Abstract
Pouchitis is a frequent complication in ulcerative colitis patients after proctocolectomy with ileal pouch–anal anastomosis. It is an unspecific inflammation of the pouch with unknown aetiology. First-line treatment for acute and chronic pouchitis is antibiotics. Some cases of severe chronic refractory pouchitis may benefit from biological treatment. Anti-tumour necrosis factor should be recommended as the first option, leaving the new biologicals for multirefractory patients. Permanent ileostomy may be an option in severe cases, after failure of medical treatment. Prophylaxis therapy with a probiotic mixture is recommended after the first episode of pouchitis, whereas it is not clear whether probiotics are useful for all patients after surgery. Here, we present a case report and review the treatment options in different forms of pouchitis.
Keywords: Ulcerative colitis, pouchitis, probiotics, antibiotics, anti-TNF
Case report
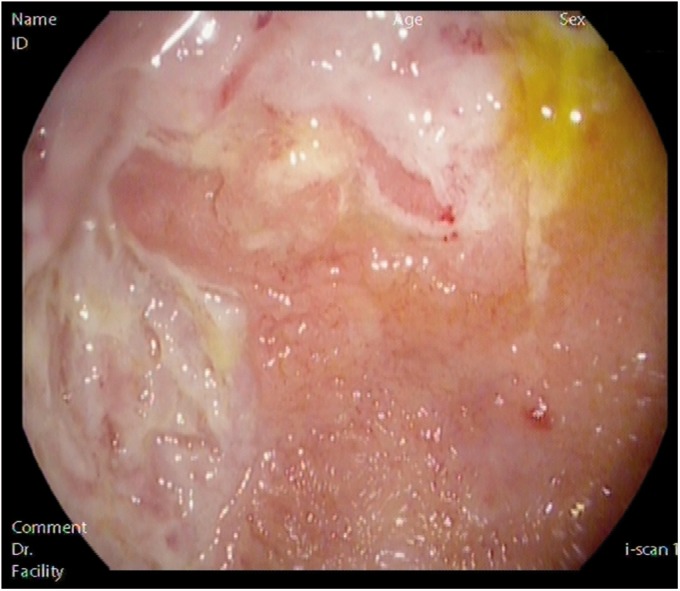
A 40-year-old man underwent proctocolectomy with ileal pouch–anal anastomosis (IPAA) for refractory ulcerative colitis (UC), maintaining remission for 3 years after surgery. At this point, he developed abdominal pain and around 15 loose bloody stools per day. Pouch endoscopy and histopathological analysis were consistent with pouchitis (Figure 1). Antibiotics, probiotics, budesonide and infliximab were given with no response; adalimumab was then started and symptoms were controlled with this drug for 3 years. After that, clinical relapse motivated adalimumab intensification, without response; therefore, the patient swapped to vedolizumab. Since the patient has always refused permanent ileostomy, he has recently started ustekinumab therapy, being aware of the lack of strong evidence regarding its efficacy.
Figure 1.
Endoscopic image of pouchitis.
Diagnosis
Despite advances in medical treatment for UC, between 6 and 15% of patients eventually require surgery due to refractory disease, dysplasia or colorectal cancer (CRC).1 Proctocolectomy with IPAA has been considered the first-line surgical treatment since 19892 and pouchitis is the most frequent complication, affecting almost one-half of patients within 5 years after surgery.3 Pouchitis is an unspecific inflammation of the ileal reservoir whose pathogenesis is still unknown. In order to diagnose pouchitis, clinical manifestations, as well as compatible endoscopic and histological findings, are required.4 After proctocolectomy with IPAA, patients usually have four to eight bowel movements, and about 700 cc of soft or liquid stool every day, compared with lower rates in healthy subjects. The most typically reported symptoms of pouchitis are watery diarrhoea, abdominal pain or cramps, tenesmus, urgency, faecal incontinence, fever and, less frequently, extraintestinal manifestations.5 Patients can also report rectal bleeding, but this is more often related to cuffitis than to pouchitis. Nevertheless, this clinical setting is not specific to pouchitis, and this is why the diagnosis has to be confirmed by endoscopic and histological abnormalities, allowing us to rule out other entities such as irritable pouch syndrome or concurrent surgery-related mechanical conditions. Pouch endoscopy should include not only the careful examination of the pouch, but also the afferent ileal limb, the anastomosis and the rectal cuff (if present). Taking into account the fact that some patients develop a stenosis of the pouch–anal anastomosis, doctors usually choose a gastroscope to perform the endoscopy, due to its flexibility and reduced diameter.6 The main endoscopic features of pouchitis include erythema, oedema, friability, haemorrhage, absent vascular pattern, erosions and ulcerations. Small ulcers confined to the anastomosis are frequent, but they do not necessarily establish the diagnosis of pouchitis or Crohn’s disease.7 Thus, this area should be avoided when taking biopsies as its foreign-body granulomas can be misunderstood as Crohn’s disease of the pouch. In spite of the normal appearance of the mucosa and according to some experts, we recommend that biopsies are taken from the pouch and the afferent limb, in order to distinguish between pouchitis and Crohn’s disease, Citomegalovirus (CMV) infection, ischaemia and dysplasia.
Microscopic findings of pouchitis include chronic and acute inflammation changes such as villous atrophy, crypt distortion and chronic inflammatory infiltrates, together with crypt abscesses, mucosal ulceration and neutrophilic infiltrates.8
There are different diagnostic criteria for pouchitis, but the most widely used index is the Pouchitis Disease Activity Index (PDAI) (Table 1), which includes clinical, endoscopic and histological parameters. A modified index without histological criteria has been developed (mPDAI) due to the PDAI’s complexity in clinical practice. Both indices have high correlations with each another, and both PDAI ≥ 7 and mPDAI ≥ 5 establish a diagnosis of pouchitis.9,10
Table 1.
Pouchitis Disease Activity Index.
| Variable | Score |
|---|---|
| 1. Clinical criteria | |
| Postoperative stool frequency | |
| Usual stool frequency | 0 |
| One to two stools/day more than is postoperatively usual | 1 |
| Three or more stools/day than is postoperatively usual | 2 |
| Rectal bleeding | |
| None or rare | 0 |
| Present daily | 1 |
| Faecal urgency or abdominal cramping | |
| None | 0 |
| Occasional | 1 |
| Usual | 2 |
| Fever (temperature ≥ 38℃) | |
| No | 0 |
| Yes | 1 |
| 2. Endoscopic criteria | |
| Oedema | 1 |
| Granularity | 1 |
| Friability | 1 |
| Loss of vascular pattern | 1 |
| Mucosal exudate | 1 |
| Ulcerations | 1 |
| 3. Histological criteria | |
| Polymorphonuclear infiltration | |
| Mild | 1 |
| Moderate and crypt abscess | 2 |
| Severe and crypt abscess | 3 |
| Ulceration due to a field with low increase | |
| <25% | 1 |
| 25–50% | 2 |
| >50% | 3 |
Pouchitis is defined as Pouchitis Disease Activity Index ≥ 7.
Natural history of pouchitis
Multiple studies suggest that the first episode of pouchitis most probably occurs in the first year following surgery. The course of pouchitis may be similar to that of UC, since it can appear as a chronic condition characterized by continuous inflammation that displays episodic symptomatic exacerbations. In some patients, it can also be limited to one isolated episode or very infrequent flares.
The condition may be classified into distinct entities depending on its evolution. Regarding the duration of symptoms, it is classified as acute (<4 weeks) or chronic (>4 weeks), and regarding its course, pouchitis can be infrequent (less than three episodes per year), recurrent (more than three episodes per year) or continuous. After a single episode of acute pouchitis, 39% of patients will respond to antibiotic treatment without recurrence while 61% will have at least one recurrent episode.11
Several studies have identified different risk factors for pouchitis. Thus, smoking has been pointed out as a risk factor for acute pouchitis, while it seems to protect against chronic pouchitis. Some clinical factors related to chronic pouchitis are extraintestinal manifestations,12,13 an elevated platelet count and a long duration of IPAA14 (Table 2).
Table 2.
Risk factors associated with pouchitis.
| Extraintestinal manifestations |
| Thrombocytosis |
| Long duration of ileal pouch–anal anastomosis |
| Non-smoker |
| Primary sclerosing cholangitis |
| Anti-neutrophil cytoplasmic antibodies |
| Postoperative non-steroidal anti-inflammatory drug usage |
According to the response to antibiotics, patients are divided into responsive (good response), dependent (need for maintenance therapy) or refractory (no response) groups. Previous studies have shown that refractory or frequently relapsing pouchitis appears in 5–19% of patients with acute pouchitis.
Considering the aetiology, pouchitis is classified as idiopathic or secondary to infections (e.g. CMV or Clostridium difficile), surgical complications, non-steroidal anti-inflammatory drug intake or other autoimmune conditions (e.g. primary sclerosing cholangitis or Crohn's disease of the pouch).
The cumulative probability of pouch failure, and consequently the need for definitive ileostomy and excision of the pouch, ranges from 3–15% in large series. This often occurs due to refractory pouchitis.15
Pouchitis may be complicated by fistulae, abscesses and stricture of the pouch–anal anastomosis. Another important complication is the development of a pouch neoplasia, which is defined by the appearance of low- or high-grade dysplasia, or CRC at the anal transitional zone, cuff, pouch body or afferent limb.4,16 In the Cleveland Clinic, a study of 3203 patients with IPAA revealed cumulative incidences of pouch neoplasia of 1.3 and 5.1% at 10 and 25 years after surgery, respectively.17
The presence of precolectomy dysplasia or CRC has been shown to increase the risk of getting pouch neoplasia by 4- and 25-fold, respectively. This hazard is also raised in patients with concomitant primary sclerosing cholangitis, a family history of CRC and chronic pouch inflammation secondary to different entities (e.g. chronic pouchitis). The best management and surveillance strategies in these patients remain uncertain, but the performance of regular surveillance biopsies of the pouch may be recommended6 (Table 3). This strategy is recommended even in patients with mucosectomy, since it does not avoid the risk of pouch neoplasia.18 The efficacy of surveillance is not clear in cases of low-grade dysplasia. Wu et al. have reported that it may be recommended to take biopsies in these patients at 3–6-month intervals. If dysplasia disappears, it could be reasonable to lengthen this periodicity from several months to 1 year, but if the patient has risk factors for progression, surgical intervention may be needed.19
Table 3.
Patients who need regular surveillance.
| Precolectomy colon dysplasia or colorectal cancer |
| Primary sclerosing cholangitis |
| Family history of colorectal cancer |
| Chronic pouchitis |
| Chronic cuffitis |
| Crohn's disease of the pouch |
Treatment dilemmas
Should a first pouchitis episode be prevented after surgery?
Due to the high risk of developing pouchitis after IPAA, a great dilemma for gastroenterologists is to decide whether prophylaxis treatment will be necessary in all patients after surgery. In a well-designed trial of probiotics for primary prevention of pouchitis, 40 patients were randomized to a specific probiotic mixture with eight bacterial strains that included Lactobacilli (Lactobacillus casei, L. plantarum, L. acidophilus and L. delbrueckii ssp. bulgaricus), Bifidobacteria (Bifidobacterium longum, B. breve and B. infantis), and Streptococcus thermophilus or placebo. Of the patients treated with this probiotic mixture (currently called De Simone formulation), 10% developed pouchitis after 12 months, compared with 40% of the placebo-treated group (risk ratio (RR) 1.5, 95% confidence interval (CI) 1.02–2.1), and their quality of life was also better.20
The same probiotic mixture was evaluated in an open-label randomized study in 31 pouchitis patients, comparing it with a group of patients without any treatment. Despite no new case of pouchitis appearing in the patients undergoing treatment, there was no statistically significant difference (RR 1.10, 95% CI 0.89–1.36) because the rate of pouchitis in the control group was very low (8%).21
Regarding conventional drugs, an uncontrolled retrospective study with sulfasalazine 2 g showed efficacy. After 68 months, pouchitis developed in 15% of patients on sulfasalazine in comparison with 64.5% not taking any drug.22
With these results, and as figures from the probiotic mixture trial have not been replied and other drug studies are not of high quality, prophylaxis in all patients cannot be recommended. A recommendation could be to treat only patients with a high risk of pouchitis after surgery with a probiotic mixture.
How to treat acute pouchitis?
Antibiotics are the first-line treatment for acute pouchitis, with response rates near to 80%. Both metronidazole and ciprofloxacin are effective in pouchitis patients. However, randomized trials are scarce. Only one head-to-head study has compared these two antibiotics. Over 14 days, 16 patients were randomized for ciprofloxacin 1 g/day (n = 7) or metronidazole 20 mg/kg/day (n = 9). PDAI scores improved with both antibiotics, but were more significant in those that had been treated with ciprofloxacin (p = 0.002). Regarding specific items like symptom and endoscopic scores, again the benefits were higher with ciprofloxacin (p = 0.03). Another important finding of the study was observed regarding adverse events, because while no patients in the ciprofloxacin group were referred, those one in three metronidazole-treated patients reported adverse events.23 Other antibiotic agents, including amoxicillin-clavulanic acid and rifaximin, have been also investigated and showed some effect in case series with a limited number of patients. Despite some positive results with probiotic mixtures and budesonide, in our opinion, these treatments are only recommended in cases of intolerance or non-response to antibiotics.4
Is prophylaxis treatment necessary after a first pouchitis episode?
Between 7 and 20% of patients who suffer a first pouchitis episode will relapse and develop chronic pouchitis. This is the reason why prophylaxis treatment with a probiotic mixture that includes Lactobacilli (L. casei, L. plantarum, L. acidophilus and L. delbrueckii ssp. bulgaricus), Bifidobacteria (B. longum, B. breve and B. infantis) and Streptococcus thermophilus is indicated. Robust data have come from a randomized controlled trial performed in 40 patients who achieved remission with antibiotics, and were randomized to a probiotic mixture (dose of 6 g/day) or placebo for 9 months for the prevention of new episodes. All patients treated with placebo presented a new episode, while only 15% of those treated with the probiotic mixture developed chronic pouchitis.24 Compatible results were reported in a very similar trial performed with the same probiotic mixture.25 A joint analysis of both studies that included 76 patients showed that 85% of patients who received the probiotic mixture maintained remission compared with only 3% treated with placebo (RR 20.24, 95% CI 4.28–95.81).26 In open-label studies, the response rates to probiotic mixtures were not as high as in the randomized controlled trials. In a study of 31 patients with antibiotic-dependent pouchitis, who received a probiotic mixture as maintenance therapy after the induction of remission with ciprofloxacin, 81% had stopped the probiotic at 8 months because of a lack of efficacy or adverse effects.27 Different options like rifaximin or mesalamine did not show similar efficacy, so in all patients we recommend the use of a probiotic mixture after a first episode of pouchitis in order to prevent new episodes.
What is the treatment for chronic pouchitis?
Treatment with a combination of antibiotics for ≥ 4 weeks is the chosen next step in patients who do not responded to 2-week treatment with an antibiotic. Ciprofloxacin with metronidazole or rifaximin is the most recommended combination, based on well-designed studies that showed high rates of response (around 80%), but also increased risk of adverse effects due to prolonged use of antibiotics. Another problem is that a percentage of patients who respond initially can them lose their ability to respond and become antibiotic-dependent. In such cases, other therapies should be evaluated.28
In cases with an absence of response to antibiotic combination, the next step is to change this treatment to locally active steroids. An open study of 20 patients showed remission in 75% of patients treated with a high dose of budesonide (9 mg) for 8 weeks, together with improved quality of life. Beclomethasone dipropionate, at a higher dose than is usual (10 mg daily) for 8 weeks, was also effective in another open-label study in antibiotic-refractory patients.29
Do immunosuppressive or biological drugs play a role in chronic refractory pouchitis?
Due to an absence of data about the use of thiopurines or methotrexate for the treatment of chronic refractory pouchitis, we cannot recommend these drugs in such patients. Regarding cyclosporine, despite some cases having being treated with enemas, evidence is too scarce to recommend its use in clinical practice.
Regarding biological therapy, anti-tumour necrosis factor (TNF) drugs have been more frequently evaluated. In the absence of clinical trials, as with other drugs regarding chronic refractory pouchitis, open series have shown their potential utility in these patients. First reports of data from a small case series with infliximab were optimistic, with high short-term remission rates, but two larger series published from Ferrante et al. and Barreiro-de Acosta et al. have shown that after 1 year, response rates were around 50%.30,31 Data with adalimumab have been limited to patients who have failed infliximab, and after 1 year of treatment with this drug, 50% had avoided permanent ileostomy.32 In cases of pouchitis with anti-TNF treatment failure, vedolizumab and ustekinumab have shown efficacy in recent case series and, in our opinion, are alternative options in order to avoid permanent ileostomy.33,34
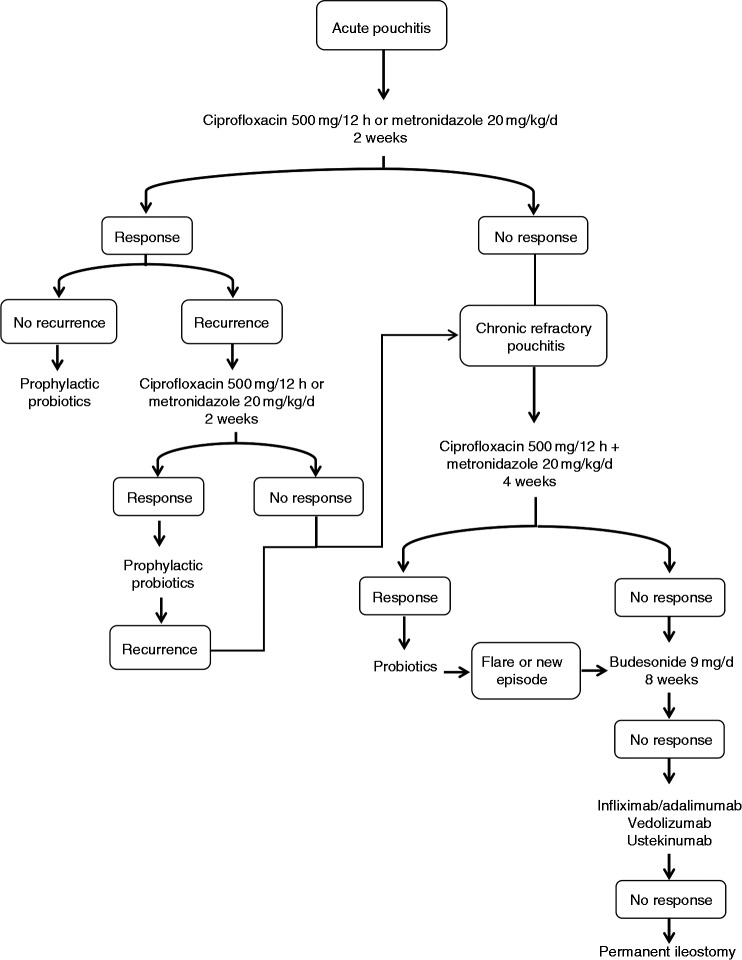
In Figure 2, we propose an algorithm for the treatment of pouchitis. Despite all these treatment options, around 25% of patients will still require permanent ileostomy.35
Figure 2.
Treatment algorithm for pouchitis.
Conclusions
In our opinion, pouchitis treatment represents one of the biggest gaps of knowledge in inflammatory bowel disease treatment, fundamentally due to a lack of well-designed studies because of the heterogeneity of patients and their limited numbers per centre. More specific studies are needed; until they become available the following of expert opinion recommendations from physicians with significant expertise remains a good option.
Acknowledgements
Author contributions: M.B.A., I.B.R. and C.C.S. drafted the review; M.B.A. and J.E.D.M. did the final review and wrote the final article.
Declaration of conflicting interests
M.B.A. has served as a speaker, consultant and advisory member for, or has received research funding from, MSD, Abbvie, Takeda and Janssen. I.B.R., C.C.S. and J.E.D.M. have nothing to disclose.
References
- 1.Parragi L, Fournier N, Zeitz J, et al. Colectomy rates in ulcerative colitis are low and decreasing: 10-year follow-up data from the Swiss IBD cohort study. J Crohns Colitis 2018; 12: 811–818. [DOI] [PubMed] [Google Scholar]
- 2.Williams NS. Restorative proctocolectomy is the first choice elective surgical treatment for ulcerative colitis. Br J Surg 1989; 76: 1109–1110. [DOI] [PubMed] [Google Scholar]
- 3.Ferrante M, Declerck S, De Hertogh G, et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis 2008; 14: 20–28. [DOI] [PubMed] [Google Scholar]
- 4.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 5.Shen B, Achkar JP, Lashner BA, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology 2001; 121: 261–267. [DOI] [PubMed] [Google Scholar]
- 6.Pardi DS, Shen B. Endoscopy in the management of patients after ileal pouch surgery for ulcerative colitis. Endoscopy 2008; 40: 529–533. [DOI] [PubMed] [Google Scholar]
- 7.Pemberton JH. The problem with pouchitis. Gastroenterology 1993; 104: 1209–1211. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd NA, Jass JR, Duval I, et al. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol 1987; 40: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc 1994; 69: 409–415. [DOI] [PubMed] [Google Scholar]
- 10.Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003; 46: 748–753. [DOI] [PubMed] [Google Scholar]
- 11.Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol 2013; 11: 1538–1549. [DOI] [PubMed] [Google Scholar]
- 12.Lohmuller JL, Pemberton JH, Dozois RR, et al. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg 1990; 211: 622–627. discussion 627–629. [PMC free article] [PubMed] [Google Scholar]
- 13.Hata K, Okada S, Shinagawa T, et al. Meta-analysis of the association of extraintestinal manifestations with the development of pouchitis in patients with ulcerative colitis. BJS Open 2019; 3: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreiro-de Acosta M, Gutierrez A, Rodríguez-Lago I, et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on pouchitis in ulcerative colitis. Part 1: epidemiology, diagnosis and prognosis. Gastroenterol Hepatol 2019; 42: 568–578. [DOI] [PubMed] [Google Scholar]
- 15.Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg 2003; 238: 605–614. discussion 614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan F, Shen B. Inflammation and neoplasia of the pouch in inflammatory bowel disease. Curr Gastroenterol Rep 2019; 21: 10–10. [DOI] [PubMed] [Google Scholar]
- 17.Kariv R, Remzi FH, Lian L, et al. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology 2010; 139: 806–812. 812.e1–2. [DOI] [PubMed] [Google Scholar]
- 18.M’Koma AE, Moses HL, Adunyah SE. Inflammatory bowel disease-associated colorectal cancer: proctocolectomy and mucosectomy do not necessarily eliminate pouch-related cancer incidences. Int J Colorectal Dis 2011; 26: 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XR, Remzi FH, Liu XL, et al. Disease course and management strategy of pouch neoplasia in patients with underlying inflammatory bowel diseases. Inflamm Bowel Dis 2014; 20: 2073–2082. [DOI] [PubMed] [Google Scholar]
- 20.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 2003; 124: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 21.Pronio A, Montesani C, Butteroni C, et al. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis 2008; 14: 662–668. [DOI] [PubMed] [Google Scholar]
- 22.Scaioli E, Sartini A, Liverani E, et al. Sulfasalazine in prevention of pouchitis after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Dig Dis Sci 2017; 62: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 23.Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis 2001; 7: 301–305. [DOI] [PubMed] [Google Scholar]
- 24.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119: 305–309. [DOI] [PubMed] [Google Scholar]
- 25.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004; 53: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Stroud AM, Holubar SD, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev 2015; 11: CD001176–CD001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen B, Brzezinski A, Fazio VW, et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther 2005; 22: 721–728. [DOI] [PubMed] [Google Scholar]
- 28.Mimura T, Rizzello F, Helwig U, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther 2002; 16: 909–917. [DOI] [PubMed] [Google Scholar]
- 29.Gionchetti P, Calabrese C, Calafiore A, et al. Oral beclomethasone dipropionate in chronic refractory pouchitis. J Crohns Colitis 2014; 8: 649–653. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante M, D’Haens G, Dewit O, et al. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis 2010; 16: 243–249. [DOI] [PubMed] [Google Scholar]
- 31.Barreiro-de Acosta M, García-Bosch O, Souto R, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis 2012; 18: 812–817. [DOI] [PubMed] [Google Scholar]
- 32.Barreiro-De Acosta M, García-Bosch O, Gordillo J, et al. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol 2012; 24: 756–758. [DOI] [PubMed] [Google Scholar]
- 33.Bär F, Kühbacher T, Dietrich NA, et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther 2018; 47: 581–587. [DOI] [PubMed] [Google Scholar]
- 34.Ollech JE, Rubin DT, Glick L, et al. Ustekinumab is effective for the treatment of chronic antibiotic-refractory pouchitis. Dig Dis Sci 2019; 64: 3596–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen B, Yu C, Lian L, et al. Prediction of late-onset pouch failure in patients with restorative proctocolectomy with a nomogram. J Crohns Colitis 2012; 6: 198–206. [DOI] [PubMed] [Google Scholar]