Abstract
Background
Cannabis benefits patients with inflammatory bowel disease (IBD). Cannabinoid receptors are expressed in gut immune cells and in epithelial cells of inflamed guts. Mucosal healing (MH) requires epithelial layer restoration.
Objective
To analyze the effects of CB2 agonist on parameters implicated in gut inflammation and MH.
Methods
Mucosal samples from areas of inflamed/uninflamed colon from 16 patients with IBD were cultured without/with cannabinoid receptor 2 (CB2) agonist (JWH-133, 10 µM, 6 hours (hr)), and analyzed for epithelial/stromal cell proliferation, apoptosis (secretome matrix metalloproteinase 9 (MMP9) activity, which impairs epithelial permeability) and interleukin-8 (IL-8) levels (n = 5–9). In addition, Caco-2 (colon carcinoma epithelial cells) were cultured with biopsy secretomes (48 hr), and analyzed for phenotype and protein markers of proliferation (proliferating cell nuclear antigen), autophagy (LC3IIB) and permeability (Zonula occludens-1) (n = 4–6).
Results
Uninflamed tissue had higher epithelial proliferation (Ki67: 50%↑, p < 0.05), and reduced secretome MMP9 activity and IL-8 levels (>50%↓, p < 0.05) compared to inflamed tissue. Treatment with CB2 agonist had no effect on epithelial apoptosis, but increased epithelial Ki67 expression (25%), and reduced secretome MMP9 and IL-8 levels in inflamed biopsies. Secretomes of CB2-treated biopsies increased Caco-2 number, migration, proliferating cell nuclear antigen and LC3IIB expression (all, p < 0.05), but had no effect on ZO-1.
Conclusion
Using ex vivo and in vitro human models, we demonstrated that manipulating the cannabinoid system affects colon cells and secretome characteristics that facilitate MH in IBD.
Keywords: Inflammatory bowel disease, colon biopsy culture, cannabinoid receptor 2, mucosal healing, colon epithelial cells
Key Summary
The established knowledge on this subject
Following gut inflammation and intestinal barrier breach, a process of immune cell interaction with epithelial and stromal cells begins to reestablish the intestinal barrier. This process includes the proliferation of epithelial cells. Improving this process facilitates mucosal healing.
Current research models of inflammatory bowel disease (IBD) use either a single cell type, which does not reflect the complex cellular interactions occurring during inflammation, or animal models of chemically induced colitis, which are not identical to human IBD.
Experimental studies and recent clinical trials suggest that treatment with cannabis benefits patients with IBD.
Cannabinoids have anti-inflammatory effects and regulate epithelial permeability, but the mechanisms of these effects are not well characterized.
Significant findings of this study
The current study established a reliable, short-culture human biopsy model, which preserves the dynamic cross talk between all cells in the organ.
Using this model and a cell line model that was exposed to the biopsy secretomes (the soluble IBD microenvironment), we demonstrated that cannabinoid receptor 2 agonist: facilitates colon epithelial cell proliferation, facilitates colon epithelial cell autophagy, has no significant effect on ZO-1 levels, and reduces secretome matrix metalloproteinase 9 enzyme activity and IL-8 levels.
These effects may promote mucosal healing in IBD patients.
Introduction
Inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, idiopathic, inflammatory, gastrointestinal (GI) disorders. The pathogenesis of IBD is multifactorial and related to dysregulated immune responses to environmental factors in genetically susceptible hosts.1 The gut epithelial cells act as first responders in the event of a barrier breach that induces inflammation. Upon being triggered, they secrete immunomodulatory substances, including interleukin-8 (IL-8) and matrix metalloproteinases (MMPs), that facilitate inflammation.2,3 Eventually, the inflamed microenvironment leads to increased epithelial apoptosis4 with little or no epithelial cell division at the acute phase,5 altogether leading to reduced ability of the epithelial layer to serve as a barrier.6,7 Autophagy of the epithelial cells may limit intestinal inflammation.8 Following gut damage and during chronic inflammation,5 a process of epithelial restitution begins, including epithelial cell migration, proliferation and differentiation. This leads to repair of the epithelial barrier and mucosal healing (MH).9,10 MMP9 has been suggested to be a surrogate marker for MH in IBD3 and mucosal concentrations of IL-8 have been found to be positively correlated with endoscopic scores.11
From a therapeutic perspective, IBD remains a challenge, as about 40% of patients do not respond to biological therapy.12 The challenge is increased due to a lack of good experimental models of IBD, as the currently used animal models have limited ability to predict human responses to therapies.13 Thus, additional therapies for IBD, with novel mechanisms of action and appropriate biological models to evaluate their effects, are needed.
The endocannabinoid system plays a role in the pathogenesis of IBD.14 Cannabinoid receptors such as cannabinoid receptor 2 (CB2) are expressed throughout the GI tract14,15 and their levels are altered during inflammation.15 Cannabinoids have anti-inflammatory effects16 and they modulate gut epithelial cell permeability.17 Experimental studies in rodents14 and recent clinical trials, including from our group, have suggested that treatment with cannabis may benefit patients with IBD.15,18–20 Yet, it is not clear whether the beneficial changes seen in patients were induced by central cannabis effects (psychoactive), peripheral (anti-inflammatory and/or MH) effects or both. While some cannabinoid receptors are expressed in gut epithelial cells, others (such as CB2) are expressed mainly on immune cells, especially on subepithelial macrophages and plasma cells.21 Therefore, a human biological model that contains epithelial, stromal and immune cells is needed to study the effect of cannabinoids on IBD patients. The current study aimed to establish a reliable short-culture human biopsy model and use it to investigate the effects of a CB2 agonist on colonic inflammation and MH.
Materials and methods
Study design
In this study, we performed the following four sequential experiments: (a) develop and validate a human biopsy model by testing the viability of the biopsies over time; (b) demonstrate that the ex vivo biopsies maintain their original morphologic and phenotypic characteristics of inflamed and uninflamed colonic areas; (c) investigate the effects of CB2 agonist on epithelial and stromal cell proliferation and death, and on factors related to MH and inflammation in cultures and secretomes (the soluble microenvironment) of colonic biopsies; and (d) investigate the effects of secretomes collected from cultured biopsies, either untreated or treated with CB2, on the phenotypes of Caco-2 colon carcinoma cells.22,23
Mucosal samples
Mucosal samples were collected from three healthy subjects and 16 IBD patients (nine with CD and 7 with UC; eight males and eight females; ages: 24–67) scheduled for routine follow-up colonoscopy at the Division of Gastroenterology and Hepatology, Meir Medical Center. Biopsy samples were collected by the gastroenterologist from inflamed and uninflamed areas of the patients' colons during endoscopy procedures. Uninflamed areas had intact, clear-shining mucosa, no ulcers and good observation of blood vessels for a segment of ≥10 cm, while inflamed areas were defined as areas of congestion with disappearance of blood vessels and the presence of ulcers. IBD patients had different levels of severity (Clinical Disease Activity Index (CDAI): 40–443 and Simple Clinical Colitis Activity Index (SCCAI): 0–12) and were treated with a variety of medications, including infliximab (one with UC and one with CD), adalimumab (two with CD), vedolizumab (one with UC), azathioprine (one with UC and one with CD), antibiotics (one with UC), steroids (one with CD) or no treatment (four with CD and three with UC).
Colon biopsy culture
In each experiment, on average three to six biopsies (∼10 mg wet weight) were taken from uninflamed/inflamed areas from each patient. One of the biopsies was immediately sent for immunohistochemistry evaluation (time = 0), and the rest were transferred to inserts (Millipore) and placed inside six-well plates containing 1.1 ml medium ((Dulbecco's Modified Eagle Medium (DMEM)/F-12(HAM) supplemented with 10% heat-inactivated fetal calf serum, leupeptin (50 µg/ml), phenylmethylsulfonyl fluoride before (PMSF) (1 mM), trypsin inhibitor (50 µg/ml), non-essential amino acids, antibiotics (penicillin 100 units/ml), streptomycin (100 µg/ml) and gentamicin (50µg/ml)) with/without CB2 agonist (JWH-133, 10 µM) or its solvent (ethanol 100%), and incubated for 6 hours (hr) at 37℃ in 5% CO2. At time 0 and after 6 hr of culture, biopsies were fixed in 4% formaldehyde and embedded in paraffin for immunohistochemistry assessment.
Immunohistochemistry
Paraffin sections were deparaffinized in xylene and alcohol, rinsed in phosphate-buffered saline (PBS), immersed in citrate buffer (pH 6.0) and microwaved for 15 minutes (min). Endogenous peroxidase activity was quenched in 3% H2O2. Samples were blocked using goat serum and incubated with primary antibodies overnight (Ki67/cleaved caspase 3 (proliferation24 and apoptosis4 markers), rabbit immunoglobulin G, dilution 1:150 (Zytomed Systems, Germany)). After washing, slides were incubated with horseradish peroxidase-labeled polymer conjugated to secondary antibody (Zytomed Systems), washed and developed with a 3-amino-9-ethylcarbazole chromogen system (Covance Research Products, USA). Isotype-matched control antibodies demonstrated an absence of non-specific staining.
Evaluation of epithelial and stromal cell proliferation and apoptosis
Following immunohistochemistry staining, two pictures were taken from different areas of each biopsy via microscopy. Then, the number of Ki67 and caspase 3-positive and -negative cells were counted in four crypts from two randomized sections from each photograph (epithelial cells), and in two areas of the picture (containing ∼ 50 stromal cells, no differentiation was done between myeloid, lymphoid and mesenchymal cells). Analyses were done on n = 3 (six pictures) for healthy people and n = 5–9 (10–18) pictures) for IBD patients.
Caco-2 cell growth
Caco-2 cells (kindly provided by Dr Koltai, The Volcani Institute, Rishon-Lezion, Israel) were grown in DMEM media supplemented with 2 mM L-glutamine, antibiotics (50 µg/ml streptomycin and 100 units/ml penicillin) and plasmocin (5 µg/ml). We used Caco-2 cells in the proliferating phase, which has a transcriptional program that resembles the mitotic cells in the crypt.25
IL-8 enzyme-linked immunosorbent assay
Cytokine levels were measured in the biopsy secretomes using human IL-8 standard 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) enzyme-linked immunosorbent assay development kits (Peprotech), according to manufacturer’s instructions.
MMP activity
Was evaluated using gelatin zymography as previously described by Dr Tartakover-Matlaon.26
Analyzing the effects of secretomes on epithelial cells
Secretomes collected from the biopsy cultures were applied to Caco-2 cells. After 48 hr, we assessed Caco-2 cell phenotypes and extracted proteins from the cells (n = 4–6).
Viability assay
In 96-well plates, 25,000 cells were cultured with the biopsy secretomes. After 48 hr, alamarBlue reagent was added to the wells for 3–4 hr at 37℃ and fluorescence was read using Infinite F200 (Tecan Group Ltd, Männedorf, Switzerland) (excitation 535 nm and emission 595 nm).
Scratch assay
The confluent Caco-2 cell monolayer was wounded by a pipette tip and biopsy secretomes were added to the cells. Wound closure was measured by microscopy 48 hr after plating. Results are presented as the relative percentage of closure compared with time 0. The wounds were measured using ImageJ software: http://rsbweb.nih.gov/ij/.
Cell counts and death
Total cell counts, as well as the respective proportion of live/dead cells, were assayed using Trypan blue dye. Cells were automatically counted by Countess (Invitrogen). Live cells remained unstained, while dead cells stained with the dye.
Protein extraction and western blotting
Caco-2 cells were washed with PBS and lysed in buffer (as previously described26) on ice for 10 min. Protein lysate from 105 cells was mixed (1:5) with loading buffer, denatured for 10 min at 65℃, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene-difluoride membrane. After blocking (5% dry milk), membranes were incubated at 4℃ overnight with primary antibodies (proliferating cell nuclear antigen (PCNA) 1:500, Microtubule associated protein 1 light chain 3 beta 2 (LC3IIB) 1:500, Zonula occludens-1 (ZO-1) 1:1000) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 1:1000). Bound antibodies were visualized using peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) followed by enhanced chemiluminescence detection (Pierce, Rockford, USA). Optical densities were visualized and measured as arbitrary units via an LAS3000 Imager (Fugifilm). Results were normalized to GAPDH using the Multi Gauge V3.0 program (Fugifilm).
Ethical considerations
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional Human Research Committee. The research was approved by the Meir Medical Center Ethics Committee (21/07/2016-20/7/2020). All patients gave informed consent.
Statistical analyses
A Student’s t-test using Excel software was used to analyze differences between pictures in the different cohorts. Paired tests were used when both samples consisted of the same test patients (inflamed versus uninflamed) and unpaired when both samples consisted of distinct test subjects (healthy versus IBD patients). An effect was considered significant when p ≤ 0.05. Comparisons were found to be statistically different with adequate power (∼80%) using sample/size calculators: http://statulator.com/SampleSize/ss2PM.html for comparing paired differences and https://www.stat.ubc.ca/∼rollin/stats/ssize/n2.html for independent samples. Results are presented as mean + standard error.
Results
Development and validation of human biopsy model
We collected biopsies from healthy subjects and from uninflamed colon areas of IBD patients, and evaluated their epithelial and stromal cell proliferation (Ki67 staining) and apoptosis (fragmented caspase 3 staining), before (time 0) or after 6 hr of culture. Since ethanol was the CB2 agonist solvent, we also analyzed the effect of ethanol (equivalent dilution to that used in JWH-133-treated biopsies, 1:10,000) on the cultured biopsies.
After culture, alamarBlue staining indicated biopsy viability (Figure 1(a)) and most crypts remained morphologically intact (Figure 1(g)). The patterns of Ki67 expression were similar at time 0 and after 6 hr of culture (Figure 1(c), (d) and (g)). The numbers of proliferating cells in the crypts and stroma of uninflamed areas of IBD patients were significantly higher (Figure 1(c) and (d)) compared to those of healthy people. Culture conditions were associated with slight increases in epithelial (∼10%) and stromal (∼6%) cell apoptosis in all biopsies (Figure 1(e) and (f)). These findings demonstrate that our model of biopsies cultured for 6 hr supports the survival and proliferation of cells, reliably reflected true biological events and enabling the analysis of treatments on the biopsies.
Figure 1.
Culture of colon biopsies from healthy people and non-inflamed areas of patients with inflammatory bowel disease.
Biopsies were taken during endoscopy from healthy people and non-inflamed areas of inflammatory bowel disease patients. For each patient, one biopsy was immediately fixed in formaldehyde (time 0), and the rest cultured for 6 hours in medium with or without ethanol. Then the biopsies were analyzed by immunohistochemistry for evaluation of stromal/epithelial cell Ki67 (proliferation marker) and cleaved caspase 3 (apoptotic marker) expression. In the first three experiments with tissue from healthy/ inflammatory bowel disease patients, one biopsy was cultured with alamarBlue to validate tissue viability. Upon culture with alamarBlue the medium turned red, reflecting the presence of viable cells. Representative photomicrographs of biopsies cultured with (left) or without (right) alamarBlue (a); colon biopsy stained with isotype match control antibody (b); graphs present Ki67 and cleaved caspase 3 expression in epithelial ((c) and (e)) and stromal ((d) and (f)) cells, and representative figures of biopsies stained for Ki67 and cleaved caspase 3 at time 0 and after 6-hour culture (G). *indicates statistically significant results (p < 0.05). n = 3 biopsies (six pictures) from healthy people and 5–7 biopsies (10–14 pictures) from different inflammatory bowel disease patients (0 or 6 hours, respectively).
6 hr C: biopsies cultured for 6 hr in medium; 6 hr Eth: biopsies cultured for 6 hr in medium that contain ethanol; Cas3: caspase 3; hr: hour; IBD: inflammatory bowel disease; IL-8: interleukin-8; N: non-inflamed areas.
Demonstration that the ex vivo biopsies obtained from IBD patients maintain the characteristics of inflamed and uninflamed colonic areas
For the next phase, we showed that biopsies cultured for 6 hr maintained the original morphologic and phenotypic characteristics of the inflamed and uninflamed colonic areas. For this purpose, biopsies from inflamed/uninflamed areas of the colon from IBD patients were analyzed for epithelial/stromal cell proliferation (Ki67 staining) and apoptosis (fragmented caspase 3 staining), before (time 0) and after 6 hr of culture with/without ethanol (the CB2 solvent).
Cellular phenotype of inflamed and uninflamed biopsies
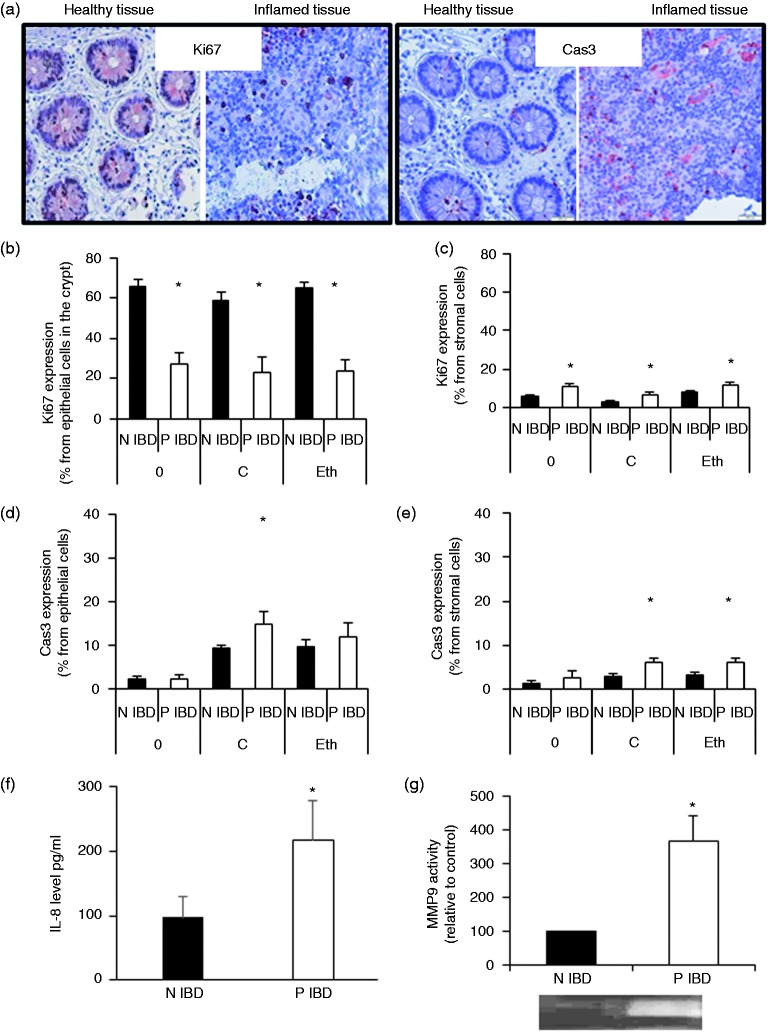
We observed more cells in the stroma surrounding the crypts in the native (time 0) and 6-hr cultured inflamed biopsies compared to uninflamed biopsies (Figure 2(a)). In addition, inflamed biopsies had slightly increased proliferation and apoptosis in stromal cells (Figure 2(c) and (e)), and fewer epithelial cells per crypt circumference. These findings were similar at time 0 and after 6 hr of culture in both the inflamed and uninflamed biopsies (uninflamed: 28 and 25 cells, and inflamed: 18 and 17 cells at time 0 and 6 hr, respectively). The smaller numbers of cells between the inflamed and uninflamed biopsies could be explained by the decrease in epithelial cell proliferation (Figure 2(b), similar at time 0 and 6 hr) and the small increase in apoptosis (Figure 2(d), 6 hr) in the inflamed cultured biopsies.
Figure 2.
Cultured biopsies from inflamed colon areas retain characteristics similar to those found in vivo.
Biopsies were taken during endoscopy from inflamed and uninflamed areas of inflammatory bowel disease patients. For each immunohistochemical analysis two biopsies (N and P) were immediately fixed in formaldehyde (time 0) for Ki67 expression (proliferation) and cleaved caspase 3 expression (apoptosis), and the rest of the biopsies were cultured with (one N and one P)/without (one N and one P) ethanol for 6 hours, and then analyzed for stromal/epithelial cell Ki67 and cleaved caspase 3 expression. Moreover, secretomes collected from P and N (N inflammatory bowel disease) cultured biopsies were analyzed for matrix metalloproteinase 9 activity and interleukin-8 levels. Presented are representative photomicrographs of inflamed/non-inflamed biopsies of inflammatory bowel disease patients stained for Ki67 and cleaved caspase 3 (a), graphs that show Ki67 and cleaved caspase 3 expression in epithelial ((b) and (d)) and stromal ((c) and (e)) cells, and graphs that show interleukin-8 levels (f) and matrix metalloproteinase 9 activity (g) in the secretomes. (b) to (e) Time 0 and biopsies cultured for 6 hours in medium: n = 8–9 biopsies (16–18 pictures) and biopsies cultured for 6 hours with ethanol in the medium: n = 5–8 patients (10–16 pictures); (f) n = 8; (g) n = 5. *indicates statistically significant results (p < 0.05).
0: time 0; C: biopsies cultured for 6 hours in medium; Eth: biopsies cultured for 6 hours with ethanol in the medium; IBD: inflammatory bowel disease; IL-8: interleukin-8; MMP9: matrix metalloproteinase 9; N: uninflamed; P: inflamed.
Inflammatory markers in inflamed biopsies compared with uninflamed biopsies
IL-8 levels and MMP9 activities in the secretomes collected from inflamed cultured biopsies were significantly increased, as compared with secretomes collected from uninflamed biopsies (Figure 2(f) and (g)).
Investigation of the effects of CB2 agonist on factors related to MH and inflammation
Next, we added the CB2 agonist JWH-133 to the IBD biopsies for 6 hr, and analyzed its effects on epithelial and stromal cell proliferation and death, as well as on IL-8 levels and MMP9 activities in cultured biopsy secretomes.
The effect of CB2 agonist on the cellular phenotype of inflamed and uninflamed biopsies
Proliferation of epithelial cells in inflamed biopsies varied among patients (3–52%, Ki67-positive cells). Yet, all responded with increased proliferation to CB2 agonist (11–66% Ki67-positive cells, 10–250% increase; Figure 3(a), p < 0.05). JWH-133 had no effect on the proliferation of epithelial cells in uninflamed areas. Few cells (<10%) were found to be proliferating in the stroma and CB2 agonist modestly reduced proliferation in these cells (3–4%, Figure 3(b), p < 0.05). Furthermore, CB2 agonist had only a marginal and non-significant effect on cell apoptosis (Figure 3(c) and (d)).
Figure 3.
The effect of cannabinoid receptor 2 agonist on cultured inflammatory bowel disease biopsies.
Biopsies were taken during endoscopy from inflamed and uninflamed areas of eight inflammatory bowel disease patients and exposed to JWH-133 (10 µM) or to ethanol (control) (the JWH-133 solvent) for 6 hours. At the end of the experiments, the biopsies were analyzed by immunohistochemistry for stromal/epithelial cell Ki67 and cleaved caspase 3 expression. Secretomes collected from inflamed and non-inflamed cultured biopsies from inflammatory bowel disease patients were analyzed for matrix metalloproteinase 9 activity and interleukin-8 levels. Presented are graphs that show Ki67 and cleaved caspase 3 expression in epithelial ((a) and (c)) and stromal ((b) and (d)) cells, and graphs that show matrix metalloproteinase 9 activity (E) and interleukin-8 levels (F) in the secretomes. *indicates statistically significant results (p < 0.05). (a) to (d) n = 5 biopsies (10 pictures); (e) n = 5 patients; (f) n = 8 patients.
ACB2: biopsies cultured for 6 hours with CB2 agonist (JWH-133) in the medium; Eth: biopsies cultured for 6 hours with ethanol in the medium; IBD: inflammatory bowel disease; MMP9: matrix metalloproteinase 9; N: uninflamed; P: inflamed.
Effect of CB2 agonist on inflammatory and MH factors in secretomes from cultured biopsies
Treatment with CB2 agonist significantly reduced MMP9 activity in the secretomes collected from cultured inflamed biopsies compared to those not treated with CB2 agonist (40% reduction, p < 0.05, Figure 3(e)). Furthermore, IL-8 levels were reduced by CB2 agonist in six out of seven uninflamed biopsies and in four out of six inflamed biopsies, although this observation was not statistically significant (p = 0.13 for all biopsies, Figure 3(f)).
Effects of secretomes collected from untreated and CB2-treated biopsies on colon carcinoma cell phenotypes
Next, the biopsy secretomes were added to Caco-2 cells for 48 hr and the phenotypes were analyzed. Secretomes from cultured inflamed biopsies reduced Caco-2 cell migration (28%, Figure 4(c) and (g), p < 0.05) and proliferation, as demonstrated by reduced viability (25%↓, Figure 4(a)), number (30%↓, Figure 4(b)) and PCNA expression (23%↓, Figure 4(d); all p < 0.05) compared to secretomes from uninflamed biopsies. The reduced proliferation helps explain the decreased epithelial cell numbers found in the inflamed biopsies that secreted these supernatants (Table1). However, Caco-2 cells cultured with secretomes collected from inflamed biopsies treated with CB2 agonist did not reduce PCNA expression, survival and migration (Figure 5(a) to (d)). Epithelial cell autophagy and epithelial barrier function limit intestinal inflammation. We found that secretomes collected from inflamed areas treated with the CB2 agonist significantly increased the level of LC3IIB (autophagy marker, 33%, Figure 5(e), p < 0.05), but had no significant effect on ZO-1 level (tight junction protein, data not shown) compared with secretomes from inflamed biopsies. Treatment of Caco-2 cells with the CB2 agonist without the secretomes did not affect cell numbers, but it did facilitate Caco-2 cell migration (50%, p < 0.05).
Figure 4.

Secretome collected from the inflamed biopsies with impaired colon carcinoma cell phenotypes.
Biopsies were taken during endoscopy from inflamed and non-inflamed areas of inflammatory bowel disease patients and cultured for 6 hours. Then the secretomes were collected from the biopsies and added to Caco-2 cells for 48 hours. Following culture, the cells were harvested and analyzed for cell phenotype, and proliferating cell nuclear antigen and LC3IIB expression. Presented are graphs that show viability (alamarBlue (a)), number (automatic counter (b)), survival (Trypan blue (f)), migration ((c) and (g) scratch assay), proliferating cell nuclear antigen expression (d) and LC3IIB expression (e). *indicates statistically significant results (p < 0.05). (b), (c), (e) and (f) n = 6; (d) and (e) n = 5.
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; hr: hour; N: uninflamed; P: inflamed; PCNA: proliferating cell nuclear antigen.
Table 1.
The effect of cannabinoid receptor 2 agonist on epithelial cell proliferation in the ex vivo and in vitro assays.
| Treatment | Proliferation |
|
|---|---|---|
| Control (%) | +CB2 agonist | |
| Inflamed tissue versus non-inflamed tissue (6 hr) | Ki67: 65 versus 25% | Ki67: 65 versus 35% |
| Caco-2 + inflamed secretome versus Caco-2 + non-inflamed secretome (48 hr) | Viability: 25↓ | Equal |
| Number: 30↓ | ||
| PCNA level: 23↓ | ||
CB2: cannabinoid receptor 2; hr: hour; PCNA: proliferating cell nuclear antigen.
Figure 5.
Secretome collected from the inflamed biopsies impaired the colon carcinoma cell phenotype, whereas secretomes from cannabinoid receptor 2-treated biopsies did not.
Biopsies were taken during endoscopy from inflamed and non-inflamed areas of IBD patients and cultured for 6 hours with ethanol (control) or cannabinoid receptor 2 agonist). Then the secretomes were collected from the biopsies and added to Caco-2 cells for 48 hours. Following culture, the cells were harvested and analyzed for cell phenotype, and proliferating cell nuclear antigen and LC3IIB expression. Presented are graphs that show cell number (automatic counter (b)), survival (Trypan blue (c)), migration ((d) scratch assay), proliferating cell nuclear antigen expression (a) and LC3IIB expression (e). * indicates statistically significant results (p < 0.05). n = 4.
ACB2: cannabinoid receptor 2 agonist; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IBD: inflammatory bowel disease; N: uninflamed; P: inflamed; PCNA: proliferating cell nuclear antigen.
Discussion
Previous studies have underscored the importance of immune cell interactions with epithelial and stromal cells in shaping the intestinal barrier. Therefore, we cultured gut mucosa, which preserves the dynamic cross talk between all cells in the organ.
First, we validated the ex vivo human colonic biopsy culture. We used short 6-hr cultures, and demonstrated that the crypts preserved the same level of proliferation and apoptosis increased only slightly (6–10%). These findings were similar for biopsies collected from healthy subjects and from IBD patients. Then, we used this system to compare biopsies from inflamed and uninflamed areas.
As expected, we observed increased stromal cell proliferation and reduced epithelial cell proliferation in the inflamed biopsies, as compared to the uninflamed biopsies. These characteristics remained similar after 6 hr of culture. These findings reflect the aberrant infiltration and activation of immune cells in the gut of IBD patients, representing hallmarks of the disease27 and of the intestinal epithelial damage found in IBD. We also evaluated the levels of the pro-inflammatory factors MMP9 and IL-8. Previous reports have shown that they are affected by cannabinoids.3,18 Our findings of increased IL-8 and MMP9 activity in secretomes collected from inflamed biopsies compared to those from uninflamed biopsies correspond to increased IL-8 levels found in patients with IBD, and increased MMP9 levels found in CD and even more so in UC patients,3,28 which correlates with UC disease activity.3 Taken together, these results demonstrate that our model reliably reflects mucosal biological events, and preserves the native differences between inflamed and uninflamed areas.
Next, we analyzed the effects of CB2 agonist on the epithelial/stromal cell phenotype, IL-8 levels and MMP9 activity. The most significant effect of the CB2 agonist was its pro-proliferative effect on epithelial cells and its ability to inhibit MMP9 activity in the inflamed biopsies. Previous data have shown that cannabinoids can regulate cell proliferation and protein kinase cascades, which are involved in cell proliferation and survival.29,30 In the human colon, CB2 receptors are either absent or very weakly expressed in the inflamed epithelium31; yet, they are expressed in lamina propria plasma cells and in activated macrophages.23,31 The increased epithelial cell proliferation may result from a direct effect of the CB2 agonist on the epithelial cells, or because of cross talk between stromal cells (that contain CB2) and the epithelial cells.
The effect of the agonist on secretome composition was evident in the in vitro studies. While the inflamed secretome reduced Caco-2 cell proliferation and migration, secretomes collected from CB2 agonist-treated biopsies had no such effects. Caco-2 cells have been used as an in vitro model to study intestinal epithelium.22,23 Data regarding the expression of CB2 in Caco-2 are controversial.21 We found that adding the agonist to Caco-2 cells had no effect on cell numbers. Therefore, the proliferation-promoting effect of secretomes collected from CB2 agonist-treated inflamed biopsies on Caco-2 cells was probably a result of the effect of the agonist on the composition of the biopsy secretomes.
We also found that the CB2 agonist facilitated autophagy but not ZO-1 expression in cells exposed to secretomes collected from inflamed biopsies. Studies have repeatedly shown a link between IBD and damaged autophagy,32 and between IBD and increased intestinal permeability.33 The increased autophagy induced by the CB2 agonist could be an additional mechanism for its beneficial effect in IBD patients. Other studies in different disease and biological models have shown that cannabinoids regulate normal/cancer cell autophagy.34 Furthermore, while cannabinoids have been found to be involved in mediating Caco-2 tight junction expression,12,26 as in our study, CB2 was not involved in this process.33
Our results are also consistent with previous data showing reduced levels of MMP9 messenger RNA in IBD biopsies treated with cannabis extract18 and with animal models, which showed that CB2 agonists were beneficial in ameliorating chemical-induced colitis.15 Yet, to the best of our knowledge, this is the first study to demonstrate in human culture that cannabis facilitates normal colon epithelial cell proliferation and autophagy in the Caco-2 cell line. We used short-term biopsy cultures. While this short period limited our ability to assess long-term parameters, using this model and cell culture we were able to demonstrate that CB2 agonist positively affects MH. Since CB2 is expressed mainly in peripheral and inflammatory tissues, and less in the brain, and since MH is the goal of modern IBD therapy,35 improving these characteristics by modulating the activity of the CB2 receptor is expected to benefit IBD patients clinically with fewer psychoactive affects.
Acknowledgements
We thank Faye Schreiber for the English editing.
Declaration of conflicting interests
There is no conflict of interest.
Ethics approval
The protocol was approved by the local Ethics Committee.
Funding
This study was funded in part by The Josefina Maus and Gabriela Cesarman Chair for Research in Liver Diseases, Tel Aviv University to F.K. It was also funded in part by internal departmental resources.
Informed consent
The data collection was performed after obtaining the patient’s informed consent.
References
- 1.Katsanos KH, Papadakis KA. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver 2017; 11: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walana W, Ye Y, Li M, et al. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed Pharmacother 2018; 103: 253–261. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm 2015; 2015: 964131–964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J 2016; 283: 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol 2012; 7: 35–60. [DOI] [PubMed] [Google Scholar]
- 6.Martini E, Krug SM, Siegmund B, et al. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2017; 4: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke P, Shao BZ, Xu ZQ, et al. Intestinal autophagy and its pharmacological control in inflammatory bowel disease. Front Immunol 2016; 7: 695–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pott J, Maloy KJ. Epithelial autophagy controls chronic colitis by reducing TNF-induced apoptosis. Autophagy 2018; 14: 1460–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol 2016; 51: 11–21. [DOI] [PubMed] [Google Scholar]
- 11.Pearl DS, Shah K, Whittaker MA, et al. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J Crohns Colitis 2013; 7: 481–489. [DOI] [PubMed] [Google Scholar]
- 12.Plichta DR, Graham DB, Subramanian S, et al. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell 2019; 178: 1041–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizarro TT, Stappenbeck TS, Rieder F, et al. Challenges in IBD research: preclinical human IBD mechanisms. Inflamm Bowel Dis 2019; 25: S5–S12. [DOI] [PubMed] [Google Scholar]
- 14.Hasenoehrl C, Storr M, Schicho R. Cannabinoids for treating inflammatory bowel diseases: where are we and where do we go? Expert Rev Gastroenterol Hepatol 2017; 11: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrose T, Simmons A. Cannabis, cannabinoids and the endocannabinoid system-is there therapeutic potential for inflammatory bowel disease? J Crohns Colitis 2019; 13: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya N, Penukonda S, Shcheglova T, et al. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci USA 2017; 114: 5005–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gigli S, Seguella L, Pesce M, et al. Cannabidiol restores intestinal barrier dysfunction and inhibits the apoptotic process induced by Clostridium difficile toxin A in Caco-2 cells. United European Gastroenterol J 2017; 5: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nallathambi R, Mazuz M, Ion A, et al. Anti-inflammatory activity in colon models is derived from delta9-tetrahydrocannabinolic acid that interacts with additional compounds in cannabis extracts. Cannabis Cannabinoid Res 2017; 2: 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naftali T, Bar-Lev Schlieder L, Konikoff F, et al. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. United European Gastroenterol J 2019; 31: 1376–1381. [DOI] [PubMed] [Google Scholar]
- 20.Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013; 11: 1276–1280.e1. [DOI] [PubMed] [Google Scholar]
- 21.Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005; 129: 437–453. [DOI] [PubMed] [Google Scholar]
- 22.Chopra DP, Dombkowski AA, Stemmer PM, et al. Intestinal epithelial cells in vitro. Stem Cells Dev 2010; 19: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ghadban S, Kaissi S, Homaidan FR, et al. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci Rep 2016; 6: 29783–29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma 2018; 127: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saaf AM, Halbleib JM, Chen X, et al. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell 2007; 18: 4245–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein Shochet G, Drucker L, Pomeranz M, et al. First trimester human placenta prevents breast cancer cell attachment to the matrix: the role of extracellular matrix. Mol Carcinog 2017; 56: 62–74. [DOI] [PubMed] [Google Scholar]
- 27.Larmonier CB, Shehab KW, Ghishan FK, et al. T lymphocyte dynamics in inflammatory bowel diseases: role of the microbiome. Biomed Res Int 2015; 2015: 504638–504638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pujada A, Walter L, Patel A, et al. Matrix metalloproteinase MMP9 maintains epithelial barrier function and preserves mucosal lining in colitis associated cancer. Oncotarget 2017; 8: 94650–94665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Martinez E, Martin-Ruiz A, Martin P, et al. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 2016; 7: 68781–68791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res 2013; 52: 633–650. [DOI] [PubMed] [Google Scholar]
- 31.Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol 2008; 153: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooper KM, Casanova V, Kemp S, et al. The inflammatory bowel disease drug azathioprine induces autophagy via mTORC1 and the unfolded protein response sensor PERK. Inflamm Bowel Dis 2019; 25: 1481–1496. [DOI] [PubMed] [Google Scholar]
- 33.Alhamoruni A, Wright KL, Larvin M, et al. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol 2012; 165: 2598–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa L, Amaral C, Teixeira N, et al. Cannabinoid-induced autophagy: protective or death role? Prostaglandins Other Lipid Mediat 2016; 122: 54–63. [DOI] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Abreu MT, Dubinsky MC. A noninvasive method to assess mucosal healing in patients* with Crohn’s disease. Gastroenterol Hepatol (N Y) 2018; 14: 1–12. [PMC free article] [PubMed] [Google Scholar]