Immune-mediated hemolytic anemia (HA) is one of the possible complications after both solid organ and allogeneic stem cell transplantation (ASCT), potentially associated with significant morbidity, including severe hemolysis and acute renal failure1. Death from massive hemolysis has also been reported in the literature2. Passenger lymphocyte syndrome (PLS) is occasionally a cause of hemolysis in these patients. PLS occurs between day 5 and 15 post transplant when a minor ABO-incompatibility exists between the donor and the recipient (most common A+ recipient, O+ donor). Immunocompetent donor B-lymphocytes transferred passively with the graft maintain their ability to generate antibodies which bind to the recipient’s RBCs causing hemolysis3–6. The treatment of PLS has been mainly supportive, with or without immunosuppression1, 6. This report describes the first experience with the use of rituximab (Rituxan®, Genentech, CA) for the treatment of PLS in an ASCT patient, and reviews the accumulated data regarding the transfusion requirement for patients with PLS after ASCT.
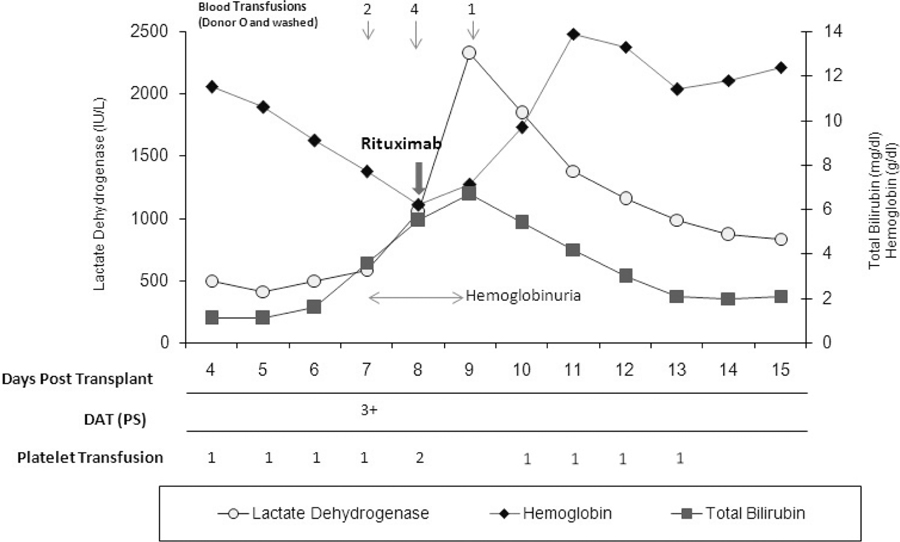
A 65-year-old woman with chronic myelomonocytic leukemia was admitted for an ASCT from a 10/10 HLA-matched unrelated donor. A minor ABO-mismatch was present between the donor and the recipient (group A, Rh-positive) and the donor (group O, Rh-positive). The preparative regimen consisted of gemtuzumab ozogamicin 2mg/m2 on day –12, fludarabine 25mg/m2 iv days –7 to −4, melphalan 70mg/m2 on days −4 and –3. GVHD prophylaxis was employed with rabbit ATG 0.5 mg/kg on day −3 and 1.25 mg/kg on days −2 and −1, tacrolimus and “mini-methotrexate” (MTX) 5mg/m2 on days 1, 3, 6 and 11 post transplant7. The patient received a bone marrow blood graft containing 3.1x106 CD34+ cells/kg and 21.8x104 CD3+ cells/kg. No graft manipulation was performed. The post-transplant course was uneventful until day 6 when the patient developed mild indirect bilirubinemia (iBr) and increased LDH. This evolved over the next two days to full HA, with rapid drop in hemoglobin (Hgb) to 6g/dl, increase LDH to 1051 IU/L, further rise in iBr to 3.0mg/dl (total Br 3.6mg/dl), low haptoglobin (22mg/dl) and hemoglobinuria. The peripheral blood smear revealed numerous spherocytes. On day 7, a direct Coombs test (DAT) was strongly positive (3+) for IgG and complement. Anti-A IgG antibodies were elutriated off the surface of the RBCs. Patient’s plasma showed no evidence of anti RBC antibodies. The patient began transfusion with packed RBCs (PRBCs) and started steroids, methylprednisolone 2.5mg/kg/day. Due to extensive hemolysis one dose of rituximab 375mg/kg was given on day 8 after transplant. This was associated with a rapid resolution of the hemolytic process, which lasted for only 3 days (Figure 1). The patient received at total of 7 units of PRBCs, 2 units on day 7, 4 units on day 8, one unit on day 9. A repeat DAT was not performed until day 20 post transplant, when it was found to be negative.
Figure 1.
Changes in laboratory parameters in a patient with PLS-associated hemolysis after rituximab administration.
PS, polyspecific (anti-IgG and C3); Transfused platelets were single donor, irradiated and leukocyte depleted.
PLS is an intriguing immunological phenomenon described both in solid organ and ASCT1. Several risk factors for the development of PLS have been postulated in the literature including the degree of mismatch, peripheral stem cell source, amount of lymphoid tissue transplanted and the use of cyclosporine/tacrolimus without methotrexate for GVHD prophylaxis1, 6, 8. Treatment has been transfusion with donor ABO compatible PRBCs (O+ PRBCs are used in this setting) steroids, occasionally plasma or red cell exchange and adequate kidney perfusion1, 6. Rituximab, a chimeric monoclonoal antibody directed against CD 20+ B-lymphocytes, has been tried in HA of other causes, and its use in PLS would make intuitive sense. Rituximab has been successfully used in one pediatric solid organ transplantation case and in two pediatric cases of late onset hemolytic anemia5, 9, 10. Our patient displayed typical features of PLS for which rituximab, in addition to O+ PRBCs and steroids, aborted the massive intravascular hemolysis soon after its onset. MTX has also been shown to mitigate hemolysis secondary to PLS, however pretreated patients with MTX and ATG still appear to be susceptible to the development of this clinical entity3, 8. A lower dose of MTX as in the “mini-methotrexate” regimen received by this patient could be less efficacious in preventing PLS. Such patients may benefit from the use of rituximab in case severe hemolysis develops.
We reviewed 27 cases of PLS associated with ASCT reported in the English literature (search on 11/21/07 PubMed/Ovid/Medline for passenger lymphocyte syndrome/minor ABO mismatch/ASCT) for which the duration of hemolysis and the number of PRBCs units transfused were reported (Table 1). The median number of days of hemolysis was 8 (range 4–30) while the median number of PRBCs transfused was 9 (range 5–31). In the only other PLS case treated with rituximab the duration of hemolysis lasted for 5 days and the number of PRBCs units transfused was also 75. Although the experience is very limited, these findings suggest that rituximab may be an effective therapy to minimize severe hemolysis associated with PLS. Further evaluation could better determine its efficacy and provide more insight into the mechanisms of action in this disease.
Table 1.
Duration of hemolysis and transfusion requirement in 27 patients with PLS post AHSCT.
| Patient Characteristic | Hemolysis | |||||
|---|---|---|---|---|---|---|
| References | Age | Dx | Start Date | Resolution Date | Days Total | RBC Units Transfused |
| Hows, J. et al. Blood 1986;67:177–181 | 17 | CGL | 10 | 15 | 6 | 9 |
| 13 | CGL | 16 | 22 | 7 | 6 | |
| 24 | SAA | 10 | 19 | 10 | 14 | |
| 11 | AML | 9 | 18 | 10 | 7 | |
| 26 | ALL | 11 | 14 | 4 | 6 | |
| 32 | SAA | 9 | 16 | 8 | 5 | |
| Toren, A. et al. Blood 1996;87:843–4 | 12 | ALL | 8 | 14 | 7 | 7 |
| Greeno, E.W. et al. Transfusion 1996;36:71–4 | 37 | CML | 7 | 22 | 16 | 16 |
| Bornhauser, M. et al. BMT 1997;19:295–7 | 23 | CML | 12 | 17 | 6 | 10 |
| Oziel-Taieb, S. et al. BMT 1997;19:1155–6 | 38 | MM | 8 | 18 | 11 | 12 |
| Moog, R. et al. Beitr Infus Trans 1997;34:150–2 | 19 | AML | 9 | 16 | 8 | NR |
| Laurencet, F.M. et al. Hematol Cell Ther 1997;39:159–62 | 37 | MM | 12 | 22 | 11 | 9 |
| Salmon, J.P. et al. Transfusion 1999;39:824–7 | 16 | AML | 7 | 13 | 7 | NR |
| Leo, A. et al. Transfusion 2000;40:632–6 | 50 | AML | 17 | 24 | 8 | 10 |
| Tiplady, C.W. et al. Transfus Med 2001;11:455–8 | 28 | ALL | 9 | 14 | 6 | 17 |
| Bolan, C.D. et al. Br J Hematol 2001;112:787–95 | 55 | CLL | 8 | 16 | 9 | 6 |
| 38 | AML | 6 | 13 | 8 | 8 | |
| 38 | NHL | 10 | 13 | 4 | 9 | |
| Hoegler, W. et al. Med Pediatr Oncol 2002;38:143–4 | 7 | ALL | 8 | 17 | 10 | 7 |
| Worel, N. et al. Transfusion 2002;42:1293–301 | 35 | ALL | 9 | 22 | 14 | NR |
| 33 | AML | 10 | 21 | 12 | NR | |
| 47 | NHL | 7 | 25 | 19 | NR | |
| 42 | AML | 8 | 11 | 4 | NR | |
| Reed, M. et al. Arch Pathol Lab Med 2003;127:1366–8 | 61 | AML | NR | NR | NR | NR |
| Noborio, K. et al. Leuk Lymphoma 2003;44:357–9 | 35 | CTCL | 8 | 12 | 5 | NR |
| Curtin, N.J. et al. Leuk Lymphoma 2005;27:206–8 | 54 | TPLL | 11 | 22 | 12 | 8 |
| Nair, V. et al. BMT 2007;39:805–6 | 13 | ALL | 12 | 18 | 7 | 31 |
Reference:
- [1].Yazer MH, Triulzi DJ. Immune hemolysis following ABO-mismatched stem cell or solid organ transplantation. Curr Opin Hematol 2007. November;14(6):664–70. [DOI] [PubMed] [Google Scholar]
- [2].Horn B, Viele M, Mentzer W, Mogck N, DeSantes K, Cowan M. Autoimmune hemolytic anemia in patients with SCID after T cell-depleted BM and PBSC transplantation. Bone Marrow Transplant 1999. November;24(9):1009–13. [DOI] [PubMed] [Google Scholar]
- [3].Hows J, Beddow K, Gordon-Smith E, Branch DR, Spruce W, Sniecinski I, et al. Donor-derived red blood cell antibodies and immune hemolysis after allogeneic bone marrow transplantation. Blood. 1986 January 1, 1986;67(1):177–81. [PubMed] [Google Scholar]
- [4].Toren A, Dacosta Y, Manny N, Varadi G, Or R, Nagler A. Passenger B-lymphocyte-induced severe hemolytic disease after allogeneic peripheral blood stem cell transplantation. Blood. 1996. January 15;87(2):843–4. [PubMed] [Google Scholar]
- [5].Panaro F, DeChristopher PJ, Rondelli D, Testa G, Sankary H, Popescu M, et al. Severe hemolytic anemia due to passenger lymphocytes after living-related bowel transplant. Clin Transplant 2004. June;18(3):332–5. [DOI] [PubMed] [Google Scholar]
- [6].Sokol RJ, Stamps R, Booker DJ, Scott FM, Laidlaw ST, Vandenberghe EA, et al. Posttransplant immune-mediated hemolysis. Transfusion. 2002. February;42(2):198–204. [DOI] [PubMed] [Google Scholar]
- [7].de Lima M, Champlin RE, Thall PF, Wang X, Martin TG 3rd, Cook JD, et al. Phase I/II study of gemtuzumab ozogamicin added to fludarabine, melphalan and allogeneic hematopoietic stem cell transplantation for high-risk CD33 positive myeloid leukemias and myelodysplastic syndrome. Leukemia. 2007. November 8. [DOI] [PubMed]
- [8].Gajewski JL, Petz LD, Calhoun L, O’Rourke S, Landaw EM, Lyddane NR, et al. Hemolysis of transfused group O red blood cells in minor ABO-incompatible unrelated-donor bone marrow transplants in patients receiving cyclosporine without posttransplant methotrexate. Blood. 1992 June 1, 1992;79(11):3076–85. [PubMed] [Google Scholar]
- [9].Corti P, Bonanomi S, Vallinoto C, Balduzzi A, Uderzo C, Cazzaniga G, et al. Rituximab for immune hemolytic anemia following T- and B-Cell-depleted hematopoietic stem cell transplantation. Acta Haematol 2003;109(1):43–5. [DOI] [PubMed] [Google Scholar]
- [10].Ship A, May W, Lucas K. Anti-CD20 monoclonal antibody therapy for autoimmune hemolytic anemia following T cell-depleted, haplo-identical stem cell transplantation. Bone Marrow Transplant 2002. February;29(4):365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]