Abstract
Stricturing Crohn’s disease (CD) is a significant clinical problem. The presence of a stricture may be suggested by clinical symptoms. Cross-sectional imaging using computed tomography or magnetic resonance enterography is essential in diagnosing strictures as it allows further characterization and evaluation for complications such as abscess, fistulizing disease or malignancy. Managing small bowel stricturing CD should be approached in a multidisciplinary fashion. Medical therapy can be considered in strictures which are not associated with complications, with most of the data supporting anti-TNF strategies in this setting. If the disease is refractory to medical therapy, endoscopic therapy or surgery should be performed. Endoscopic balloon dilation (EBD) is an option for short, uncomplicated and straight strictures that are within reach of a colonoscope. Although EBD has good short-term outcomes, repeat dilation is often required. Surgical options mainly include resection and strictureplasty. Strictures refractory to medical therapy, not amenable or refractory to EBD, or associated with complications or malignancy should be managed surgically. However, surgery may also be considered at an earlier stage depending on disease characteristics and patient preference. Postoperative recurrence is common, highlighting the importance of careful monitoring of the patient postoperatively and optimization of medical management accordingly. There is a pressing need to develop anti-fibrotics for the treatment of stricturing CD. This requires the development of standardized diagnostic criteria, patient-reported outcome measures and validation of endpoints in fibrostenotic CD. The STAR consortium is pioneering this effort in order to allow development and testing of anti-fibrotics in future clinical trials.
Keywords: Stricture, stenosis, fibrosis, endoscopic balloon dilation, surgery, inflammatory bowel disease, Crohn’s disease
Clinical case
A 35-year-old male patient with a known history of ileocolonic Crohn’s disease (CD) presents with post-prandial abdominal pain, abdominal distention, nausea and vomiting for 1 month. Magnetic resonance enterography (MRE) shows wall thickening and contrast enhancement in the distal ileum, over a segment of approximately 5 cm in length. It is associated with luminal narrowing and pre-stenotic small bowel dilation of up to 3 cm. There are no associated sinus tracts or fistulas.
Natural history of stricturing Crohn’s disease
Although most patients with CD present with an inflammatory phenotype at time of diagnosis, about 10% of patients exhibit a stricturing phenotype.1 According to population-based studies using the Montreal classification, the probability of progression to stricturing CD is about 15% at 10 years and 21.6% at 20 years.2 Strictures are a main indication for surgery in CD. About 40–70% of patients overall require surgical treatment for a complication (e.g. stricture, fistula, abscess) 10 years after diagnosis.3 Unfortunately, postoperative recurrence is common and usually occurs at the ileocolonic anastomotic site, driving re-stricturing and need for redo surgery.4
The development of fibrostenosis is likely the result of a combination of inflammation-dependent and -independent processes. Although fibrosis in inflammatory bowel disease (IBD) has been traditionally viewed as a consequence of inflammation only,2,5 no change in progression to fibrostenosing CD has been shown despite the introduction of anti-TNF therapy, even early after diagnosis.6–8 This may be explained by the fact that at time of diagnosis (which is considered an “early” treatment point in most publications) tissue damage has already occurred and the process became independent of inflammation. Fibrosis results from the activation of mesenchymal cells, which in turn leads to the excessive accumulation of extracellular matrix. This appears to originate from different profibrotic pathways involving molecules such as TGF-β, tyrosine kinases, IL-13, IL-36, etc. Inflammation is also an important driver of fibrosis through the release of profibrotic factors. A detailed discussion of the pathogenesis of fibrosis is beyond the scope of this review but has been described elsewhere.5
Fistulizing disease is often found in conjunction with stricturing disease. It is commonly thought to be due to progression of disease from an underlying stenosis, given the fact that most internal fistulae are upstream of the stricture and originate from the area of pre-stenotic dilation. This theory, however, is largely anecdotal and has not been supported by prospective data.5 In a retrospective study, an underlying stricture was found in most patients with internal fistulizing CD, such that fistulas had a positive predictive value of 86.2% for the diagnosis of a stricture.9
Although no accurate predicting factors have been identified for the development of stricturing disease, several risk factors were associated with the development of a stenosis in the prospective multicenter TREAT registry and in the ACCENT I trial: duration of disease, severity of disease, ileal location of disease, and new corticosteroid use.10 Infliximab was not found to be associated with increased rates of stenosis, which helped debunk the opinion that anti-TNF therapy may drive stricture formation through rapid healing. Only colonic disease was shown to be protective.10
Strictures can occur anywhere in the gastrointestinal tract, but are most commonly found in the small bowel and follow the distribution of inflammation in CD.11 Colonic CD strictures are less common and associated with a higher rate of dysplasia. In a large French retrospective study, 2.4% of colon strictures were ultimately found to be associated with dysplasia or cancer despite thorough sampling during colonoscopy which did not show any signs of dysplasia or malignancy prior to resection.12 Strictures can also occur in ulcerative colitis (UC) and are found in 2–11.2% of patients.5 Although their malignant potential should be strongly considered, more than 70% of strictures in UC are in fact benign.13 In a series of 59 patients with UC and strictures, some of the factors associated with malignant potential were a location proximal to the splenic flexure, duration of disease longer than 20 years, and strictures associated with large bowel obstruction.13
Diagnosis
Stricturing CD may be diagnosed by different modalities. Clinically, it may be suggested by obstructive symptoms, such as post-prandial abdominal pain, distention, nausea, vomiting and dietary restrictions. However, symptoms correlate poorly with the presence of a stricture and further testing is essential.14 Endoscopy typically shows a narrowed lumen which cannot be traversed. Endoscopic scores such as SES-CD and CDEIS have incorporated stenosis as part of their scoring system and have defined stenosis as an area that is impossible or difficult to pass with an adult colonoscope.15,16 In an effort to standardize the diagnosis of stricturing disease, the CrOhN’s disease anti-fibrotic STRICTure therapies (CONSTRICT group), an international panel of experts, has devised the endoscopic definition of a stricture as “inability to pass an adult colonoscope through the narrowed area without prior endoscopic dilation with a reasonable amount of pressure applied.”14
Although biopsies should be performed in order to rule out underlying dysplasia or malignancy, it is important to note that biopsies may miss underlying dysplasia and cannot inform about the deeper layers of the intestine. Furthermore, there are no validated histology scoring systems to quantify the severity of fibrosis. A recent systematic review led by the Stenosis Therapy and Research (STAR) consortium found significant heterogeneity in histopathologic scores used to evaluate fibrostenotic CD, therefore highlighting the importance of the development of a validated histologic index to be used as a possible endpoint in future clinical trials.17 This work is currently ongoing.
Cross-sectional imaging is essential as it allows further characterization of the strictures and evaluation for complications such as abscess or fistulizing disease. Abdominal ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) can identify stenosis with varying degrees of diagnostic accuracy.18–22 Sensitivity and specificity for each modality can be found in Table 1.
Table 1.
Diagnostic accuracy of ultrasound, CT enterography and MR enterography in identification of strictures in studies comparing imaging to histopathology. Values are provided as ranges. Adapted from Bettenworth et al.18
| Ultrasound | CT enterography (only one study available) | MR enterography | |
|---|---|---|---|
| Sensitivity | 80%27–100%19 | 100%20 | 75%21–100%22 |
| Specificity | 63.3%19–75%27 | 100%20 | 91%22–96%21 |
In order to standardize radiologic diagnostic criteria, the CONSTRICT group defined a stricture on cross-sectional imaging as having the following characteristics: localized luminal narrowing, bowel wall thickening and pre-stricture dilation (Table 2). Although both CT enterography and magnetic resonance enterography (MRE) were found to be highly accurate, the latter was proposed as the preferred diagnostic modality given the absence of radiation exposure.14
Table 2.
Definition of small bowel strictures according to the CONSTRICT criteria. The combination of all three features should be present to diagnose a stricture on cross-sectional imaging. Adapted from Rieder et al.14
| Naïve small bowel stricture | Anastomotic small bowel stricture |
|---|---|
| 1. Localized luminal narrowing | 1. Localized luminal narrowing |
| ▪ Decrease in lumen diameter by >50% as compared with a normal, appropriately distended adjacent bowel | ▪ Decrease in lumen diameter by > 50% as compared with a normal, appropriately distended adjacent bowel |
| 2. Bowel wall thickening | 2. Bowel wall thickening |
| ▪ Increased wall thickness by 25% in the maximally thickened segment, as compared with a normally distended adjacent bowel | ▪ Maximal wall thickness >3 mm in an appropriately distended bowel |
| 3. Pre-stenotic dilation | 3. Pre-stenotic dilation |
| ▪ Proximal bowel diameter >3 cm | ▪ Proximal bowel diameter >3 cm |
Determining the degree of inflammation and fibrosis in a stricture can help guide management, but is often difficult as both elements coexist in most patients.5 MRE appears to be the most accurate in assessing the degree of fibrosis and inflammation, though no imaging modality to date can reliably quantify the degree of fibrosis in stricturing CD.18,23 Indeed, MRI was shown to differentiate between mild–moderate and severe fibrosis deposition with a sensitivity of 94% and a specificity of 89% using percentage of enhancement gain.24
Novel imaging modalities are being evaluated, such as magnetization transfer MRI, MR with dynamic contrast enhancement and ultrasound elastography; however, these are not ready to be used in clinical practice.5
Current management
Managing small bowel stricturing CD should be approached in a multidisciplinary fashion, involving input from gastroenterologists, colorectal surgeons, radiologists and pathologists, if necessary.23 In the acute setting of a CD-related small bowel obstruction, the patient should be hospitalized and promptly evaluated both clinically and by cross-sectional imaging in order to assess the disease and rule out complications such as perforation, abscess or fistulizing disease as well as any signs of underlying malignancy. Patients are initially kept nil per os until decompression is achieved. Hydration, electrolyte replacement and insertion of a nasogastric tube are often required; the C-reactive protein and abdominal X-rays are often reviewed daily. Intravenous corticosteroid therapy is typically started if there is an inflammatory component, despite limited supporting evidence. One study found that 25/26 patients with acute obstruction responded to intravenous corticosteroids within 72 h; however, only 28% remained obstruction-free at 52 months.25 In the case of persistent obstruction, biologic treatment, endoscopic balloon dilation (EBD), surgery or a combination thereof are often necessary. The management depends on stricture characteristics (such as inflammatory component, length, location), patient preference and the presence of complications such as a phlegmon, fistula, or abscess.26
Medical therapy
Anti-tumor necrosis factor (anti-TNF) agents have been evaluated in the medical management of stricturing CD. Although they were initially thought to increase stricturing through healing inflammation, more recent data, including data from the TREAT registry and the ACCENT I trial, do not support this finding and have found them to be safe and effective.10,27–30 Adalimumab was evaluated in a prospective observational cohort study in patients with stricturing small bowel CD (the CREOLE study). At week 24, 64% of patients were still on adalimumab without the need for additional therapy (e.g. EBD or surgery), and 50.7% of patients remained surgery free at 4 years.31 Other biologics such as vedolizumab or ustekinumab have not been evaluated in this setting.
In a randomized controlled trial which included 72 patients with sub-occlusive ileal CD randomized to either azathioprine or mesalamine, azathioprine led to a lower re-hospitalization rate as compared with mesalamine.32 A post-hoc analysis subsequently showed a lower rate of recurrent sub-occlusion with azathioprine.33 A summary of medical therapies and their evidence in stricturing CD can be found in Table 3.
Table 3.
Summary of medical therapies and their evidence in small bowel stricturing Crohn’s disease
| Medical therapy | Studies | Study design |
|---|---|---|
| Corticosteroids | ▪ Yaffe and Korelitz25 | ▪ Retrospective cohort |
| Azathioprine | ▪ De Souza et al.32 | ▪ Randomized controlled trial |
| ▪ Vidigal et al.33 | ▪ Post-hoc analysis of randomized controlled trial | |
| Adalimumab | ▪ Bouhnik et al.31 | ▪ Prospective cohort |
| Infliximab | ▪ Pallotta et al.27 | ▪ Prospective cohort |
| ▪ Holtmann et al.28 | ▪ Retrospective cohort | |
| ▪ Pelletier et al.29 | ▪ Retrospective cohort | |
| ▪ Allocca et al.30 | ▪ Retrospective cohort |
Endoscopic therapy
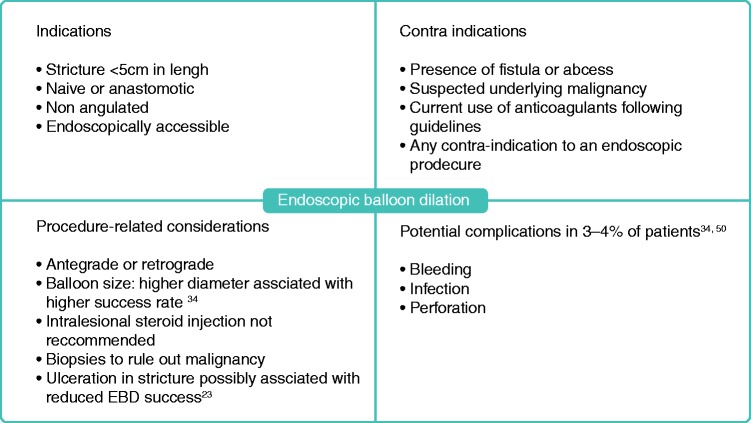
If the disease is refractory to medical therapy, endoscopic therapy or surgery should be performed. EBD is an option for strictures that are short (<5 cm long), straight, and accessible with an endoscope or colonoscope. It should not be performed in the setting of complications, i.e. penetrating disease or suspected malignancy.5 EBD can be attempted in naïve and anastomotic strictures with similar success rates.34 EBD is most commonly performed using a through-the-scope balloon, in either retrograde or anterograde fashion, and can be performed in upper gastrointestinal, small bowel and colonic strictures.26 However, duodenal strictures appear to be five times more likely to necessitate earlier surgery after dilation compared with strictures in the jejunum, ileum or colon.34 Although EBD can be performed in colonic strictures, surgery should be considered given the higher risk of malignancy in this setting compared with small bowel strictures.12 A practical guide to EBD can be found in Figure 1.
Figure 1.
Key considerations prior to endoscopic balloon dilation of IBD strictures.
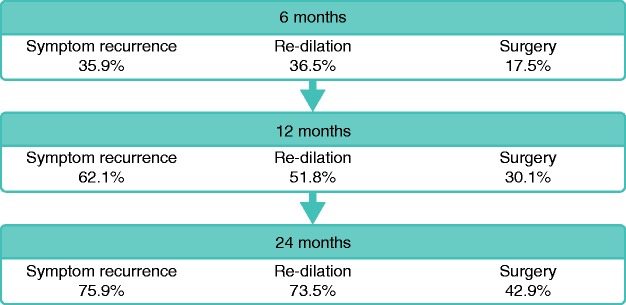
A systematic review including 1463 patients and a total of 3213 EBD procedures found a technical success rate of 89.1%, with clinical efficacy in 80.8% of cases; 42.9% of patients underwent surgical resection at 24 months. Complications occurred in 2.8% of procedures and included perforation, fever and bleeding.34 Ulceration in the stricture was not associated with increased rates of complications.34 However, available studies were observational and therefore susceptible to bias toward possible avoidance of EBD in patients with underlying inflammation. Factors such as length <5 cm, absence of ulcerations in the stricture, and technically successful EBD were found to be associated with clinically successful short-term outcomes.23 However, repeat dilation is often required—up to 73.5% at 2 years in the previously mentioned study 34 —but does not appear to be associated with an increased risk of complications over time.23 A summary of short-term and long-term outcomes of EBD can be found in Figure 2.
Figure 2.
Short and long-term outcomes of EBD: Symptom recurrence, need for re-dilation and need for surgery at 6, 12 and 24 months. Adapted from Bettenworth et al.34
Other endoscopic modalities have been evaluated such as stent insertion, intralesional steroid or anti-TNF injection but cannot be recommended for routine practice given limited evidence.26 Needle-knife stricturotomy has been found to have promising short-term outcomes and warrants further exploration, in particular in controlled settings and with a thorough evaluation of complication rates.35
Surgery
Surgical options include segmental resection and strictureplasty. Strictures refractory to medical therapy, not amenable or refractory to EBD, or associated with complications or malignancy should be managed surgically.23 However, the timing of surgery appears to be important. Early resection, either at diagnosis or shortly after diagnosis in ileocecal stricturing CD was found to be associated with longer clinical remission,36 decreased risk of repeat surgery37 and decreased overall exposure to steroids and biologic therapies.38 Therefore the decision to proceed with surgery earlier on after stricture diagnosis should be considered and based on disease and stricture characteristics as well as patient preference.23
Strictureplasty is a bowel-sparing approach. It is preferred in patients with prior extensive resections or at risk of short bowel syndrome. Contra-indications include suspected malignancy, penetrating complications, malnutrition and colonic strictures. The Heineke–Mikulicz technique is typically performed for short strictures (<10 cm) and the Finney technique for intermediate length strictures (10–20 cm), whereas the “non-conventional” strictureplasty such as the Michelassi is rarely performed for very long areas of continuous disease.39 Interestingly, recurrence at the site of strictureplasty is uncommon and most recurrences occur at a different site.39 An ultrasound study has in fact shown a decreased bowel wall thickness at the site of strictureplasty over time, and observational data indicate that regression of stricturing disease occurs.40–42 For upper gastrointestinal stricturing CD, bypass surgery is an additional surgical option.26
Of note, smoking cessation and optimization of nutritional status and anemia are important steps in management and should be addressed in all patients. In addition, patients may need to be on medical therapy postoperatively according to risk factors, and should be monitored closely for recurrence regardless of medical treatment.23,43
Perspective
Despite recent advances in the management of IBD, surgery is often necessary and postoperative recurrence is common.4 Current biologic therapies address inflammation, but no available agents target fibrosis. Several promising molecules are being evaluated.44 AMA0825, a Rho-associated protein kinase inhibitor, was found to reverse intestinal fibrosis in mice and reduce the secretion of pro-fibrotic markers in CD biopsies.45 Tranilast exhibits anti-fibrotic properties through reduction of TGF-β activity in rats.46 It was evaluated in a prospective study in patients with asymptomatic CD strictures and was associated with higher rates of asymptomatic patients compared with controls over a median observation period of 782 days.47 The peroxisome proliferator-activated receptor gamma agonist GED-0507-34 indicated anti-fibrotic properties in mice.48 More recently, an antibody to the IL-36 receptor was found to decrease fibrosis in mice with chronic intestinal inflammation.49 More detailed mechanistic information can be found in a separate review on this topic.5
There is a pressing need to develop and evaluate anti-fibrotics for the treatment of stricturing CD. This requires the development of standardized diagnostic criteria, patient-reported outcome measures (PROs) and validation of endpoints for fibrostenotic CD. The STAR consortium, an international group of experts, is laying the groundwork for this and has proposed consensus-based definitions of strictures in CD, as well as diagnostic criteria. Endpoints to be used in future clinical trials are currently being built, which includes PRO, radiology and histopathology indices.14 The PRO and radiology indices are being validated in a prospective clinical study.
Acknowledgment
SE and FR planned, conducted the study and drafted the manuscript. All authors have contributed to drafting the manuscript and approved the final version of the manuscript.
Declaration of Conflicting Interests
FR is on the advisory board or consultant for AbbVie, Celgene, Receptos, Thetis, UCB, Samsung, Pliant, Boehringer-Ingelheim, Helmsley, RedX, Thetis, Gossamer, Pfizer, Gilead, Takeda and Roche. BC is on the speaker’s bureau for Takeda.
Funding
This work was supported by the Helmsley Charitable Trust through the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium.
References
- 1.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001; 49: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010; 139: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: A systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017; 152: 340–350.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeuring SFG, Van Den Heuvel TRA, Liu LYL, et al. Improvements in the long-term outcome of Crohn’s Disease over the past two decades and the relation to changes in medical management: Results from the population-based IBDSL cohort. Am J Gastroenterol 2017; 112: 325–336. [DOI] [PubMed] [Google Scholar]
- 7.Lazarev M, Ullman T, Schraut WH, et al. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: Experience from a large tertiary care center. Inflamm Bowel Dis 2010; 16: 830–835. [DOI] [PubMed] [Google Scholar]
- 8.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017; 389: 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jürgens M, Brand S, Laubender RP, et al. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn’s disease independent of the IL23R genotype. J Gastroenterol 2010; 45: 721–731. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol 2006; 101: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 11.Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology 1985; 88: 1818–1825. [DOI] [PubMed] [Google Scholar]
- 12.Fumery M, Pineton de Chambrun G, Stefanescu C, et al. Detection of dysplasia or cancer in 3.5% of patients with inflammatory bowel disease and colonic strictures. Clin Gastroenterol Hepatol 2015; 13: 1770–1775. [DOI] [PubMed] [Google Scholar]
- 13.Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut 1992; 33: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther 2018; 48: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: A prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989; 30: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 17.Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn’s disease: A systematic review. Gastroenterology. Epub ahead of print 2019. DOI: 10.1053/j.gastro.2019.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: A systematic review. Gut 2019; 68: 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn’s disease: Clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther 2003; 18: 749–756. [DOI] [PubMed] [Google Scholar]
- 20.Vogel J, Da Luz Moreira A, Baker M, et al. CT enterography for Crohn’s disease: Accurate preoperative diagnostic imaging. Dis Colon Rectum 2007; 50: 1761–1769. [DOI] [PubMed] [Google Scholar]
- 21.Pous-Serrano S, Frasson M, Palasí Giménez R, et al. Accuracy of magnetic resonance enterography in the preoperative assessment of patients with Crohn’s disease of the small bowel. Color Dis 2017; 19: O126–O133. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Hakim A, Alexakis C, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: Correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol 2015; 30: 86–91. [DOI] [PubMed] [Google Scholar]
- 23.Rieder F, Latella G, Magro F, et al. European Crohn’s and colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J Crohn’s Colitis 2016; 10: 873–885. [DOI] [PubMed] [Google Scholar]
- 24.Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–440. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol 1983; 5: 211–215. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, Holubar SD, Rieder F. How I approach the management of stricturing Crohn’s disease. Am J Gastroenterol 2019; 114: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 27.Pallotta N, Barberani F, Hassan N-A, et al. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J Gastroenterol 2008; 14: 1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtmann M, Wanitschke R, Helisch A, et al. [Anti-TNF antibodies in the treatment of inflammatory intestinal stenoses in Crohn’s disease]. Z Gastroenterol 2003; 41: 11–17. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier AL, Kalisazan B, Wienckiewicz J, et al. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment Pharmacol Ther 2009; 29: 279–285. [DOI] [PubMed] [Google Scholar]
- 30.Allocca M, Bonifacio C, Fiorino G, et al. Efficacy of tumour necrosis factor antagonists in stricturing Crohn’s disease: A tertiary center real-life experience. Dig Liver Dis 2017; 49: 872–877. [DOI] [PubMed] [Google Scholar]
- 31.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2017; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza GS, Vidigal FM, Chebli LA, et al. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in sub-occlusive ileocecal Crohn’s disease patients. Med Sci Monit 2013; 19: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidigal FM, de Souza GS, Chebli LA, et al. Azathioprine is more effective than mesalazine at preventing recurrent bowel obstruction in patients with ileocecal Crohn’s disease. Med Sci Monit 2014; 20: 2165–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis 2017; 23: 133–142. [DOI] [PubMed] [Google Scholar]
- 35.Lan N, Shen B. Endoscopic stricturotomy with needle knife in the treatment of strictures from inflammatory bowel disease. Inflamm Bowel Dis 2017; 23: 502–513. [DOI] [PubMed] [Google Scholar]
- 36.Aratari A, Papi C, Leandro G, et al. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther 2007; 26: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 37.Latella G, Cocco A, Angelucci E, et al. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis 2009; 41: 269–276. [DOI] [PubMed] [Google Scholar]
- 38.Golovics PA, Lakatos L, Nagy A, et al. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol 2013; 19: 7701–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strong SA. Strictureplasty in complex Crohn’s disease: Beyond the basics. Clin Colon Rectal Surg 2019; 32: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: A prospective study. Ann Surg 2001; 233: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Buck van Overstraeten A, Vermeire S, Vanbeckevoort D, et al. Modified side-to-side isoperistaltic strictureplasty over the ileocaecal valve: An alternative to ileocaecal resection in extensive terminal ileal Crohn’s disease. J Crohns Colitis 2016; 10: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazio VW, Tjandra JJ, Lavery IC, et al. Long-term follow-up of strictureplasty in Crohn’s disease. Dis Colon Rectum 1993; 36: 355–361. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: Management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113: 481–517. [DOI] [PubMed] [Google Scholar]
- 44.Pariente B, Hu S, Bettenworth D, et al. Treatments for Crohn’s disease-associated bowel damage: A systematic review. Clin Gastroenterol Hepatol 2019; 17: 847–856. [DOI] [PubMed] [Google Scholar]
- 45.Holvoet T, Devriese S, Castermans K, et al. Treatment of intestinal fibrosis in experimental inflammatory bowel disease by the pleiotropic actions of a local rho kinase inhibitor. Gastroenterology 2017; 153: 1054–1067. [DOI] [PubMed] [Google Scholar]
- 46.Martin J, Kelly D, Mifsud S, et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-? Cardiovasc Res 2005; 65: 694–701. [DOI] [PubMed] [Google Scholar]
- 47.Oshitani N, Yamagami H, Watanabe K, et al. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn’s disease. Gut 2007; 56: 599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speca S, Rousseaux C, Dubuquoy C, et al. Novel PPARγ modulator GED-0507-34 Levo ameliorates inflammation-driven intestinal fibrosis. Inflamm Bowel Dis 2016; 22: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019; 156: 1082–1097.e11. [DOI] [PubMed] [Google Scholar]
- 50.Navaneethan U, Lourdusamy V, Njei B, et al. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: A systematic review and meta-analysis of non-randomized trials. Surg Endosc 2016; 30: 5434–5443. [DOI] [PubMed] [Google Scholar]