Abstract
Background
The magnitude and drivers of the risk of serious viral infections in Inflammatory Bowel diseases (IBD) are unclear.
Objective
The objective of this study was to assess the incidence and risk factors for systemic serious viral infections in IBD patients.
Methods
Using MICISTA, a database detailing prospective characteristics and complications of IBD, we identified patients that were followed for IBD in 2005–2014 outside the context of organ transplantation, HIV infection or chronic viral hepatitis. We estimated incidences of systemic serious viral infections, defined by the need for hospitalization or permanent organ damage. Standardized incidence ratios (SIRs) were calculated using the French hospital database. We performed a case-control study nested in MICISTA for assessing the role of exposure to IBD drugs and IBD clinical activity in the risk of developing infection.
Results
We identified 31 patients with serious viral infections among 2645 patients followed for 15,383 person-years. We observed 13 cases of cytomegalovirus, 10 Epstein–Barr virus, 5 varicella zoster virus and 3 herpes simplex virus infections. No deaths occurred. The incidence rate of infections in patients with IBD was 2.02/1000 person-years, and the SIR was 3.09 (95% confidence interval (CI), 1.98–4.20; p = 0.0002) in the study population. By multivariate analysis, increased risk of infection was associated with exposure to thiopurines (odds ratio (OR), 3.48; 95% CI, 1.36–8.90; p = 0.009), and clinically active IBD at onset of infection (OR, 3.35; 95% CI, 1.23–9.23; p = 0.02).
Conclusions
The incidence of systemic serious viral infections in patients with IBD is tripled compared to general population. Clinically active IBD and exposure to thiopurines are the main drivers of the risk.
Keywords: Viral infections, immune-suppressive therapy, thiopurines, cytomegalovirus, Epstein–Barr virus, hemophagocytic lymphohistiocytosis
Key Summary
Established knowledge
Most patients with Inflammatory Bowel diseases (IBD) are exposed for prolonged periods to various immunosuppressive drugs, but the respective role of IBD clinical activity and IBD drugs in promoting serious viral infection is unclear.
Significant findings of the study
The risk of systemic serious viral infection (SVI) was three-fold higher in our cohort compared with the general population, but the resulting individual absolute risks remained acceptable
The main and independent drivers of systemic SVI are clinically active IBD and exposure to thiopurines
Excess hospitalizations due to SVI contribute to the human and financial burden associated with management of IBD.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are lifetime diseases. Most of the patients with IBD are currently exposed for prolonged periods to immunosuppressive drugs, including small molecules (thiopurines and methotrexate) and biologics, mainly anti-tumor necrosis factor (TNF) agents.1
Serious infections are usually defined as infections that require hospitalization or result in death or permanent organ damage.2–4 Focusing on viral infections, patients with IBD may first develop serious viral infections (SVIs) that are not related to IBD activity or treatment. They may also develop SVI triggered by intestinal inflammation, mainly cytomegalovirus (CMV) colitis,5,6 and Epstein–Barr Virus (EBV) systemic reactivation.7 Finally, they may develop SVI attributable to the immunosuppressive action of IBD drugs, knowing that the magnitude of this effect may substantially differ among drugs.4
Clinical research on systemic viral infections in IBD has been mainly focused on herpes zoster.8–10 The promoting effect of immunosuppressive agents on serious infections, including SVI, has been also repeatedly reported.10–17 However, in these studies, the respective role of IBD clinical activity and IBD drugs has not been addressed. The aim of this study was to assess the incidence and risk factors of systemic SVI in patients with IBD, prospectively followed in a referral IBD center.
Methods
Data sources
MICISTA
MICISTA is an IBD-specific prospective electronic-form database dedicated to clinical research created by a senior gastroenterologist (J.C.) in 1994. All patients with a permanent diagnosis of IBD followed in our IBD unit are enrolled in the database at the time of the first face-to-face contact in the IBD unit. The following items are recorded upon entry in the database: socio-demographic details, initial (at diagnosis) and cumulative Montreal phenotype, smoking status, and history of IBD surgery. The database is then updated prospectively as long as patients are regularly or intermittently followed in our IBD unit. 18 A regular follow-up is defined as at least one face-to-face contact with the patient (visit or hospitalization) per calendar year in our IBD unit. At each contact in the IBD unit, clinical IBD activity is scored as follows: 0, no digestive symptoms; 1, mild symptoms that may be attributable to IBD activity, postoperative functional sequelae or associated irritable bowel syndrome; 2, symptoms that are attributable to IBD and are compatible with usual home and/or professional activities; 3, symptoms that are attributable to IBD and are not compatible with sustained home and/or professional activities; 4, hospitalization for IBD flare; 5, intestinal resection. At every face-to-face contact with the patient at the IBD unit, IBD treatment is scored as follows: 0, no IBD drug; 1, oral 5-amino-salicylates; 2, low doses of systemic corticosteroids (up to 10 mg a day of prednisone/prednisolone, 8 mg a day of methylprednisolone or 3 mg a day of budesonide); 4, high doses of systemic corticosteroids; 4, thiopurines or methotrexate; 5, anti-TNF agent; 6, vedolizumab or ustekinumab. At the end of each calendar year of follow-up, the annual clinical IBD activity of each patient is rated as the highest clinical score recorded within the calendar year, and the annual status of IBD treatment is rated as the highest score of IBD drugs used within the calendar year.
At each face-to-face contact with patients in the IBD unit, physicians are required to fill in the database with the date and type of the following events that have occurred in the time interval between the last and the current contact: IBD-related surgery, intestinal dysplasia or cancer, systemic cancer, and serious infection (defined as infections that require hospitalization or result in permanent organ damage).
The use of the MICISTA database for the purpose of observational studies has been authorized by the French data protection agency (Commission Nationale Informatique et Liberté) decision DR-2016-373 on 5 September 2016.
French National Hospital Discharge Database
The French National Hospital Discharge Database (Programme de Médicalisation des Systèmes d’Information (PMSI)) covers all public and private hospitals in France. The standardized discharge summary includes patient’s demographics, primary and associated discharge diagnosis codes (WHO International Classification of Diseases, 10th revision (ICD-10)13), medical and surgical procedures performed, length of stay, entry, and in-hospital mortality. A unique anonymous identifier allows the linking of all hospital claims of the patient since January 2008 and tracking the occurrence and progression of chronic conditions over time.
Patient selection
We considered for enrollment in the study population all patients aged 18 years or older with at least a one-year period of regular follow-up in the MICISTA database between 1 January 2005 and 31 December 2014. We excluded patients with chronic (more than 6 months) replicative infection by hepatitis B virus, hepatitis C virus or human immunodeficiency virus (HIV). We also excluded organ transplant recipients.
For each individual patient, the observational time started on 1 January 2005 for those patients previously enrolled in the MICISTA database, or the day of entry into the MICISTA database for those patients who were enrolled in the MICISTA database between 1 January 2005 and 31 December 2013. The observational time ended on the day of death, the day of first symptoms of systemic SVI, the day of end of regular follow-up, or on 31 December 2014.
Definition and selection criteria of systemic SVI
SVI were defined as viral infections requiring hospitalization or resulting in death or permanent organ damage. We excluded patients hospitalized for IBD flares associated with CMV colonic infection, defined as follows: intracellular inclusion bodies visualized with standard hematoxylin and eosin staining of colon tissue, or detection of CMV DNA in colonic tissue by quantitative PCR; no evidence for systemic organ damage; no elevation of blood liver tests of more than two times the upper limit of the normal range; no mononucleosis-like syndrome in blood count. We also excluded the incident cases of replicative infection by hepatitis B virus, hepatitis C virus or HIV.
Calculation of incidence and SIRs of systemic SVI
The date of the event of SVI was defined as the date of the first symptoms attributable to SVI. To avoid selection bias, patients who had symptoms attributable to SVI at the time of entry into the observational period were not considered as incident cases in the analysis. For determination of incidence of SVI according to exposure to IBD drugs, we considered the number of events related to the cumulative numbers of calendar years that were individually rated by the highest score of IBD drugs used within calendar years of follow-up. For subgroup analyses, age at entry into the observational period was grouped as older than 18 years but younger than 35 years, 35–65 years, and older than 65 years.
Data for calculating the incidence of systemic SVI in the general population were obtained from the French National Hospital Discharge Database (PMSI) between 2009 and 2013. Cases of hospital stays associated with a hospitalization ICD-10 code of systemic SVI (see the list in Supplementary Table 1) were identified in the PMSI database. The general population at risk was defined as all adults residing in France. As in our study population, we excluded patients with chronic infection by hepatitis B virus, hepatitis C virus or HIV, and organ transplant recipients. We obtained the expected number of cases of SVI in the general population by multiplying the person-years at risk in each 10-year age group by the corresponding sex-specific and age-specific incidence rate for each year between 2009 and 2013. The cases of SVI that required hospitalization and were identified in the MICISTA database were then divided by the expected number of cases to determine the SIRs. Confidence intervals (CI) for SIRs were calculated with an exact method based on the Poisson distribution.
Characterization of the cases of systemic SVI identified in the MICISTA database
A specific case report form was developed for all patients diagnosed with systemic SVI. Data were extracted by a junior gastroenterologist (A.W) and a senior gastroenterologist (L.B) from a review of medical files (including all hospitalization reports related to SVI) of patients managed for systemic SVI in our hospital. In the remaining patients, data were extracted from hospitalization or out-visit reports associated with SVI that were specifically requested by physicians treating patients. Thus, we were able to verify that all patients were referred to infection specialist for diagnostic confirmation and therapeutic advice.
We considered the exposure to IBD drugs and clinical activity of IBD on the first day of symptoms attributable to systemic SVI. Clinically active IBD was defined by the presence of symptoms clearly attributable to IBD (which corresponds to values 2 to 5 of the MICISTA score).
Nested case-control study and statistical analysis
To assess the respective roles of exposure to drugs and clinical IBD activity on the risk of systemic SVI, we performed a 4:1 case-control study. The selection process was performed in the MICISTA database: patients were matched for age (up to 5 years older or younger), gender, and IBD subtype (CD on one side, ulcerative colitis or IBD unclassified on the other).
We included the following patient characteristics on the first day of SVI or at the matched observation time in controls, in univariate conditional regression: exposure to IBD drugs and clinical activity of IBD, smoking status, patient body mass index (BMI), level of education, Montreal localization of disease, prior intestinal surgery, and clinically active perianal lesions. Items of interest in controls were extracted by a junior gastroenterologist (A.W) and a senior gastroenterologist (L.B) from a review of medical files of patients selected in the MICISTA database. Variables significant at p < 0.20 were entered into a multivariate logistic regression model with a backward variable elimination procedure to assess the strength of the associations while controlling for possible confounding variables.
For all subgroup comparisons of the study, proportions were compared using Fisher exact test and continuous outcomes were compared using the Kruskal–Wallis test. All tests were 2-tailed at a 5% significance level.
Results
Selection, baseline characteristics and follow-up time of the study population
We identified in the MICISTA database 2821 patients over the age of 18 with at least a one-year period of regular follow-up between 1 January 2005 and 31 December 2014 (Figure 1). Among them, we excluded 176 patients who were transplant recipients or chronically (more than 6 months) infected with hepatitis B virus, hepatitis C virus or HIV.
Figure 1.
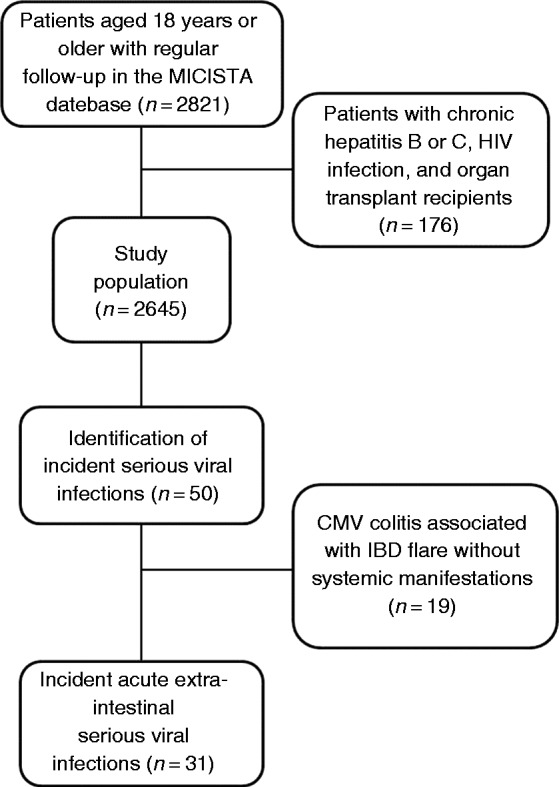
Flow diagram for study population selection and identification of systemic serious viral infections.
The study population eventually included 2645 patients (44.3% males, 68.6% with CD), followed for a total of 15,383 person-years. The median follow-up time was 6.1 years (interquartile range (IQR), 2.9–9.4). Patient characteristics at entry into the observation period are provided in Table 1. The median age of patients at entry into the observation period was 34.4 years (IQR, 25.2–46.8). The median time interval between the diagnosis of IBD and the entry into the observation period was 6.3 years (IQR, 1.5–13.5).
Table 1.
Characteristics of the study population at entry into the observation period.
| Patients, n | 2645 |
| Median age, years (IQR) | 34.4 (25.2–46.8) |
| Median time from diagnosis of IBD, years (IQR) | 6.3 (1.5–13.5) |
| Male sex, n (%) | 1172 (44.3) |
| Median BMI (IQR) | 21.9 (19.6–24.6) |
| BMI <18.5, n (%) | 394 (14.9) |
| BMI ≥30, n (%) | 148 (5.6) |
| Active smokers, n (%) | 673 (25.4) |
| CD, n (%) | |
| Segments ever involveda | 1814 (68.6) |
| L1, n (%) | 493 (27.2) |
| L2, n (%) | 488 (26.9) |
| L3, n (%) | 813 (44.8) |
| L4, n (%) | 20 (1.1) |
| perineal disease, n (%) | 430 (23.7) |
| Ulcerative colitis or IBD unclassified, n (%) | |
| Segments ever involveda | 831 (31.4) |
| E1, n (%) | 89 (10.7) |
| E2, n (%) | 356 (42.8) |
| E3, n (%) | 386 (46.5) |
IQR: interquartile range; IBD: inflammatory bowel disease; BMI: body mass index; CD: Crohn’s disease.
According to Montreal classification.
Incidence and SIR of systemic SVI
No patients had symptoms attributable to SVI at the time of entry into the observational period. Among the 50 patients who developed acute SVI during the observation period, we excluded 19 patients who developed CMV colitis associated with an IBD flare without systemic manifestations (Figure 1). No patient developed infection by hepatitis B virus, hepatitis C virus or HIV during the observation period. Finally, we identified 31 patients who developed 31 cases of systemic SVI during the observation period. The median age of patients at time of SVI diagnosis was 40.8 (30.5–53.3) years and the duration of IBD prior to diagnosis of SVI was 12.6 (7.1–20.8) years.
The incidence rate of systemic SVI in patients with IBD was 2.02 per 1000 (95% CI, 1.95–2.08) person-years in the total study population. Incidence rates of systemic SVI by age class, gender, IBD subtype, and exposure to IBD drugs are detailed in Supplementary Table 2. The SIR of systemic SVI in patients with IBD was 3.09 (95% CI, 1.98–4.20, p = 0.002) in the total study population. The SIR of systemic SVI by age class, gender, IBD subtype, and exposure to IBD drugs are listed in Table 2. Among age classes, the highest SIR was observed in patients under 35 years. The SIR of systemic SVI was higher in patients with UC or IBD unclassified than in patients with CD. Regarding IBD drugs, the highest SIR was observed in patients exposed to immunomodulators within a calendar year of occurrence of SVI.
Table 2.
Standardized incidence ratios (SIRs) of systemic SVI according to age, gender, IBD subtype and exposure to IBD drugs.
| Person-years | Reported casesa | Expected cases | SIR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| All patients | 15,383 | 30 | 9.7 | 3.09 | 1.98–4.20 | 0.0002 |
| Age classb | ||||||
| At least 18 and less than 35 years | 6311 | 19 | 3.7 | 5.1 | 2.81–7.40 | 0.0005 |
| 35 to 65 years | 7833 | 9 | 4.5 | 1.98 | 0.69–3.28 | 0.14 |
| More than 65 years | 1238 | 2 | 1.5 | 1.32 | 0–3.15 | 0.73 |
| Gender | ||||||
| Male | 6720 | 14 | 4.3 | 3.23 | 1.54–4.92 | 0.01 |
| Female | 8663 | 16 | 5.4 | 2.98 | 1.52–4.44 | 0.008 |
| IBD subtype | ||||||
| Crohn’s disease | 10,893 | 17 | 6.8 | 2.51 | 1.32–3.70 | 0.03 |
| Ulcerative colitis or IBD, unclassified | 4490 | 13 | 2.9 | 4.44 | 2.03–6.85 | 0.005 |
| Exposure to IBD drugsc | ||||||
| No treatment or 5-amino-salicylates | 5773 | 4 | 3.9 | 1.03 | 0.02–2.05 | 0.95 |
| Systemic corticosteroids | 934 | 1 | 0.6 | 1.61 | 0.00–4.76 | 0.71 |
| Immunomodulatorsd | 4836 | 19 | 3 | 6.43 | 3.54–9.32 | 0.0002 |
| Anti-TNF agents | 3840 | 6 | 2.3 | 2.65 | 0.53–4.78 | 0.13 |
IBD: inflammatory bowel disease; SVI: serious viral infection; TNF: tumor necrosing factor; CI: confidence interval.
Analysis restricted to SVI requiring hospitalization (30 out of 31).
At entry into the observation period.
Maximal treatment, according to MICISTA IBD drug scale (see Method section) during the calendar year of occurrence of SVI.
Thiopurines or methotrexate.
Characteristics of SVI
Distribution of SVI according to pathogens and clinical expression is shown in Table 3. Among the 31 patients who developed SVI, 15 had CMV systemic infection, 8 had EBV infection, 3 had herpes simplex virus (HSV) infection and 5 had varicella zoster virus (VZV) infection.
Table 3.
Characteristics of systemic severe viral infections according to pathogens and clinical expression.
| Pathogen | n | Subgroups | n | Specific presentation | n |
|---|---|---|---|---|---|
| CMV | 15 | Primary infection | 10 | Mononucleosis syndrome | 8 |
| isolated | 2 | ||||
| with hepatitis | 3 | ||||
| with pericarditis | 1 | ||||
| with cutaneous manifestations | 1 | ||||
| with hepatitis and pneumonitis | 1 | ||||
| Isolated hepatitis | 1 | ||||
| IBD flare with high viremia and without colitis | 1 | ||||
| Reactivation | 5 | IBD flare | 4 | ||
| with hepatitis | 2 | ||||
| with hemolytic anemia | 1 | ||||
| with high viremia and without colitis | 1 | ||||
| CMV colitis and CMV-induced hepatitis | 1 | ||||
| EBV | 8 | Primary infection Reactivation | 5 3 | Mononucleosis syndrome | 4 |
| isolated | 1 | ||||
| with severe neutropenia | 2 | ||||
| with hepatitis | 1 | ||||
| Hemophagocytic lymphohistiocytosis | 1 | ||||
| Hemophagocytic lymphohistiocytosis | 2 | ||||
| IBD flare with EBV-induced hepatitis | 1 | ||||
| HSV | 3 | 3 | Severe esophagitis | 1 | |
| Facial nerve paralysis | 1 | ||||
| Cutaneous disseminated infection | 1 | ||||
| VZV | 5 | Varicella Herpes zoster | 3 2 | Severe cutaneous manifestation | 2 |
| With hepatitis | 1 | ||||
| Multidermatomal | 2 |
CMV: cytomegalovirus; EBV: Epstein–Barr virus; VZV: varicella zoster virus; HSV: herpes simplex virus.
Characteristics of incident SVI are shown in Table 4. All but one (97%) patient required hospitalization, as the remaining patient who developed permanent HSV facial nerve paralysis was treated in an ambulatory setting. IBD was clinically active at clinical onset of SVI in 35% of patients. A total of 5 out of the 10 patients with CMV primary infection and 4 of the 5 patients with reactivation of CMV infection had clinically active IBD at clinical onset of SVI. None of the five patients with EBV primary infection and one of the three patients with reactivation of EBV infection had clinically active IBD at clinical onset of SVI. EBV-induced hemophagocytic lymphohistiocytosis (HLH) was diagnosed according to international diagnostic criteria in three patients.19 These three patients were exposed to thiopurines and one of them had clinically active IBD at onset of symptoms attributable to systemic HLH. Immunosuppressive drugs were stopped on admission in all patients. Only one patient received corticosteroids, immunoglobulins, etoposide, and rituximab while the two others had spontaneous resolution of the disease. All patients recovered without sequelae.
Table 4.
Characteristics of incident cases of systemic SVI.
| Patient number | Sex | IBD type | Age at diagnosis of IBD | Age at clinical onset of SVI | Virus | Infection type | Exposure to IBD drugs at clinical onset of SVI | Mononucleosis syndrome or systemic symptoms | Clinical or biological expression | Clinically active IBD at clinical onset of SVI | IBD treatment prior to SVI suspended | Antiviral treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | CD | 20 | 22 | CMV | Primary Infection | Anti-TNF agent | Yes | Mononucleosis Syndrome | Yes | Yes | IV |
| 2 | F | UC | 21 | 22 | CMV | Reactivation | 5-ASA | No | Hepatitis | Yes | Yes | IV |
| 3 | F | UC | 19 | 23 | CMV | Primary infection | MTX | Yes | Pneumonitis, Hepatitis | No | Yes | IV |
| 4 | M | UC | 13 | 27 | CMV | Primary infection | AZA | Yes | Mononucleosis syndrome | No | No | No |
| 5 | F | CD | 22 | 29 | CMV | Primary infection | AZA | Yes | Hepatitis | Yes | Yes | Oral |
| 6 | F | UC | 19 | 30 | CMV | Primary infection | 5-ASA, AZA | Yes | Cutaneous | Yes | Yes | IV |
| 7 | F | UC | 19 | 34 | CMV | Primary infection | AZA, Anti-TNF agent | Yes | Hepatitis | No | Yes | No |
| 8 | M | CD | 20 | 35 | CMV | Reactivation | 5-ASA | No | Hemolytic anemia | Yes | Yes | IV |
| 9 | M | CD | 25 | 36 | CMV | Primary infection | AZA | Yes | Mononucleosis syndrome | No | Yes | IV |
| 10 | F | UC | 22 | 37 | CMV | Primary infection | AZA | Yes | Pericarditis | No | Yes | IV |
| 11 | F | UC | 37 | 39 | CMV | Reactivation | 5-ASA, AZA | No | Hepatitis | Yes | Yes | IV |
| 12 | F | CD | 21 | 42 | CMV | Primary infection | AZA | Yes | Hepatitis | Yes | Yes | IV |
| 13 | F | UDC | 22 | 45 | CMV | Primary infection | AZA | Yes | Mononucleosis syndrome | Yes | Yes | IV |
| 14 | M | UC | 48 | 51 | CMV | Reactivation | Corticosteroids Anti-TNF agent, | No | Hepatitis | No | Yes | Oral |
| 15 | M | UC | 50 | 79 | CMV | Reactivation | 5-ASA, MTX, Corticosteroids | Yes | Mononucleosis syndrome | Yes | Yes | IV |
| 16 | F | CD | 8 | 20 | EBV | Primary infection | AZA | Yes | Neutropenia | No | Yes | No |
| 17 | M | CD | 5 | 20 | EBV | Primary infection | AZA, Anti-TNF agent | Yes | Mononucleosis | No | Yes | No |
| 18 | F | CD | 15 | 21 | EBV | Primary infection | 5-ASA, AZA | Yes | HLH | No | Yes | No |
| 19 | F | UC | 22 | 23 | EBV | Reactivation | 5-ASA, Corticosteroids | No | Hepatitis | No | Yes | No |
| 20 | M | CD | 14 | 24 | EBV | Primary infection | 5-ASA, AZA | Yes | Hepatitis | No | Yes | No |
| 21 | F | CD | 20 | 25 | EBV | Primary infection | 5-ASA, AZA | Yes | Neutropenia | No | Yes | No |
| 22 | M | CD | 37 | 43 | EBV | Reactivation | AZA | Yes | HLH | Yes | Yes | No |
| 23 | F | CD | 19 | 53 | EBV | Reactivation | AZA | Yes | HLH | No | Yes | No |
| 24 | M | CD | 18 | 19 | HSV | Primary infection | Corticosteroids | No | Esophagitis | Yes | Yes | IV |
| 25 | M | CD | 25 | 32 | HSV | Reactivation | MTX, Anti-TNF agent | No | Severe labial lesions | No | Yes | IV |
| 26 | F | CD | 27 | 36 | HSV | Primary infection | AZA | No | Facial paralysis | No | Yes | IV |
| 27 | F | CD | 17 | 18 | VZV | Reactivation | AZA, Anti-TNF agent | No | Multidermatomal shingles | No | Yes | Oral |
| 28 | M | UC | 8 | 22 | VZV | Primary infection | 5-ASA, AZA | No | Varicella, Hepatitis | No | Yes | No |
| 29 | M | CD | 23 | 23 | VZV | Reactivation | AZA | No | Multidermatomal shingles | No | Yes | Oral |
| 30 | F | CD | 6 | 28 | VZV | Primary infection | MTX | No | Varicella | No | No | No |
| 31 | M | UC | 56 | 78 | VZV | Primary infection | None | No | Varicella | No | Yes | IV |
IBD: inflammatory bowel disease; SVI: serious viral infections; M: male; F: female; CD: Crohn’s disease; UC: ulcerative colitis; TNF: tumor necrosis factor; IV: intravenous; 5-ASA: 5-amino-salicylates; MTX: methotrexate; AZA: azathioprine; HLH: hemophagocytic lymphohistiocytosis.
Anti-viral therapy was administered in 61% of patients, including 13 of the 15 patients who developed systemic CMV infection. Out of the 31 patients who developed SVI, 20 (65%) were exposed to thiopurines at clinical onset of SVI. Out of the eight patients who developed EBV infection, seven (88%) were exposed to thiopurines at clinical onset of SVI. SVI occurrence led to transient suspension (drug withheld until SVI resolution) of immunosuppressive therapy in 58% of cases, and permanent drug withdrawal for the rest of the follow-up in 35% of cases. All patients eventually recovered without sequelae, except a patient with permanent attenuated HSV facial nerve paralysis. No permanent end-organ damage or death was reported.
Risk factors of systemic SVI in the study population
Nested case-control study for assessing risk factors of SVI included 31 cases and 124 controls. Non-matched characteristics of IBD were not significantly differently distributed between cases and controls (Supplementary Table 3). Among the 13 factors that were entered in the univariate analysis, only two factors, clinically active IBD at clinical onset of SVI and exposure to thiopurines at clinical onset of SVI, reached the eligibility level (p < 0.20) for being entered in the multivariate model. By multivariate analysis, increased risk of SVI was associated with exposure to thiopurines (odds ratio (OR), 3.48; 95% CI, 1.36–8.90; p = 0.009), and clinically active disease (OR, 3.35; 95% CI, 1.23-9.23; p = 0.02) at clinical onset of SVI. Values of OR associated with IBD clinical activity and exposure to IBD drugs at clinical onset of SVI, by univariate and multivariate analysis, are shown in Table 5.
Table 5.
Impact of IBD clinical activity and exposure to IBD drugs at clinical onset of SVI in cases on the risk of SVI.
| Cases of SVI (n = 31) | Controls (n = 124) | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Clinically active disease | 13 (41.9) | 23 (18.6) | 3.86 | 1.46–10.17 | 0.006 | 3.35 | 1.23-9.23 | 0.02 |
| 5-ASA | 10 (32.3) | 53 (42.7) | 0.62 | 0.26–1.46 | 0.27 | – | – | – |
| Corticosteroids | 3 (9.7) | 12 (9.7) | 1.00 | 0.25–4.00 | 1 | – | – | – |
| Methotrexate | 4 (12.9) | 11 (8.9) | 1.53 | 0.44–5.21 | 0.50 | |||
| Azathioprine or 6-mercaptopurine | 20 (64.5) | 42 (33.9) | 3.94 | 1.55–10.02 | 0.004 | 3.48 | 1.36–8.90 | 0.009 |
| Anti-TNF agent | 6 (19.4) | 21 (16.9) | 1.18 | 0.43–3.25 | 0.75 | – | – | – |
| Combination therapya | 4 (12.9) | 11 (8.9) | 1.47 | 0.45–4.75 | 0.51 | – | – | – |
IBD: inflammatory bowel disease; SVI: serious viral infection; CI: confidence interval.
Anti-TNF agent and azathioprine, 6-mercaptopurine or methotrexate.
Discussion
We have shown that the risk of systemic SVI is tripled in patients with IBD followed in IBD referral centers, compared to individuals of the general population. The excess risk is mainly related to IBD clinical activity and exposure to thiopurines. The resulting individual absolute risks remains acceptable but excess hospitalizations due to SVI contribute to the human and financial burden of IBD.20 Owing to the increasing use of biosimilars, the cost of hospitalization has again began to tend to be, in France as in other countries,21 the main cost associated with the management of IBD.
The main limitation of this study is the recruitment of patients restricted to a referral IBD center, with an over-representation of severe forms of IBD. To illustrate this, the overall incidence of SVI was doubled in our referral population compared to general French population exposed to immunosuppressants.4 Another limitation is that approximately 10% of the patients quit our center every year for various reasons. However, within the individual observation time of each patient, information on severe infections is exhaustive since the question on intervals between serious infections is systematically asked at each face to face contact with the patients. A significant strength of our study was to integrate the prospective monitoring of IBD clinical activity, which allowed the distinction between complications of IBD drugs and complications of IBD itself. This causality assessment is not possible in studies using medico-administrative databases.3,4
Patients with silent chronic CMV infection may develop CMV-mediated colitis, without systemic manifestations of CMV infection, in the context of an IBD flare.6 Conversely, we observed that half of the patients with systemic CMV primary infection had intestinal manifestations considered as clinically active IBD at clinical onset of SVI, suggesting that primary CMV infection may provoke or worsen intestinal inflammation. Most of the patients with systemic signs of reactivation of CMV infection had clinically active IBD at clinical onset of SVI, suggesting at least a partial role of IBD activity in the pathogenesis of infection. In relation to this latter observation, it had been previously observed that a significant increase of viral load may occur in the context of a severe IBD flare,7 before the initiation of immune-suppressive therapy. Among immunosuppressive drugs, thiopurines may promote CMV replication7,22 while exposure to anti-TNF agents has no clear independent effect on CMV activation.23,24 EBV primary infection was never associated with digestive symptoms in our study.
It is established that patients exposed to corticosteroids are at increased risk of serious infections in general.11,13 In our study, this may be due to a low number of patients chronically exposed to corticosteroids, we could not identify exposure to corticosteroids as an independent risk factor of SVI.
It has been repeatedly reported that thiopurines promote both primary viral infections22 and reactivation of latent chronic infections.8,9,11,13,25,26 Fatal forms of primary VZV27 and EBV primary infection22 represent the most challenging problem. Fatal forms of varicella are not associated with HLH and can be prevented by vaccinating patients against VZV before starting immunosuppressive therapy. Fatal forms of HLH that occur in patients with IBD are mainly due to EBV primary infection,28 and are almost exclusively observed in patients exposed to thiopurines.22,28 This specific risk, together with the risk of fatal post-mononucleosis lymphoproliferation,28,29 can be reduced by limiting the use of thiopurines in EBV-seronegative patients.30 In EBV-seronegative patients exposed to thiopurines, clinicians should measure EBV viral load and look at biological signs of HLH in case of unexplained fever, splenomegaly or bicytopenia. Prompt consultation with a hematologist may lead to earlier treatment of HLH that relies increasingly on a severity case-by-case basis for the use of rituximab and etoposide.31,32
Increasing age is an established risk factor of severe infection in general in patients exposed to immunosuppressive drugs.4,13,33,34 The case for SVI is more complex. Young people are more prone to develop severe forms of primary viral infections whereas the risk of zoster increases linearly with age.8 This dual impact may explain why, in our study population, the highest incidence rates of SVI according to age classes was observed in patients under the age of 35 years.
The incidence of SVI was numerically higher in patients with UC than in patients with CD. This can be attributed to the greater incidence of systemic CMV infection in patients with UC, in our study as well as in the literature.6
In an ideal word, the risk of SVI in patients with IBD could get closer to that of the general population with the use of IBD drugs that prevent IBD-related SVI by way of sustained mucosal healing, whilst not promoting SVI via immunosuppressive effects. However, mucosal healing is currently achieved in a minority of patients and all immunosuppressive drug classes that are currently used promote infections. Thiopurines promote more SVI than anti-TNF agents,4,9 but the converse is true for opportunistic bacterial infections.4
In conclusion, our study provides a comprehensive snapshot in the field of SVI caused by IBD and IBD drugs prior to the arrival of tofacitinib, a molecule that is associated with a marked excess risk of herpes zoster.10
Supplemental Material
Supplemental material, UEG889763 Supplemetal Material for Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines by Andrew Wisniewski, Julien Kirchgesner, Philippe Seksik, Cécilia Landman, Anne Bourrier, Isabelle Nion-Larmurier, Philippe Marteau, Jacques Cosnes, Harry Sokol, Laurent Beaugerie and the Saint-Antoine IBD network in United European Gastroenterology Journal
Acknowledgments
AW and JK contributed equally to this manuscript.
AW, JK, and LB were jointly responsible for the study concept and design, collection, assembly, and analysis of data, and drafting and revision of the article. JC took part in collection, assembly, and analysis of data. All the byline authors took part in the revision of the manuscript and approved the final version of the manuscript.
There was no external writing assistance.
Contributor Information
Collaborators: Lionel Arrivé, Laurent Beaugerie, Anne Bourrier, Marine Camus, Najim Chafai, Edouard Chambenois, Ulriikka Chaput, Clotilde Debove, Charlotte Delattre, Xavier Dray, Jean-François Fléjou, Guillaume Le Gall, Nadia Hoyeau, Julien Kirchgesner, Cécilia Landman, Jérémie H Lefèvre, Philippe Marteau, Chloé Martineau, Laurence Monnier-Cholley, Isabelle Nion-Larmurier, Violaine Ozenne, Yann Parc, Philippe Seksik, Harry Sokol, Magali Svrcek, and Emmanuel Tiret
Declaration of conflicting interests
The authors disclose the following: Andrew Wisniewski has received consulting fees from Abbvie, Janssen, Takeda and Pfizer. Philippe Seksik has received honoraria from Takeda, Merk MSD, Biocodex, Astellas, Ferring pharmaceuticals and Abbvie. Cécilia Landman has received consulting fees from Abbvie, Hospira-Pfizer, Janssen-Cilag and Ferring pharmaceuticals, and research support from Biocodex. Philippe Marteau has received payments for lectures/speakers bureau participation from Abbvie, Ferring Pharmaceuticals, and Hospira, Pfizer. Harry Sokol has received consulting fees from MSD, Danone, Takeda, Abbvie, Astellas, Enterome, Maat, Novartis, BMS, and research support from Biocodex. Laurent Beaugerie has received consulting fees from Janssen, Pfizer and Allergan, lecture fees from Abbvie, Janssen, MSD, Ferring Pharmaceuticals, Mayoly-Spendler, Takeda and Tillots, and research support from Abbott, Ferring Pharmaceuticals, Hospira-Pfizer, Janssen, MSD, Takeda and Tillots. Julien Kirchgesner, Anne Bourrier, Isabelle Nino-Larmurier and Jacques Cosnes disclose no conflicts.
Ethics approval
In France, submission to Ethics Committee is not requested for pure observational studies.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Informed consent
In France, signed consents are not requested for pure observational studies.
Supplemental material
Supplemental Material for this article is available online.
References
- 1.Kirchgesner JJ, Lemaitre M, Rudnichi A, et al. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009–2014. Aliment Pharmacol Ther 2017; 45: 37–49. [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L, Kirchgesner J. Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019; 17: 370–379. [DOI] [PubMed] [Google Scholar]
- 3.Nyboe Andersen N, Pasternak B, Friis-Moller N, et al. Association between tumour necrosis factor-alpha inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ 2015; 350: h2809–h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018; 155: 337–346.e10. [DOI] [PubMed] [Google Scholar]
- 5.Sager K, Alam S, Bond A, et al. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41: 725–733. [DOI] [PubMed] [Google Scholar]
- 6.Siegmund B. Cytomegalovirus infection associated with inflammatory bowel disease. Lancet Gastroenterol Hepatol 2017; 2: 369–376. [DOI] [PubMed] [Google Scholar]
- 7.Reijasse D, Le Pendeven C, Cosnes J, et al. Epstein–Barr virus viral load in Crohn’s disease: effect of immunosuppressive therapy. Inflamm Bowel Dis 2004; 10: 85–90. [DOI] [PubMed] [Google Scholar]
- 8.Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006; 4: 1483–1490. [DOI] [PubMed] [Google Scholar]
- 9.Khan N, Patel D, Trivedi C, et al. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol 2018; 16: 1919–1927.e3. [DOI] [PubMed] [Google Scholar]
- 10.Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018; 24: 2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134: 929–936. [DOI] [PubMed] [Google Scholar]
- 12.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut 2009; 58: 501–508. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107: 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol 2013; 108: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 15.Naganuma M, Kunisaki R, Yoshimura N, et al. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol 2013; 48: 595–600. [DOI] [PubMed] [Google Scholar]
- 16.Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration adverse event reporting system. J Gastrointestin Liver Dis 2013; 22: 269–276. [PubMed] [Google Scholar]
- 17.Osterman MT, Haynes K, Delzell E, et al. Effectiveness and safety of immunomodulators with anti-tumor necrosis factor therapy in Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1293–1301 e5. quiz e70, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology 2001; 120: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 19.Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124–131. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NH, Khera R, Ohno-Machado L, et al. Annual burden and costs of hospitalization for high-need, high-cost patients with chronic gastrointestinal and liver diseases. Clin Gastroenterol Hepatol 2018; 16: 1284–1292.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boschetti G, Nancey S, Daniel F, et al. Costs of Crohn’s disease according to severity states in France: a prospective observational study and statistical modeling over 10 years. Inflamm Bowel Dis 2016; 22: 2924–2932. [DOI] [PubMed] [Google Scholar]
- 22.Biank VF, Sheth MK, Talano J, et al. Association of Crohn’s disease, thiopurines, and primary Epstein-Barr virus infection with hemophagocytic lymphohistiocytosis. J Pediatr 2011; 159: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Ovidio V, Vernia P, Gentile G, et al. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol 2008; 43: 180–183. [DOI] [PubMed] [Google Scholar]
- 24.Pillet S, Jarlot C, Courault M, et al. Infliximab does not worsen outcomes during flare-ups associated with cytomegalovirus infection in patients with ulcerative colitis. Inflamm Bowel Dis 2015; 21: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 25.Papadakis KA, Tung JK, Binder SW, et al. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol 2001; 96: 2137–2142. [DOI] [PubMed] [Google Scholar]
- 26.Long MD, Martin C, Sandler RS, et al. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springfeld C, Sauerbrei A, Filusch A, et al. Fatal varicella in an immunocompromised adult associated with a European genotype E2 variant of varicella zoster virus. J Clin Virol 2009; 44: 70–73. [DOI] [PubMed] [Google Scholar]
- 28.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374: 1617–1625. [DOI] [PubMed] [Google Scholar]
- 29.de Francisco R, Castaño-García A, Martínez-González S, et al. Impact of Epstein–Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther 2018; 48: 723–730. [DOI] [PubMed] [Google Scholar]
- 30.Beaugerie L. Lymphoma: the bête noire of the long-term use of thiopurines in adult and elderly patients with inflammatory bowel disease. Gastroenterology 2013; 145: 927–930. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald MP, Armstrong L, Hague R, et al. A case of EBV driven haemophagocytic lymphohistiocytosis complicating a teenage Crohn’s disease patient on azathioprine, successfully treated with rituximab. J Crohns Colitis 2013; 7: 314–317. [DOI] [PubMed] [Google Scholar]
- 32.Thompson G, Pepperell D, Lawrence I, et al. Crohn’s disease complicated by Epstein–Barr virus-driven haemophagocytic lymphohistiocytosis successfully treated with rituximab. BMJ Case Rep 2017; 2017: bcr2016218578–bcr2016218578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011; 9: 30–35. [DOI] [PubMed] [Google Scholar]
- 34.Broekman MMTJ, Coenen MJH, Wanten GJ, et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther 2017; 46: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG889763 Supplemetal Material for Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines by Andrew Wisniewski, Julien Kirchgesner, Philippe Seksik, Cécilia Landman, Anne Bourrier, Isabelle Nion-Larmurier, Philippe Marteau, Jacques Cosnes, Harry Sokol, Laurent Beaugerie and the Saint-Antoine IBD network in United European Gastroenterology Journal


