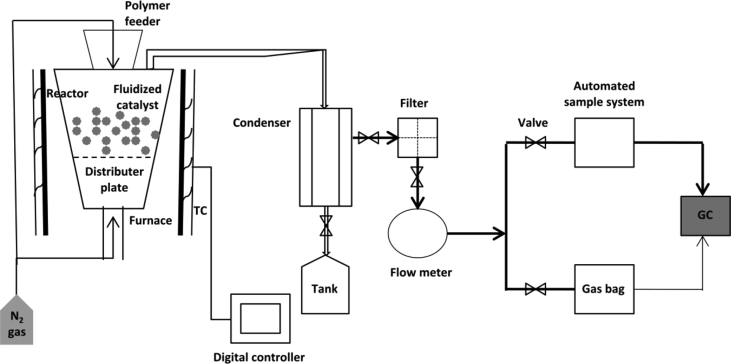
Whereas in catalytic fluidised-bed reactor the catalyst is adequately fluidized and the polymer feed system is designed to enable polymer particles, purged under nitrogen as a fluidizing gas, to reach the top of the reactor and at t = 0 min dropping freely into the fluidized bed. As the polymer particles wets the catalyst surface capillary action rules upon to drag them into the macropores of the catalyst and at optimum polymer/catalyst ratio (4:1) [157], the outer part of the catalyst particles remain free from polymer melts to move freely and maintain the fluidized bed at fluidized stage [78]. Minimum particle sizes for catalyst as well as the polymer sample are chosen to avoid entrainment and maximize interaction between them. The fluidized bed reactor system for catalytic pyrolysis is schematically represented in Fig. 4.
Fig. 4.
Schematic representation of fluidized bed reactor system for catalytic polymer pyrolysis process.
2.2.2.3. Characteristics of catalysts
There are chiefly two types of catalyst systems employed in the catalytic cracking of polymers: (i) homogeneous catalysis, involving one phase, in which the sample material and the catalyst substance are brought in contact in the same phase, ensuring high catalytic activity together with selectivity; and (ii) heterogeneous catalysis, involving more than one phase, the sample material and the catalyst reside in different phases [8, 62]. The catalyst activity can be expressed in terms of turn over frequency (TOF), which is the number of substrate molecules catalytically converted during a time period, while selectivity is indicated by the desired final product yields [98]. Homogeneous catalysts especially used for polymer pyrolysis are classical Lewis acids, such as AlCl3, FeCl3, TiCl4 and TiCl3, fused metal tetrachloroaluminates [M(AlCl4)n] where (M = Li, Na, K, Mg, Ca or Ba and n = 1 or 2), etc. [29]. However limitations exist in the practicality of homogenous catalysis due to difficulties in the separation of the catalyst from the final product after reaction completion. Therefore, heterogeneous catalysis is preferred in catalytic pyrolysis process for the ease of separation of the mixed fluid products from the solid catalyst and since the costly catalyst recovery is economically demanded. The deactivated catalysts can be possibly regenerated by oxidation of the deposited coke at 600 °C in the presence of air for 3 h [49].
Heterogeneous catalysts applied for cracking of polymers may be conventionally classified into: a) solid acids such as clays e.g. saponite, montmorillonite, etc., silica-alumina (having different mole ratios of SiO2:Al2O3), alumina, zeolites (alumino-silicates), fluid catalytic cracking (FCC) catalysts, etc.; b) mesostructured catalysts like mobile crystalline material (MCM-41), etc.; c) non-acidic solids such as microporous silica catalysts (silicalite), amorphous mesoporous silica-gel (Q3), crystalline mesoporous folded silica material (FSM), etc.; and d) others of the kind metal supported on carbon e.g. Fe/activated charcoal, etc.; basic oxides e.g. MgO, ZnO, TiO2, Fe2O3, Al2O3, red mud (main constituents include Fe2O3, Al2O3, TiO2, SiO2, CaO, Na2O, MgO), etc.; alkali or alkaline metal carbonates e.g. MgCO3, CaCO3, BaCO3 [35,49,64,65,76,78,81,158]. Textural properties like BET surface area, pore size, dimension along with Si/Al ratio, thermal stability and acid strength are the chief characteristics of acid catalysts. Textural characteristics also control molecular accessibility for reactions at the catalytic sites, which is crucial for large polymer molecules. Acidity of the catalyst has an important role in catalytic pyrolysis thus both Lewis acid and Bronsted acid sites are taken into consideration during the acidity measurement, majority of them being located within the catalyst pores [159]. Porosity is another governing feature of the catalyst [73] and its activity affecting through selective adsorption mechanism.
2.2.2.4. General mechanism of action
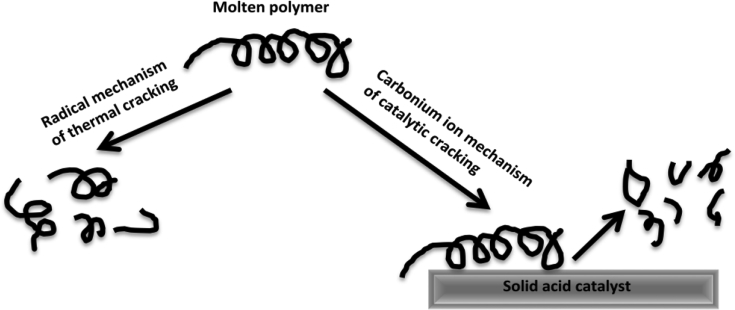
Generally, the mechanism of polymer degradation over acidic solid catalysts is assumed to proceed similarly in the manner of carbonium ion mechanism for hydrocarbon cracking [49]. Possible mechanism of polymer degradation over solid acid catalysts is shown in Fig. 5. Initially, the thermal cracking occurs over the external surface of the solid catalyst, the internal pores of the catalyst behave as channels thereafter for selective passage and further breakdown of larger hydrocarbons into smaller ones, where product selectivity takes place [160]. Mainly the gases are produced inside the small sized pores, whilst, due to the external cracking occurring on the outer active sites of the catalyst, waxes are produced [161]. Low molecular weight hydrocarbons, produced by the reactions held at the surface, are sufficiently volatile at the reaction temperature and capable of diffusing out through the molten polymer film as a final product or can even react more within the pores. The catalytic process is enhanced by microporous catalysts, having high internal crystalline structure, producing more gases and lowered liquid yield but with improved quality than in the cases of catalysts possessing macropore volume [43, 73]. In addition, multi-dimensional catalysts provide more surface area therefore allows more cracking than mono-dimensional catalysts [81].
Fig. 5.
Illustration of polymer cracking over solid acid catalyst [49].
Although different mechanisms of ionic as well as free radical have been postulated for catalytic polymer pyrolysis [162, 163, 164], reaction proceeding via carbonium ion in the transition state includes chief steps of (i) initiation such as H-transfer with on-chain carbonium ion formation by proton addition (at Bronsted acid sites) or random abstraction of hydride ion (at Lewis acid sites), (ii) chain/β-scission, (iii) depropagation giving rise to oligomerization/alkylation, followed by (iv) isomerization and aromatization [165]. These complex reaction procedures are influenced by the strength of acid-sites, density, distribution along with pore size of the catalysts which in turn affects the chemical composition and final product distribution [166]. Thermogravimetric analysis (TGA) technique applied for catalyst screening reveals that the catalyst potentially decreases the apparent energy of activation [83]. Following equations describes briefly the carbonium ion mechanism of catalytic pyrolysis [29, 158]:
| (1) |
| (2) |
| (3) |
Successive chain cleavage followed by depropagation yields oligomer fraction approximately ranging (C30–C80). Further cleavage thereafter of the oligomers leads to simultaneous formation of gas fraction and liquid fraction ranging (C10–C25) approx.
While, in catalytic degradation with Fe/activated charcoal in H2 atmosphere, and temperature above 380 °C, there is depolymerisation and degradation by free radical chain reaction through hydrogenation of hydrocarbon radical and abstraction of H radical from hydrocarbon or hydrocarbon radical [29, 167]:
| (4) |
| (5) |
| (6) |
Polymer degradation may proceed using reforming catalyst showing bi-functionality due to the two different active sites, metallic sites accelerate hydrogenation and dehydrogenation reactions whereas the acidic sites on support accelerate isomerization. Improvement in octane numbers of gasoline-range hydrocarbons without change in their carbon numbers is achieved. Pt/SiO2-Al2O3 with ∼0.5 wt% Pt only is the common reforming catalyst whilst composite of zeolite with Zn enhances adsorption capacity by narrowing the pores of zeolites thereby improving the sieving effect which permits passage of small molecules only. Moreover, due to increased acidity, Co-Mo/zeolite is highly efficient for catalytic conversion of waste polymeric materials into gasoline range tar [168]. The reforming reaction is illustrated through the following n-pentane isomerization [29, 158]:
| (7) |
| (8) |
| (9) |
Investigations and comparison of the effects of various types of catalysts on polymer degradation revealed the fact that more than the weak acid ones the strong acid catalysts are capable to catalyze the cracking of heavier hydrocarbons to lighter ones into gaseous products [49]. Therefore, red mud and zeolites like ZSM-5, possessing two distinct weak as well as strong acid sites, yield more gaseous and less liquid products than the silica-alumina catalysts having only weak acid sites. Whereas FSM virtually having no acidity yield liquid hydrocarbons more than 86 wt% with residues much less than that of non-catalyzed thermal pyrolysis, while non-acidic porous materials, silica-gel and silicalite, produce liquid range hydrocarbons with yields around 80 wt% and 75 wt% respectively [49]. The characteristics and effects of some catalysts in catalytic pyrolysis process of polymers are summarized in Table 4. However, zeolites are the most widely used catalysts in polymer pyrolysis with proven effectiveness to improve the quality of the obtained products [31, 81], discussed in the next section.
Table 4.
Characteristic effects of catalysts on polymer pyrolysis process [57].
| Catalyst characteristic | Effect | |
|---|---|---|
| High BET surface area | More cracking & gases produced, decrease in liquid yield | |
| High thermal stability | Appreciable activity maintained at high temperatures | |
| High internal crystalline structure | Excellent activity, enhanced cracking, increased gaseous yield | |
| High Lewis & Bronsted acid site | More cracking & gases produced, decreased impurities in liquid yield | |
| Pore volume/size: Microporous & Macroporous | Favors gaseous yield & favors liquid yield, respectively | |
| Reformed catalyst: Metal doped on acidic base | Performs dual function, improves catalytic activity | |
| Example: | ||
| Catalyst | Polymeric feedstock | |
| Red mud | PE, PP, PS, PET, PVC | Enhanced catalytic activity, increased cracking ability High temperature yields more gases & aromatic compounds |
| Na2CO3 | Tires | Decrease in reaction temperature Increases conversion with increased liquid yield |
| FCC | HDPE, LDPE, PP, PS | Increase in cracking process & reduction in energy demand High liquid yield due to less microporous area Formation of branched hydrocarbons in liquid product due to carbon-chain isomerization Decreased concentration of heavy aliphatic compounds (C20+) & increased concentration of lighter hydrocarbons (C13−) Increased C2 and C4 & decreased methane in gaseous product with increase in temperature |
| Natural zeolite (NZ), Clinoptillolite, Mordenite(1D catalyst) |
Municipal polymeric waste HDPE,PS PE |
Decreases the liquid pour point & the heavy oil fraction Increase in the gasoline fraction High liquid yield due to less microporous area Increase of aromatic compounds in oil Wax conversion into light hydrocarbons |
| Modified natural zeolite: Ni/Z, Co/Z, NiMo/Z, CoMo/Z |
LDPE | Metal impregnation (Ni, Co, Ni-Mo & Co-Mo) on NZ unaffects crystallinity, increases activity & selectivity towards cracking Composite yields high liquid, maximum gasoline at ∼ 350 °C due to high acidity Liquid (C6 - C19) contains paraffins, napthenes & olefins |
| Y-zeolite | PE | Decrease in liquid yield due to high BET surface area Increase of gasoline fraction & aromatic compounds in oil High conversion of wax to light hydrocarbons |
| ZSM-5 | HDPE, PE, PP, PS, PET, PVC | More porous & presence of enhanced Bronsted acid site in internal structure facilitates cracking process & high yield of gases at decreased pyrolysis process temperature Promotes production of i-butane in gases with high fraction of C3-C4 contents and increased concentration of H2 Produced gases have similar HHV (48–53 MJ/kg) as natural gas, applicable as an alternative Increase in gasoline & light oil yield Liquid contain high aromatics, C5-C9 compounds |
| HZSM-5 | HDPE, PE | Increase in cracking process, overall yield, volatile compounds & high conversion of wax to light hydrocarbons |
| HY-zeolite | PE, PP, PS | High yield of gaseous & liquid hydrocarbons, usually no wax Liquid produced from PS consist chiefly styrene |
2.2.2.5. Zeolites
Zeolites are a class of crystalline aluminosilicates of group 1A or 2A elements, having chief characteristics of ion exchange capabilities and open micropores functioning as molecular sieves that restrict the free diffusion of bulky large molecules within the internal structure of the catalyst [169, 170]. The catalyst is built by varying ratio of SiO2/Al2O3 depending on its type, determining its reactivity affecting final pyrolysis product selectivity and distribution, while forming three-dimensional framework with the tetrahedral sides linked by oxygen atoms developing interconnected channels and cavities of differing shape and size for cations to reside. The tetrahedral structure is the primary unit composed generally of AlO4 and SiO4 with aluminum or silicon occupied at the tetrahedral position and oxygen atom being shared by two silicon or aluminum atoms. As the AlO4 tetrahedrons possess negative charges of (-1), due to the (+3) valence of aluminum while (+4) of silicon, the charge imbalance requires balancing by cations of alkali or alkaline earth metals (especially Na+, K+, Ca+2 or Mg+2) to be present within the pores of zeolite, which also renders the possibility of ionic replacement through cation exchange. These cations when exchanged for protons, the acid sites of zeolites are created, which may be either Brønsted acid types (proton donors) or Lewis acid types (electron pair acceptors) [171]. Other adsorbates usually water molecules occupy the channels and cavities favoring ion exchange, therefore the chemical composition of zeolites are represented by the empirical formula: M2/nO.Al2O3.ySiO2.wH2O (where n is the cationic valence, y values from 2-10 and w is the quantity/moles of structural water) [66]. Though natural zeolites are more abundant having lesser cost, the synthetic zeolites hold the advantages of purity, uniformity in shapes and sizes of cavities and channels, with pre-defined chemical composition, required for specific applications e.g. petroleum cracking [172].
Chemical and physical characteristics ensuring reactivity and selectivity catalytic properties to molecular sieves may be cited as: high specific area and adsorption capacity; active acidic sites (strength and concentration directed specifically according to application); network of channels and cavities providing selectivity of shape and size to the reactant molecules, transition state species and products; along with enormous thermal stability for catalyzing various hydrocarbon reactions, inclusive cracking of polymers [173]. However, the rupturing process of polymer molecules begins on the external surface, since to access the internal reactive specific pore sites of the zeolites, the polymer chains need to be broken down in order to penetrate the small pore size. Due to the presence of microporous structure, the zeolites possess higher internal surface than the external which enables mass transfer between the two surfaces, specificity varying with pore volume factor depending upon one to another zeolite [173]. The catalytic activity level of zeolites enhances with the increase in number of acidic sites located majorly within the pores of the catalyst material [29]. Hence, the two main factors of zeolites involved in polymer catalytic cracking are the textural property of crystalline microporous structure and many active acid sites favoring hydrogen transfer reactions, thereby, enabling higher conversions into volatile gas fraction at relatively milder operating conditions between temperatures 350–500 °C [174]. Factor that drastically affects the catalytic capacity of zeolites is their deactivation by deposition of coke onto their cavities and channels, while the rate of coke formation (coking) depends mostly upon reaction conditions like pressure, temperature, reactants, not excluding structural and acidic characteristics of the catalyst [173].
Moreover, the ratio of SiO2/Al2O3 indicates the acidity of Zeolite catalysts, the basis of its being more active in cracking process with marked effect in the product fraction yield, achieving increased production of light olefins decreasing the heavy fractions and liquid yields [82]. Low ratio of SiO2/Al2O3 denotes high acidity of zeolite material with (SiO2/Al2O3 = 30) as the highest acidic catalyst among its group and cracking waxes more actively improving yield of lighter olefins (from 35.5 to 58.0 wt%) and lowering heavy fraction range C12–C20 (from 28.0 to 5.3 wt%) in comparison to the zeolite catalyst (SiO2/Al2O3 = 280) with lowest acidity. Besides, the most reduced ratio of SiO2/Al2O3 also raises the octane number with higher content of aromatics, lighter alkanes and nil amounts of sulfur. In terms of final product selectivity, various zeolites show different product preferences. Examples of widely used zeolite catalysts in catalytic pyrolysis of polymeric materials are natural zeolite (NZ) [131], β-zeolite [175, 176], Y-zeolite [81], USY [177], REY [178], ZSM-5 [179], ZSM-11 [180], clinoptilolite [181], mordenite [182], etc. The usage of zeolite catalysts in catalytic pyrolysis of real municipal polymeric wastes proved to influence the reduction of impurities in the produced tar [147]. Apart from these, FCC catalyst consists of zeolite crystals on non-zeolite acid matrix of silica–alumina along with the binder [170, 183, 184]. The chief component of FCC catalyst is usually Y-zeolite, used because of its high thermal stability, product selectivity [185] and additional quality of cost effectiveness since being a ‘reused’ catalyst.
2.3. Effect of catalyst particle size and the advent of nanostructured catalysts for pyrolysis
One of the biggest set-back countered by conventional heterogeneous catalysts, in comparison to its homogeneous counterparts, is the limitation in catalytic capacity arising due to reduced surface area lacking complete accessibility to reactant molecules, thus compelling high consumption of the valuable catalysts [186, 187]. Possible solution to both the matter of access and expense can be postulated as size reduction of the active catalyst material giving rise to concomitant enhancement of its surface to volume ratio (S/V) [188, 189]. Therefore engineering catalysts at the nanoscale can specifically achieve the targeted high S/V. Nanosized catalysts display additional unique properties not present in the macroscale bulk catalyst materials [190, 191, 192, 193, 194], further contributing towards lower process energy consumption, longer catalyst lifecycle and enhanced ease in isolating and reusing the active nanocatalysts [108]. Nanocatalysis though regarded as “soluble heterogeneous” [195] or “semi-heterogeneous” [101] catalysis, may be considered as a distinct category which combines the beneficial aspects of exclusive high activity with attainment of near 100% selective reactions and excellent yield associated with homogeneous catalysis and tremendously eased product separation along with catalyst recovery related to heterogeneous catalysis [106, 186, 196].
The effect of particle size of the catalyst on polymer pyrolysis process has sparsely been studied. Investigation of particle sizes in the nanorange of zeolites and its effect on catalytic polymer degradation discovered the fact that conversion decreases with particle size while finally the product quality increases [197]. Few nanocrystalline zeolites to be mentioned are HUSY, HMOR, Hβ, HZSM-5, etc. [8, 198], extensively applied for research purpose of polymer pyrolysis. The application of nanocrystalline HZSM-5 promotes greater gas fraction yield in the range of C3–C6, in remarkably higher concentrations, at mild temperatures and achieves raised selectivity to the obtained products than in the case of micron-sized ZSM-5. Reports exist of above 90% conversion, at temperatures below 350 °C, applying nano-HZSM-5 for cracking HDPE, LDPE and PP [199, 200]. Implying 40 wt% of polymer to catalyst ratio, left over residue was minimum ∼4.53 wt%, indicating the capability of HZSM-5 to maximize total product conversion in the pyrolysis of polymeric wastes. Catalytic pyrolysis of HDPE carried out with HZSM-5, employing 20 wt% of catalyst to polymer ratio, at 450 °C yielded 35 wt% of liquid and 65 wt% gases, whereas using the same feedstock and catalyst at 500 °C, liquid fraction was reduced to 4.4 wt% with increase in the produced gases to 86.1 wt% [201, 202]. Moreover, HZSM-5 showed superior resistance to coking when the product stream of isobutane and isopentanes stayed unaffected throughout while the olefins consisting butene and pentene increased in the process [68, 203, 204]. Hence, apart from higher efficiency HZSM-5 has extremely low rate of deactivation, rendering catalyst reuse suitable, since efficiently regenerated, thus imparting longer life cycle.
Nanocrystalline zeolites HUSY and HMOR were investigated to be equally efficient, leaving very less residue of around 7.07 wt% and 8.93 wt% respectively [205]. PP pyrolysis conducted at 360 °C with HUSY zeolite catalyst and 40 wt% of catalyst to polymer ratio resulted to obtain little quantity of liquid product of 3.75 wt% only [203]. It was proved by experimental studies that various nanocatalysts vary in product selectivity. Using a batch reactor and catalyst to polymer ratio of 10 wt% at constant temperature of 550 °C performance of HZSM-5 and HUSY was studied on HDPE and LDPE. HUSY yielded higher liquid product of 41.0 wt% and 61.6 wt% from HDPE and LDPE, respectively, compared to HZSM-5 which catalyzed the recovery of 17.3 wt% and 18.3 wt% from HDPE and LDPE, respectively, but facilitated to obtain more of the gaseous product which was 72.6 wt% and 70.7 wt% through degradation of HDPE and LDPE, respectively [74]. Same pattern of product selectivity was observed for HUSY and HZSM-5 nanocatalysts with reports of minimal amount of liquid yield of 3.75 wt% and 2.31 wt% respectively, on PP pyrolysis, employing 40 wt% of catalyst to polymer ratio, at 360 °C, Lin and Yen [203].
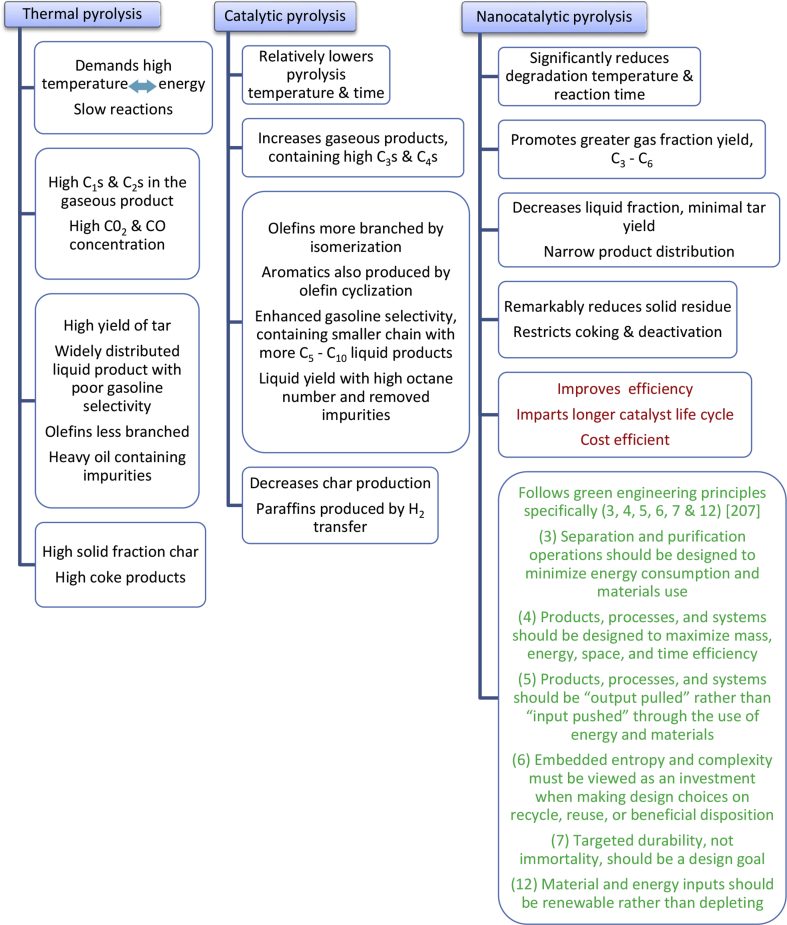
Overall, nano-zeolite catalysts improve the volatile hydrocarbon production and based on the results it can be deduced that the effectiveness of the nano-scale particles lies in the increased surface area of the catalyst material. On the contrary, high surface area together with the system of very small pores poses big difficulty in achieving handsome amounts of C5–C12 gasoline range hydrocarbons, which casts limitation on the selectivity of nanocatalysts to liquid products. Possible solution to this could be brought through the approach of nanosized particles belonging to catalysts being selective to liquid products of gasoline range such as the FCC catalyst, reformed catalyst Al-MCM-41, etc. Nowadays, a new family of mesoporous aluminosilicates, MCM, has also been developed with uniformly distributed pores of 2–20 nm in a regular array, constituting framework with broad range of Si/Al ratios and having mild acidity [29, 65]. Studies suggest that besides reducing the reaction temperature and enhancing the product character, HMCM-41 enables increased conversion of polymer mixture because of its sufficiently large pore dimensions which allow the polymer molecules to enter within and reach the acid sites in order to be accessible. Al-MCM-41 generates a higher yield of commercial gasoline type condensable hydrocarbon products, attributing to its weaker acid sites and large pore size [206]. Fig. 6, represents the advantages of the application of nanostructured catalyst system in the pyrolysis process of waste polymers.
Fig. 6.
Representation of different polymer pyrolysis process [207].
3. Conclusions
An insight into the domain of nanocatalysis for innovation in the sector of catalytic pyrolysis, both from an environmental and an economic point of view, brings out its huge potential contribution in a greener chemistry and engineering manner. The dimensions of particles as well as pores and surface composition are the crucial influencing factors for the outstanding performance of the synthesized superior nanostructured catalysts determined by (i) activity, expressing number of raw material molecules catalytically converted to products per unit time, (ii) selectivity, producing ∼100% desirable products, (iii) recoverability and durability, featuring intrinsic system facilitating separation from product mixture and its reuse indicating the catalyst lifecycle, measured by the number of catalytic cycles undergone before replacement required. Nanoscale size of the catalysts consequently confers high surface area, enhancing the catalytically active sites accessible to polymer substrate molecules, thereby increasing the TOF, promoting catalyst use in smaller quantities, reducing the cost greatly affecting the process economy.
Optimally engineered nanocatalysts yield narrower product distribution with respect to the number of carbon atoms at lower temperatures, also decreasing the degradation time and the formed solid residue, restricting coking and deactivation. Experimental variables such as reactor type, carrier gas, feedstock, temperature, retention time, etc., may also govern the obtained product composition from pyrolysis which can be carried out either with pure polymers or with polymer blends. Cracking of polymers is processed by random chain scission yielding waxes and gasoline distillates using catalysts of medium or weak acidity, whereas through strong acidic ones scission occurs at chain end producing light hydrocarbons fraction of C3-C5 olefins. The primary cracking products may be either separated or subjected to secondary reactions of oligomerization, cyclization and aromatization. Production of gases is less with more of tar and char by macroporous catalysts than shown by the microporous catalysts. Moreover, modifications like thermal and acidic, including catalyst reforming such as doping of metals or metallic nanoparticles of Ni, Co, Mo, Zn, Pd, Pt, etc. via wet impregnation can improve the activity and characteristics of the catalysts.
The liquid tar and gases obtained have high HHV applicable as alternative sources of energy while the produced char consists of high BET surface area useful in various adsorption related applications. Therefore, in this nano-era when consumption of non-biodegradable polymeric materials has become formidable and their generated wastes too, together with the growing concern of depleting non-renewable energy sources such as fossil fuels and rising demand of petroleum oil as well as natural gas, the nanocatalytic approach towards pyrolysis for energy recovery from polymeric wastes appears unambiguously attractive along with being effective. In addition to the utilization of the contained energy and the raw materials in the polymeric wastes into valuable gas, tar and char, there is efficient management of the environmental impacts.
Declarations
Author contribution statement
Poushpi Dwivedi, P. K. Mishra, Manoj Kumar Mondal, Neha Srivastava: All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The first author PD is grateful to the Science and Engineering Research Board (SERB), a statutory body of the Department of Science and Technology (DST), Government of India (GI), for granting financial support through the Project File No.: PDF/2017/002264 under the National Post Doctoral Fellowship (N-PDF) scheme.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Rowatt R.J. The plastic waste problem. Chemtech. 1993;23:56–60. [Google Scholar]
- 2.Scott G. RSC Paperbacks; Cambridge: 1999. Polymer and the Environment. 132p. [Google Scholar]
- 3.The compelling facts about plastics. Analysis of plastics production, demand and recovery for 2005 in Europe published in 2007. [http://www.kunststofflandnrw.de/modules/kln_infomaterial/files/623f1d611b6ae2b.pdf] and The compelling facts about plastics, Analysis of plastics production, demand and recovery for 2006 in Europe published in 2008 [http://www.pvc.org/PVC.org/Media-Centre/Documents-Library/The-Compelling-Facts-about-Plastics].
- 4.Plastics Europe . 2012. An Analysis of European Plastics Production, Demand and Waste Data for 2011, Plastics - the Facts 2012. [Google Scholar]
- 5.US Environmental Protection Agency, common wastes & materials: Plastics. http://www.epa.gov/osw/conserve/materials/plastics.htm (Accessed 2013).
- 6.Indian plastic industry review & outlook, plastindia foundation report [http://www.cipad.org/files/files/india_2006.pdf].
- 7.Muthaa N.H., Patel M., Premnath V. Plastics materials flow analysis for India. Resour. Conserv. Recycl. 2006;47:222–244. [Google Scholar]
- 8.Sharuddin S.D.A., Abnisa F., Daud W.Md.A.W., Aroua Md.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016;115:308–326. [Google Scholar]
- 9.Long R., editor. The Production of Polymer and Plastics Intermediates from Petroleum. Butterworth; Guildford: 1967. [Google Scholar]
- 10.Wiseman P. Wiley; New York: 1986. Petrochemicals. [Google Scholar]
- 11.Odian G. third ed. Wiley-Interscience; New York: 1991. Principles of Polymerization. [Google Scholar]
- 12.Shastri V.P. Non-degradable biocompatible polymers in medicine: past, present and future. Curr. Pharmaceut. Biotechnol. 2003;4:331–337. doi: 10.2174/1389201033489694. [DOI] [PubMed] [Google Scholar]
- 13.L.A. Utracki, International Abbreviations for Polymers and Polymer Processing, National Research, Council Canada, Industrial Materials, Institute, Boucherville, QC, Canada
- 14.Ojeda T. Polymer Science. Intech; 2013. Chapter 1, polymers and the environment; pp. 1–34. [Google Scholar]
- 15.Kaminsky W., Menzel J., Sinn H. Recycling of plastics. Conserv. Recycl. 1976;1:91–110. [Google Scholar]
- 16.Howell G.S. A ten year review of plastics recycling. J. Hazard Mater. 1992;29:143–164. [Google Scholar]
- 17.Kaminsky W., Roessler H. Olefins from wastes. Chemtech. 1992;22:108–113. [Google Scholar]
- 18.Bisio A.L., Merrieam N.C. Chap. 3. Technologies for polymer recovery: recycling and potential for energy savings. In: Bisio A.L., Xanthos M., editors. How to Manage Plastics Waste: Technology and Market Opportunities. Carl Hanser Verlag, Munich; Munich, Germany: 1994. pp. 15–31. [Google Scholar]
- 19.Kaminsky W., Schlesselmann B., Simon C. Olefins from polyolefins and mixed plastics by pyrolysis. J. Anal. Appl. Pyrolysis. 1995;32:19–27. [Google Scholar]
- 20.Lee M. Feedstock recycling: new plastic for old. Chem. Brit. 1995;31:515–516. [Google Scholar]
- 21.Brandrup J., Bittner M., Michaeli W., Menges G. Carl Hanser Verlag, Munich; New York: 1996. Recycling and Recovery of Plastics. [Google Scholar]
- 22.Al-Salem S.M., Lettieri P., Baeyens J. Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag. 2009;29:2625–2643. doi: 10.1016/j.wasman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Nahil Md.A., Miskolczi N., Huang J., Williams P.T. Processing real-world waste plastics by pyrolysis-reforming for hydrogen and high-value carbon nanotubes. Environ. Sci. Technol. 2014;48:819–826. doi: 10.1021/es402488b. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A., Kuti R. The environmental impact of plastic waste incineration. AARMS. 2016;15:231–237. [Google Scholar]
- 25.Burton R.S. Municipal solid waste pyrolysis, fluidisation and fluid particle systems. AlChE Symp. Ser. 1973;70:116–123. [Google Scholar]
- 26.Kaminsky W. Thermal recycling of polymers. J. Anal. Appl. Pyrolysis. 1985;8:439–448. [Google Scholar]
- 27.Oudhuis A.B.J., de Wit P., Tromp P.J.J., Moulijn J.A. An exploratory study of the processing of plastics by means of pyrolysis, with emphasis on PVC/aluminium combinations. J. Anal. Appl. Pyrolysis. 1991;20:321–336. [Google Scholar]
- 28.Conesa J.A., Font R.F., MarcilIa A., Garcia A.N. PyroIysis of polyethylene in a fluidised bed reactor. Energy Fuels. 1994;8:1238–1246. [Google Scholar]
- 29.Panda A.K., Singh R.K., Mishra D.K. Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew. Sustain. Energy Rev. 2010;14:233–248. [Google Scholar]
- 30.Singhabhandhu A., Tezuka T. The waste-to-energy framework for integrated multi-waste utilization: waste cooking oil, waste lubricating oil, and waste plastics. Energy. 2010;35:2544–2551. [Google Scholar]
- 31.Almeida D., Marques M.de.F. Thermal and catalytic pyrolysis of plastic waste. Polímeros. 2016;26:44–51. [Google Scholar]
- 32.Demirbas A. Pyrolysis of municipal of plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis. 2004;72:97–102. [Google Scholar]
- 33.Arabiourrutia M., Elordi G., Lopez G., Borsella E., Bilbao J., Olazar M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis. 2012;94:230–237. [Google Scholar]
- 34.Mastral J.F., Berrueco C., Ceamanos J. Theoretical prediction of product distribution of the pyrolysis of high density polyethylene. J. Anal. Appl. Pyrolysis. 2007;80:427–438. [Google Scholar]
- 35.Stelmachowski M. Thermal conversion of waste polyolefins to the mixture by hydrocarbons in the reactor with molten metal bed. Energy Convers. Manag. 2010;51:2016–2020. [Google Scholar]
- 36.Kiran N., Ekinci E., Snape C.E. Recyling of plastic wastes via pyrolysis. Resour. Conserv. Recycl. 2000;29:273–283. [Google Scholar]
- 37.Lee K.H., Shin D.H. Influence of plastic type on pyrolysis of waste thermoplastics into oil recovery. J. Korea Soc. Waste Manag. 2004;21:646–651. [Google Scholar]
- 38.Lee K.H. Pyrolysis of municipal plastic wastes separated by difference of specific gravity. J. Anal. Appl. Pyrolysis. 2007;79:362–367. [Google Scholar]
- 39.Prasad B., Kuester J.L. Process analysis of a dual fluidized bed gasification system. Ind. Eng. Chem. Res. 1988;27:304–310. [Google Scholar]
- 40.Lee K.H., Shin D.H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: influence of lapse time of reaction. Waste Manag. 2007;27:168–176. doi: 10.1016/j.wasman.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Onwudili J.A., Insura N., Williams P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: effects of temperature and residence time. J. Anal. Appl. Pyrolysis. 2009;86:293–303. [Google Scholar]
- 42.López A., Marco I.de., Caballero B.M., Laresgoiti M.F., Adrados A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011;173:62–71. [Google Scholar]
- 43.López A., Marco I.de., Caballero B.M., Laresgoiti M.F., Adrados A., Torres A. Pyrolysis of municipal plastic wastes II: influence of raw material composition under catalytic conditions. Waste Manag. 2011;31:1973–1983. doi: 10.1016/j.wasman.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Miskolczi N., Nagy R. Hydrocarbons obtained by waste plastic pyrolysis: comparative analysis of decomposition described by different kinetic models. Fuel Process. Technol. 2012;104:96–104. [Google Scholar]
- 45.Abbas-Abadi M.S., Haghighi M.N., Yeganeh H. The effect of temperature, catalyst, different carrier gases and stirrer on the produced transportation hydrocarbons of LLDPE degradation in a stirred reactor. J. Anal. Appl. Pyrolysis. 2012;95:198–204. [Google Scholar]
- 46.Abbas-Abadi M.S., Haghighi M.N., Yeganeh H. Evaluation of pyrolysis products of virgin high density polyethylene degradation using different process parameters in a stirred reactor. Fuel Process. Technol. 2013;109:90–95. [Google Scholar]
- 47.Zeaiter J. A process study on the pyrolysis of waste polyethylene. Fuel. 2014;133:276–282. [Google Scholar]
- 48.Hartulistiyosoa E., Sigiroa F.A.P.A.G., Yulianto Md. Temperature distribution of the plastics Pyrolysis process to produce fuel at 450°C. Proc. Environ. Sci. 2015;28:234–241. [Google Scholar]
- 49.Sakata Y., Uddin M.A., Muto A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis. 1999;51:135–155. [Google Scholar]
- 50.Lee K.H., Noh N.S., Shin D.H., Seo Y.H. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polym. Degrad. Stab. 2002;78:539–544. [Google Scholar]
- 51.Lee K.H., Shin D.H., Seo Y.H. Liquid-phase catalytic degradation of mixtures of waste high density polyethylene and polystyrene over spent FCC catalyst. Effect of mixing proportions of reactants. Polym. Degrad. Stab. 2004;84:123–127. [Google Scholar]
- 52.Siddiqui M.N., Redhwi H.H. Catalytic coprocessing of waste plastics and petroleum residue into liquid fuel oils. J. Anal. Appl. Pyrolysis. 2009;86:141–147. [Google Scholar]
- 53.Huang W.C., Huang M.S., Huang C.F., Chen C.C., Ou K.L. Thermochemical conversion of polymer wastes into hydrocarbon fuels over various fluidizing cracking catalysts. Fuel. 2010;89:2305–2316. [Google Scholar]
- 54.Motevasel M., Roozbehani B., Shahi A. Catalytic degradation of mixed polymers into environmental friendly and useful products. Am. J. Oil Chem. Technol. 2014;2:360–367. [Google Scholar]
- 55.Osborn P.D. Butterworth; GuiIdford: 1985. Handbook of Energy Data and Calculations. [Google Scholar]
- 56.WampIer T.P. Thermometric behaviour of polyolefins. J. Anal. Appl. Pyrolysis. 1989;5:187–195. [Google Scholar]
- 57.Miandad R., Barakat M.A., Aburiazaiza A.S., Rehan M., Nizami A.S. Catalytic pyrolysis of plastic waste: a review. Process Saf. Environ. Prot. 2016;102:822–838. [Google Scholar]
- 58.Kuester J.L. Vol. 32. ISA; 1989. Electrical power and fuels production from wastes; pp. 69–74. (Instrumentation in the Power Industry). [Google Scholar]
- 59.Ali M.F., Siddiqui M.N., Redhwi S.H.H. Study on the conversion of waste plastics/petroleum resid mixtures to transportation fuels. J. Mater. Cycles Waste Manag. 2004;6:27–34. [Google Scholar]
- 60.Lee H., Park R.su., Lee H.W., Hong Y., Lee Y., Park S.H., Jung S.C., Yoo K.S., Jeon J.K., Park Y.K. Adsorptive removal of atmospheric pollutants over Pyropia tenera chars. Carbon Letters. 2016;19:79–88. [Google Scholar]
- 61.Benedettia V., Patuzzia F., Baratieri M. Gasification char as a potential substitute of activated carbon in adsorption applications. Energy Procedia. 2017;105:712–717. [Google Scholar]
- 62.Ivanova S.R. Selective catalytic degradation of polyolefins. Prog. Polym. Sci. 1990;15:193–215. [Google Scholar]
- 63.Ohkita H., Nishiyama R., Tochihara Y., Mizushima T., Kakuta N., Morioka Y., Ueno A., Namiki Y., Tanifuji S., Katch H., Sunazuka H., Nakayama R., Kuroyanagi T. Acid properties of silica-alumina catalysts and catalytic degradation of polyethylene. Ind. Eng. Chem. Res. 1993;32:3112–3116. [Google Scholar]
- 64.Sakata Y., Uddin M.A., Koizumi K., Murata K. Catalytic degradation of polypropylene into liquid hydrocarbons using silica-alumina catalyst. Chem. Lett. 1996;0:245–246. [Google Scholar]
- 65.Aguado J., Serrano D.P., Romero M.D., Escola J.M. Catalytic conversion of polyethylene into fuels over mesoporous MCM-41. Chem. Commun. 1996;0:725–726. [Google Scholar]
- 66.Aguado J., Serrano D.P., Escola J.M. Catalytic upgrading of plastic wastes. In: Scheirs J., Kaminsky W., Orgs, editors. Feedstock Recycling and Pyrolysis of Waste Plastics. John Wiley & Sons; Hoboken: 2006. pp. 73–110. [Google Scholar]
- 67.Uddin Md.A., Koizumip K., Murata K., Sakata Y. Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Polym. Degrad. Stab. 1997;56:37–44. [Google Scholar]
- 68.Uemichi Y., Nakamura J., Itoh T., Garforth A.A., Dwyer J. Conversion of polyethylene into gasoline-range fuels by two–stage catalytic degradation using silica-alumina and HZSM-5 zeolite. Ind. Eng. Chem. Res. 1999;38:385–390. [Google Scholar]
- 69.Aguado J., Serrano D.P., Escola J.M., Garagorri E., Fernandez J.A. Catalytic conversion polyolefins into fuels over zeolite beta. Polym. Degrad. Stab. 2000;69:11–16. [Google Scholar]
- 70.Yee L.S. Catalytic degradation of polystyrene over natural clinoptilolite zeolite. Polym. Degrad. Stab. 2001;74:297–305. [Google Scholar]
- 71.Dawood A., Miura K. Catalytic pyrolysis of γ-irradiated polypropylene (PP) over HY-zeolite for enhancing the reactivity and the product selectivity. Polym. Degrad. Stab. 2002;76:45–52. [Google Scholar]
- 72.Gobin K., Monos G. Thermogravimetric study of polymer catalytic degradation over microporous catalysts. Polym. Degrad. Stab. 2004;86:225–231. [Google Scholar]
- 73.Miskolczi N., Bartha L., Deak G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stab. 2006;91:517–526. [Google Scholar]
- 74.Marcilla A., Gomez A., Valdes F. Catalytic cracking of low density polyethylene over H-beta and HZSM-5 zeolites in dynamic conditions. Polym. Degrad. Stab. 2007;92:197–204. [Google Scholar]
- 75.Lin Y.H. Production of valuable hydrocarbons by catalytic degradation of a mixture of post-consumer plastic waste in a fluidized-bed reactor. Polym. Degrad. Stab. 2009;94:1924–1931. [Google Scholar]
- 76.Lópeza A., Marcoa I.de., Caballeroa B.M., Laresgoiti M.F., Adradosa A., Aranzabal A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B Environ. 2011;104:211–219. [Google Scholar]
- 77.Sarker M., Rashid M.M. Waste plastics mixture of polystyrene and polypropylene into light grade fuel using Fe2O3 catalyst. Int. J. Renew. Energy Technol. Res. 2013;2:17–28. [Google Scholar]
- 78.Lin Y.H., Yang M.H., Yeh T.F., Ger M.D. Catalytic degradation of high density polyethylene over mesoporous and microporous catalysts in a fluidised-bed reactor. Polym. Degrad. Stab. 2004;86:121–128. [Google Scholar]
- 79.A Sharuddin S.D., Abnisa F., Daud W.M.A.W., KAroua M. Pyrolysis of plastic waste for liquid fuel production as prospective energy resource. IOP Conf. Ser. Mater. Sci. Eng. 2018;334 [Google Scholar]
- 80.Coelho A., Costa L., Marques M.M., Fonseca I.M., Lemos M.A.N.D.A., Lemos F. The effect of ZSM-5 zeolite acidity on the catalytic degradation of high-density polyethylene using simultaneous DSC/TG analysis. Appl. Catal. Gen. 2012;413–414:183–191. [Google Scholar]
- 81.Lee K.H. Effects of the types of zeolite on catalytic upgrading of pyrolysis wax oil. J. Anal. Appl. Pyrolysis. 2012;94:209–214. [Google Scholar]
- 82.Artetxe M., Lopez G., Amutio M., Elordi G., Bilbao J., Olazar M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013;52:10637–10645. [Google Scholar]
- 83.Garforth A.A., Fiddy S., Lin Y.H., Ghanbari-Siakhalu A., Sharratt P.N., Dwyer J. Catalytic degradation of high density polyethylene: an evaluation of mesoporous and microporous catalysts using thermal analysis. Thermochim. Acta. 1997;294:65–69. [Google Scholar]
- 84.Lin Y.H., Hwu W.H., Ger M.D., Yeh T.F., Dwyer J. A combined kinetic and mechanistic modeling of the catalytic of polymers. J. Mol. Catal. A Chem. 2001;171:143–151. [Google Scholar]
- 85.Marcilla A., Gomez A., Reyes-Labrta A., Giner A. Catalytic pyrolysis of polypropylene using MCM-41: kinetic model. Polym. Degrad. Stab. 2003;80:233–240. [Google Scholar]
- 86.Ali S., Garforht A.A., Harris D.H., Rawlence D.J., Uemichi Y. Polymer waste recycling over ‘‘used’’ catalysts. Catal. Today. 2002;75:247–255. [Google Scholar]
- 87.López A., Marco I.de., Caballero B.M., Adrados A., Laresgoiti M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011;31:1852–1858. doi: 10.1016/j.wasman.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Miskolczi N., Ates F., Borsodi N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013;144:370–379. doi: 10.1016/j.biortech.2013.06.109. [DOI] [PubMed] [Google Scholar]
- 89.B. Zhou, H. Trevino, Z. Wu, Z. Zhou, C. Liu, Reforming Nanocatalysts and Method of Making and Using Such Catalysts, US Patent No. 7569508, Headwaters Technology Innovation LLC, 2005.
- 90.Serrano D., Aguado J., Rodriguez J., Peral A. Catalytic cracking of polyethylene over nanocrystalline HZSM-5: catalyst deactivation and regeneration study. J. Anal. Appl. Pyrolysis. 2007;79:456–464. [Google Scholar]
- 91.Sethi B. Recycling of polymers in the presence of nanocatalysts: a green approach towards sustainable environment. Int. J. Civ. Mech. Eng. 2016;10:525–531. [Google Scholar]
- 92.Mahmood T., Malik M., Bano A., Umer J., Shaheen A. Nanocatalytic conversion of waste palm oil grade iii and poplar plant’s wood sawdust into fuel. Innov. Energy Res. 2017;6:1–7. [Google Scholar]
- 93.Gellman A.J., Shukla N. Nanocatalysis: more than speed. Nat. Mater. 2009;8:87–88. doi: 10.1038/nmat2363. [DOI] [PubMed] [Google Scholar]
- 94.Hu E.L., Davis S.M., Davis R., Scher E. Applications: catalysis by nanostructured materials. In: Roco M.C., Mirkin C.A., Hersam M.C., editors. Nanotechnology Research Directions for Societal Needs in 2020. Springer; Berlin, Germany: 2010. pp. 341–360. [Google Scholar]
- 95.Schlogl R., Abd Hamid S.B., Nanocatalysis Mature science revisited of something really new? Angew. Chem. Int. Ed. 2004;43:1628–1637. doi: 10.1002/anie.200301684. [DOI] [PubMed] [Google Scholar]
- 96.Zhou B., Balee R., Groenendaal R. Nanoparticle and nanostructure catalysts: technologies and markets. Nanotechnol. Law Bus. 2005;2:222–229. [Google Scholar]
- 97.Pacchioni G. Nanocatalysis: staying put. Nat. Mater. 2009;8:167–168. doi: 10.1038/nmat2394. [DOI] [PubMed] [Google Scholar]
- 98.Olveira S., Forster S.P., Seeger S. Nanocatalysis: academic discipline and industrial realities. J. Nanotechnol. 2014 19 pages. [Google Scholar]
- 99.Chaturvedi S., Dave P.N., Shah N.K. Applications of nano-catalyst in new era. J. Saudi Chem. Soc. 2012;16:307–325. [Google Scholar]
- 100.Rao P.N. 2010. Nanocatalysis: Applications in the Chemical Industry.http://www.nanowerk.com/spotlight/spotid=18846.php [Google Scholar]
- 101.Astruc D., Lu F., Aranzaes J.R. Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005;44:7852–7872. doi: 10.1002/anie.200500766. [DOI] [PubMed] [Google Scholar]
- 102.Shylesh S., Schunemann V., Thiel W.R. Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010;49:3428–3459. doi: 10.1002/anie.200905684. [DOI] [PubMed] [Google Scholar]
- 103.Manbeck K.A., Musselwhite N.E., Carl L.M., Kauffman C.A., Lyons O.D., Navin J.K., Marsh A.L. Factors affecting activity and selectivity during cyclohexanone hydrogenation with colloidal platinum nanocatalysts. Appl. Catal., A. 2010;384:58–64. [Google Scholar]
- 104.Somorjai G.A., Frei H., Park J.Y. Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J. Am. Chem. Soc. 2009;131:16589–16605. doi: 10.1021/ja9061954. [DOI] [PubMed] [Google Scholar]
- 105.Tang S.Y., Bourne R.A., Smith R.L., Poliak M. The 24 principles of green engineering and green chemistry: improvements productively. Green Chem. 2008;10:268–269. [Google Scholar]
- 106.Polshettiwar V., Varma R.S. Green chemistry by nanocatalysis. Green Chem. 2010;12:743–754. [Google Scholar]
- 107.Glaser J.A. Green chemistry with nanocatalysts. Clean technEnviron. Policy. 2012;14:513–520. [Google Scholar]
- 108.Kalidindi S.B., Jagirdar B.R. Nanocatalysis and prospects of green chemistry. Chem. Sus. Chem. 2012;5:65–75. doi: 10.1002/cssc.201100377. [DOI] [PubMed] [Google Scholar]
- 109.Bhowmick A.K., Stephens H.L. second ed. CRC-Press; Boca Raton, US: 2000. Handbook of Elastomers. [Google Scholar]
- 110.http://cipet.gov.in/plastics statics.html
- 111.Zannikos F., Kalligeros S., Anastopoulos G., Lois E. Converting biomass and waste plastic to solid fuel briquettes. J. Renew. Energy. 2013;2013 9 pages. [Google Scholar]
- 112.Heikkinen J.M., Hordijk J.C., de Jong W., Spliethoff H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis. 2004;71:883–900. [Google Scholar]
- 113.Jung S.H., Cho M.H., Kang B.S., Kim J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010;91:277–284. [Google Scholar]
- 114.Ahmad I., Khan M.I., Ishaq M., Khan H., Gul K., Ahmad W. Catalytic efficiency of some novel nanostructured heterogeneous solid catalysts in pyrolysis of HDPE. Polym. Degrad. Stab. 2013;98:2512–2519. [Google Scholar]
- 115.Park S.S., Seo D.K., Lee S.H., Yu T.U., Hwang J. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J. Anal. Appl. Pyrolysis. 2012;97:29–38. [Google Scholar]
- 116.Aboulkas A., El harfi K., El Bouadili A. Thermal degradation behaviors of polyethylene and polypropylene Part I: pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010;51:1363–1369. [Google Scholar]
- 117.Hong S.J., Oh S.C., Lee H.P., Kim H.T., Yoo K.O. A study on the pyrolysis characteristics of poly(vinyl chloride) J. Korean Inst. Chem. Eng. 1999;37:515–521. [Google Scholar]
- 118.Abnisa F., Daud W.M.A.W., Sahu J.N. Pyrolysis of mixtures of palm shell and polystyrene: an optional method to produce a high-grade of pyrolysis oil. Environ. Prog. Sustain. Energy. 2014;33:1026–1033. [Google Scholar]
- 119.Othman N., Basri N.E.A., Yunus M.N.M., Sidek L.M. International Conference on Construction and Building Technology. 2008. Determination of physical and chemical characteristics of electronic plastic waste (Ep-Waste) resin using proximate and ultimate analysis method; pp. 169–180. [Google Scholar]
- 120.Ashworth D.C., Elliott P., Toledano M.B. Waste incineration and adverse birth and neonatal outcomes: a systematic review. Environ. Int. 2014;69:120–132. doi: 10.1016/j.envint.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 121.Cleetus C., Thomas S., Varghese S. Synthesis of petroleum-based fuel from waste plastics and performance analysis in a CI engine. J. Energy. 2013;2013 10 pages. [Google Scholar]
- 122.The ImpEE Project at the university of Cambridge, Recycling of Plastics. © University of Cambridge; 2005. [Google Scholar]
- 123.Straus S., Madorsky S.L. Pyrolysis of styrene, acrylate, and isoprene polymers in a vacuum. J. Res. Natl. Bur. Stand. 1953;50:165–176. [Google Scholar]
- 124.Ratnasari D.K., Nahil M.A., Williams P.T. Catalytic pyrolysis of waste plastics using staged catalysis forproduction of gasoline range hydrocarbon oils. J. Anal. Appl. Pyrolysis. 2017;124:631–637. [Google Scholar]
- 125.Walendziewski J., Steininger M. Thermal and catalytic conversion of waste polyolefins. Catal. Today. 2001;65:323–330. [Google Scholar]
- 126.Mangesh V.L., Padmanabhan S., Ganesan S., Rahul D.P., Reddy T.D.K. Prospects of pyrolysis oil from plastic waste as fuel for diesel engines: a review, Frontiers in Automobile and Mechanical Engineering. IOP Conf. Ser. Mater. Sci. Eng. 2017;197:012027 1–5. [Google Scholar]
- 127.Mani M., Subash C., Nagarajan G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic Oil. Appl. Therm. Eng. 2009;29:2738–2744. [Google Scholar]
- 128.Ozcanli M. Light and heavy phases derived from waste polyethylene by thermal cracking and their usage as fuel in direct injection diesel engine. J. Sci. Ind. Res. (India) 2013;72:198–202. [Google Scholar]
- 129.Kaimal V.K., Vijayabalan P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Convers. Manag. 2015;105:951–956. [Google Scholar]
- 130.Sarker M., Kabir A., Rashid M.M., Molla M., Din Mohammad A.S.M. Waste polyethylene terephthalate (PETE-1) conversion into liquid fuel. J. Fundam. Renew. Energy Appl. 2011;1:1–5. [Google Scholar]
- 131.Syamsiro M., Saptoadi H., Norsujianto T., Noviasri P., Cheng S., Alimuddin Z., Yoshikawa K. Fuel oil production from Municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Proc. 2014;47:180–188. [Google Scholar]
- 132.Kumar S., Singh R.K. Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz. J. Chem. Eng. 2011;28:659–667. [Google Scholar]
- 133.Desai S.B., Galage C.K. Production and analysis of pyrolysis oil from waste plastic in Kolhapur city. Int. J. Eng. Res. Gen. Sci. 2015;3:590–595. [Google Scholar]
- 134.Pinto F., Costa P., Gulyurtlu I., Cabrita I. Pyrolysis of plastic wastes 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrolysis. 1998;51:39–55. [Google Scholar]
- 135.Ahmad I., Khan M.I., Khan H., Ishaq M., Tariq R., Gul K., Ahmad W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy. 2015;12:663–671. [Google Scholar]
- 136.Mani M., Nagarajan G., Sampath S. Characterization and effect of using waste plastic oil and diesel fuel blends in compression ignition engine. Energy. 2011;36:212–219. [Google Scholar]
- 137.Boundy B., Diegel S.W., Wright L., Davis S.C. fourth ed. Oak Ridge National Laboratory; US: 2011. Biomass Energy Data Book. [Google Scholar]
- 138.Frigo S., Seggiani M., Puccini M., Vitolo S. Liquid fuel production from waste tyre pyrolysis and its utilisation in a diesel engine. Fuel. 2014;116:399–408. [Google Scholar]
- 139.Mukherjee M.K., Thamotharan P.C. Performance and emission test of several blends of waste plastic oil with diesel and ethanol on four stroke twin cylinder diesel engine. IOSR J. Mech. Civ. Eng. 2014;11 2278-1684. [Google Scholar]
- 140.Nileshkumar K.D., Jani R.J., Patel T.M., Rathod G.P. Effect of blend ratio of plastic pyrolysis oil and diesel fuel on the performance of single cylinder CI engine. IJSTE - Int. J. Sci. Technol. Eng. 2015;1:2349–2784. [Google Scholar]
- 141.Lee S., Yoshida K., Yoshikawa K. Application of waste plastic pyrolysis oil in a direct injection diesel engine: for a small scale non-grid electrification. Energy Environ. Res. 2015;5:18–32. [Google Scholar]
- 142.Jindaporn J., Lertsatitthanakorn C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Process Eng. 2014;69:1437–1442. [Google Scholar]
- 143.Lopez G., Olazar M., Artetxe M., Amutio M., Elordi G., Bilbao J. Steam activation of pyrolytic tyre char at different temperatures. J. Anal. Appl. Pyrolysis. 2009;85:539–543. [Google Scholar]
- 144.Williams P.T., Williams E.A. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuel. 1998;13:188–196. [Google Scholar]
- 145.Chen D., Yin L., Wang H., He P. Pyrolysis technologies for municipal solid waste: a review. Waste Manag. 2014;34:2466–2486. doi: 10.1016/j.wasman.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Lopez A., Marcod I., Caballero B.M., Laresgoiti M.F., Adrados A., Torres A. Pyrolysis of municipal plastic waste II: influence of raw material composition under catalytic conditions. Waste Manag. 2011;31:1973–1983. doi: 10.1016/j.wasman.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 147.Miskolczi N., Angyal A., Bartha L., Valkai I. Fuel by pyrolysis of waste plastics from agricultural and packaging sectors in a pilot scale reactor. Fuel Process. Technol. 2009;90:1032–1040. [Google Scholar]
- 148.Fernandez Y., Arenillas A., Menandez J.A. Advances in Induction and Microwave Heating of mineral and Organic Materials. InTech; Spain: 2011. Microwave heating applied to pyrolysis. [Google Scholar]
- 149.Lam S.S., Chase H.A. A review on waste to energy processes using microwave pyrolysis. Energies. 2012;5:4209–4232. [Google Scholar]
- 150.Mastral F.J., Esperanza E., Garcia P., Juste M. Pyrolysis of high-density polyethylene in a fluidized bed reactor: influence of the temperature and residence time. J. Anal. Appl. Pyrolysis. 2001;63:1–15. [Google Scholar]
- 151.Sobko A.A. Generalized Vander Waals-berthelot equation of state. Dokl. Phys. 2008;53:416–419. [Google Scholar]
- 152.Murata K., Sato K., Sakata Y. Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrolysis. 2004;71:569–589. [Google Scholar]
- 153.Audisio G., Bertini F., Beltrame P.L., Carniti P. Catalytic degradation of polyolefins. Macromol. Symp. 1992;57:191–209. [Google Scholar]
- 154.Guohua L., Tomohiko S., Satomi Y., Kunio K. Catalytic degradation of high density polyethylene and polypropylene into liquid fuel in a powder-particle fluidized bed. Polym. Degrad. Stab. 2000;70:97–102. [Google Scholar]
- 155.Lin Y.H., Yang M.H. Tertiary recycling of polyethylene waste by fluidized-bed reactions in the presence of various cracking catalysts. J. Anal. Appl. Pyrolysis. 2008;83:101–109. [Google Scholar]
- 156.Scheirs J., Kaminsky W. John Willy & Sons, Ltd.; 2006. Feedstock Recycling of Waste Plastics. [Google Scholar]
- 157.Akpanudoh N.S., Gobin K., Manos G. Catalytic degradation of plastic waste to liquid fuel over commercial cracking catalysts: effect of polymer to catalyst ratio/acidity content. J. Mol. Catal. 2005;235:67–73. [Google Scholar]
- 158.Buekens A.G., Huang H. Catalytic plastics cracking for recovery of gasoline range hydrocarbons from municipal plastic wastes. Resour. Conserv. Recycl. 1998;23:163–181. [Google Scholar]
- 159.Venuto P., Landis P. Zeolite catalysis in synthetic organic chemistry. Adv. Catal. 1968;18:259–267. [Google Scholar]
- 160.Lee K.H. Thermal and catalytic degradation of pyrolytic oil from pyrolysis of municipal plastic wastes. J. Anal. Appl. Pyrolysis. 2009;85:372–379. [Google Scholar]
- 161.Lin Y.H. Conversion of waste plastic to hydrocarbon by catalytic zeolited pyrolysis. J. Chin. Inst. Environ. Eng. 2000;10:271–277. [Google Scholar]
- 162.Singh B., Sharma N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008;93:561–584. [Google Scholar]
- 163.Corma A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995;95:559–614. [Google Scholar]
- 164.Lonyi F., Valyon J. On the interpretation of theNH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mater. 2001;47:293–301. [Google Scholar]
- 165.Park D.W., Hwang E.Y., Kim J.R., Choi J.K., Kim Y.A., Woo H.C. Catalytic degradation of polyethylene over solid acid catalysts. Polym. Degrad. Stab. 1999;65:193–198. [Google Scholar]
- 166.Pinto F., Costa P., Gulyurtlu I., Cabrita I. Pyrolysis of plastic wastes 2. Effect of catalyst on product yield. J. Anal. Appl. Pyrolysis. 1999;51:57–71. [Google Scholar]
- 167.Wall L.L., Madorsky S.L., Brown D.W., Straus S. The depolymerization of polymethylene and polyethylene. J. Am. Chem. Soc. 1954;76:343–347. [Google Scholar]
- 168.Sriningsih W., Saerodji M.G., Trisunaryanti W., Triyono R. Armunanto, Falah I.I. Fuel production from LDPE plastic waste over natural zeolite supported Ni, Ni-Mo, Co and Co-Mo metals. Proc. Environ. Sci. 2014;20:215–224. [Google Scholar]
- 169.International Zeolite Association. 2005. [Google Scholar]
- 170.Degnan T.F., Jr. Applications of zeolites in petroleum refining. Top. Catal. 2000;13:349–356. [Google Scholar]
- 171.Manos G. Catalytic degradation of plastic waste to fuel over microporus materials. In: Scheirs J., Kaminsky W., Orgs, editors. Feedstock Recycling and Pyrolysis of Waste Plastics. John Wiley & Sons; Hoboken: 2006. pp. 193–208. [Google Scholar]
- 172.Hwang E.Y., Kim J.R., Choi J.K., Woo H.C., Park D.W. Performance of acid treated natural zeolites in catalytic degradation of polypropylene. J. Anal. Appl. Pyrolysis. 2002;62:351–364. [Google Scholar]
- 173.Rehan M., Miandad R., Barakat M.A., Ismail I.M.I., Almeelbi T., Gardy J., Hassanpour A., Khan M.Z., Demirbas A., Nizami A.S. Effect of zeolite catalysts on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017;119:162–175. [Google Scholar]
- 174.Li X., Shen B., Guo Q., Gao J. Effects of large pore zeolite additions in the catalytic pyrolysis catalyst on the light olefins production. Catal. Today. 2007;125:270–277. [Google Scholar]
- 175.Anunziata O.A., Pierella L.B. Conversion of polyethylene into aromatic hydrocarbons using MEL and BEA zeolites. Stud. Surf. Sci. Catal. 1999;125:481–488. [Google Scholar]
- 176.Ojha D.K., Vinu R. Resource recovery via catalytic fast pyrolysis of polystyrene using zeolites. J. Anal. Appl. Pyrolysis. 2015;113:349–359. [Google Scholar]
- 177.Garforth A.A., Lin Y.H., Sharratt P.N., Dwyer J. Catalytic polymer degradation for producing hydrocarbons over zeolites. Stud. Surf. Sci. Catal. 1999;121:197–202. [Google Scholar]
- 178.Songip A.R., Masuda T., Kuwahara H., Hashimoto K. Kinetic studies for catalytic cracking of heavy oil from waste plastics over REY zeolite. Energy and Fuels. 1994;8:131–135. [Google Scholar]
- 179.Mastral J.F., Berrueco C., Gea M., Ceamanos J. Catalytic degradation of high density polyethylene over nanocrystalline HZSM-5 Zeolite. Polym. Degrad. Stab. 2006;91:3330–3338. [Google Scholar]
- 180.Pierella L.B., Renzini S., Anunziata O.A. Catalytic degradation of high density polyethylene over microporous and mesoporous materials. Materials. 2005;81:155–159. [Google Scholar]
- 181.Kima J.R., Kima Y.A., Yoona J.H., Parka D.W., Woob H.C. Catalytic degradation of polypropylene: effect of dealumination of clinoptilolite catalyst. Polym. Degrad. Stab. 2002;75:287–294. [Google Scholar]
- 182.You Y.S., Shim J.S., Kim J.H., Seo G.l. Liquid-phase degradation of polyethylene wax over mordenite catalysts with different Si-Al molar ratios. Catal. Lett. 1999;59:221–227. [Google Scholar]
- 183.Humphries A., Wilcox J. Zeolite components and matrix composition determine FCC catalyst performance. Oil Gas J. 1989;87 [Google Scholar]
- 184.Rajagopalan K., Habib E., Jr. Understand FCC matrix technology. Hydrocarb. Process. 1992;71 [Google Scholar]
- 185.Marcilly C.R. Where and how shape selectivity of molecular sieves operates in refining and petrochemistry catalytic processes. Top. Catal. 2000;13:357–366. [Google Scholar]
- 186.Zahmakiran M., Ozkar S. Metal nanoparticles in liquid phase catalysis from recent advances to future goals. Nanoscale. 2011;3:3462–3481. doi: 10.1039/c1nr10201j. [DOI] [PubMed] [Google Scholar]
- 187.Zach M., Hagglund C., Chakarov D., Kasemo B. Nanoscience and nanotechnology for advanced energy systems. Curr. Opin. Solid State Mater. Sci. 2006;10:132–143. [Google Scholar]
- 188.Campelo J.M., Luna D., Luque R., Marinas J.M., Romero A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chem. Sus. Chem. 2009;2:18–45. doi: 10.1002/cssc.200800227. [DOI] [PubMed] [Google Scholar]
- 189.Teunissen W., Bol A.A., Geus J.W. Magnetic catalyst bodies. Catal. Today. 1999;48:329–336. [Google Scholar]
- 190.Campbell C.T. The active site in nanoparticle gold catalysis. Science. 2004;306:234–235. doi: 10.1126/science.1104246. [DOI] [PubMed] [Google Scholar]
- 191.Haruta M. When gold is not noble: catalysis by nanoparticles. Chem. Rec. 2003;3:75–87. doi: 10.1002/tcr.10053. [DOI] [PubMed] [Google Scholar]
- 192.Ma Z., Dai S. Development of novel supported gold catalysts: a materials perspective. Nano Research. 2011;4:3–32. [Google Scholar]
- 193.Jin Z., Xiao H., Zhou W., Zhang D., Peng X. Synthesis and hydrogenation application of Pt–Pd bimetallic nanocatalysts stabilized by macrocycle-modified dendrimer. R. Soc. Open Sci. 2017;4:171414. doi: 10.1098/rsos.171414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhao S., Stocks A., Rasimick B., More K., Xu H. Highly active, durable dispersed iridium nanocatalysts for PEM water electrolyzers. J. Electrochem. Soc. 2018;165:F82–F89. [Google Scholar]
- 195.Widegren J.A., Finke R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. 2003;198:317–341. [Google Scholar]
- 196.Chang J.S., Jhung S.H., Hwang Y.K., Park S.E., Hwang J.S. Syntheses and applications of nanocatalysts based on nanoporousmaterials. Int. J. Nanotechnol. 2006;3:150–180. [Google Scholar]
- 197.You Y.S., Kim J.H., Seo G. Liquid-phase catalytic degradation of polyethylene wax over MFI zeolites with different particle sizes. Polym. Degrad. Stab. 2000;70:365–371. [Google Scholar]
- 198.Le X., Bi Y., Zhou W., Chen H., Hu J. Catalytic fast pyrolysis of cellulose by integrating dispersed nickel catalyst with HZSM-5 zeolite. IOP Conf. Ser. Earth Environ. Sci. 2018;108 [Google Scholar]
- 199.Serrano D., Aguado J., Escola J., Rodrıguez J. Nanocrystalline ZSM-5: a highly active catalyst for polyolefin feedstock recycling. Stud. Surf. Sci. Catal. 2002;142:77–84. [Google Scholar]
- 200.Serrano D.P., Aguado J., Escola J.M., Rodríguez J.M. Influence of nanocrystalline HZSM-5 external surface on the catalytic cracking of polyolefins. J. Anal. Appl. Pyrolysis. 2005;74:353–360. [Google Scholar]
- 201.Seo Y.H., Lee K.H., Shin D.H. Investigation of catalytic degradation of high density, polyethylene by hydrocarbon group type analysis. J. Anal. Appl. Pyrolysis. 2003;70:383–398. [Google Scholar]
- 202.Hernandez M.D.R., Gomez A., Garcıa A.N., Agullo J., Marcilla A. Effect of the temperature in the nature and extension of the primary and secondary reactions in the thermal and HZSM-5 catalytic pyrolysis of HDPE. Appl. Catal. Gen. 2007;317:183–194. [Google Scholar]
- 203.Lin Y.H., Yen H.Y. Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym. Degrad. Stab. 2005;89:101–108. [Google Scholar]
- 204.Obeid F., Zeaiter J., Al-Muhtaseb A.H., Bouhadir K. Thermo-catalytic pyrolysis of waste polyethylene bottles in a packed bed reactor with different bed materials and catalysts. Energy Convers. Manag. 2014;85:1–6. [Google Scholar]
- 205.Garfoth A.A., Lin Y.H., Sharratt P.N., Dwyer J. Production of hydrocarbons by catalytic degradation of high density polyethylene in a laboratory fluidized bed reactor. Appl. Catal. Gen. 1998;169:331–342. [Google Scholar]
- 206.Aguado J., Serrano D.P., Sotelo J.L., Van Grieken R., Escola J.M. Influence of the operating variables on the catalytic conversion of a polyolefin mixture over HMCM-41 and nanosized HZSM-5. Ind. Eng. Chem. Res. 2001;40:5696–5704. [Google Scholar]
- 207.Anastas P.T., Zimmerman J.B. Design through the 12 principles of green engineering. Environ. Sci. Technol. 2003;37:94A–101A. doi: 10.1021/es032373g. [DOI] [PubMed] [Google Scholar]