Abstract
Background
Collection of biologic samples from the nasal cavity and paranasal sinuses is of critical importance to the study of infectious or inflammatory conditions that affect both upper and lower airways. Numerous techniques for the study of ex-vivo samples exist, with specific applications, strengths, and weaknesses associated with each of them. In this compendium we summarize the available methods for collection of primary human samples and incorporate expert discussion of the pros, cons, and applications associated with each technique.
Methods
An expert panel containing members of the American Rhinologic Society’s Research and Grants Commiee compiled this educational reference. Rationale for use and the potential advantages and disadvantages are discussed. Research protocols and key references are enumerated.
Results
Sampling of the nasal cavity and paranasal sinuses can be achieved through a number of methods. Nonspecific sinonasal secretions may be collected via forced exhalation, nasal lavage, and nasal spray aspiration. Targeted collection of sinonasal secretions may be achieved via endoscopic placement of absorbent matrices. Nasal cytology or collection of superficial epithelium may be completed via brushing or scraping of endonasal structures. Collection of mucosal biopsies may be completed via sinonasal explant or full-thickness biopsy.
Conclusion
Multiple sampling techniques are available to collect biologic samples from the sinonasal cavity. These techniques differ in their ease of application, reproducibility, sample yield, and utility for different sinonasal pathologies or research goals. An appreciation of the benefits and drawbacks of each approach will allow investigators to select the techniques most appropriate for achieving research objectives.
Keywords: nasal cavity, paranasal sinuses, clinical protocols, sinusitis, nasal lavage fluid, nasal mucosa, cytologic techniques, exosomes
The fields of rhinology and allergy have seen an explosion in the quantity and quality of research in recent years.1 It is increasingly common for studies to combine objective clinical data or basic science findings with in-vitro experiments using biologic samples obtained from the nasal cavity and paranasal sinuses. Such experiments are of critical importance in the study of inflammatory, infectious, and allergic conditions affecting the sinonasal cavity, and may also provide quantitative metrics to assess treatment response. Furthermore, such studies may help elucidate the mechanisms underlying lower airway pathology, and, with growing acceptance of the unified airway theory, biologic samples from the nasal cavity are now frequently used as proxy tissues for the study of lower airway disease given their relative ease of procurement.2,3
Numerous techniques have been devised for the sampling and collection of nasal secretions, cells, and whole tissue in an effort to objectively characterize the sinonasal milieu. These differ significantly in their ease of use, reproducibility, and applicability to different sinonasal pathologies or research objectives. In this work we discuss some commonly accepted techniques, while addressing proper protocols, applications, and potential advantages and disadvantages of each method. Although this investigation is not meant to be a comprehensive review of all methods nor a systematic comparison of sampling techniques, we hope it will serve as a valuable reference for those considering studies using human sinonasal biologic samples. Cited protocols can be found in the Appendix, with a summary of the advantages and disadvantages of various techniques compiled in Table 1, with possible indications of each listed in Table 2, and a demonstration of select techniques in Figure 1.
TABLE 1.
Advantages and disadvantages of various sample collection techniques
| Technique | Advantages | Disadvantages |
|---|---|---|
| Bulk surface fluid collection | ||
| Blowing of secretions | • Noninvasive • Fast • No topical anesthesia required • Inexpensive |
• Ability to blow nose may be limited by anatomy, subject effort, viscosity of secretions, or mucosal edema • Mucosal origin and quantity of sample may vary |
| Nasal spray aspiration | • Noninvasive • Fast • No topical anesthesia required • Inexpensive |
• Secretions must be present unless nasal saline spray is used before collection • Blind suctioning may traumatize nasal mucosa • Difficult to assess for dilution factor |
| Nasal lavage | • Fast • Nontraumatic • No need for topical anesthesia • No need for instrumentation of nasal cavity • Inexpensive |
• Requires subject compliance • Mucus and contents may be overly diluted • Variability in path of lavage fluid flow • Cannot isolate fluid from specific anatomic regions or structures |
| Focal surface fluid collection | ||
| Cotton wool | • Inexpensive • Known dilution factor |
• Artifact from insertion trauma has not been well characterized |
| Foam rubber | • Inexpensive • May have more efficient protein recovery than nasal lavage |
• Artifact from insertion trauma has not been well characterized |
| Filter paper | • Inexpensive • May be more effective at cytokine recovery than nasal lavage • Can be directed to anatomic area of interest |
• Artifact from insertion trauma has not been well characterized |
| Absorbent fibrous matrix (Leukosorb) |
• Limits dilution of soluble biomarkers • Can be directed to anatomic area of interest • Sample stability during frozen storage has been demonstrated |
• Proprietary sampling technology may be more expensive than similar alternatives • Artifact from insertion trauma has not been well characterized |
| Nasal cytology | ||
| Nasal brush | • Fast • No need for topical anesthesia • Endoscopic guidance is not needed • May recover more cells than nasal lavage |
• The ability to collect inflammatory mediators is unclear • The degree of subject discomfort is proportional to the vigorousness of brushing, which correlates with sampling yield |
| Nasal scraping | • Fast • Minimally traumatic |
• Proprietary sampling technology may be more expensive than similar alternatives • The ability to retrieve cell mediators such as interleukins and cytokines has not been documented |
| Mucosal biopsy | ||
| • Allows for study of tissue architecture including basement membrane | • Anesthetic agents are needed and may cause aberrances in ciliary beat frequency or allergic reactions • Time-consuming • Increased equipment requirements • Traumatic • Risk of epistaxis • Serial studies generally not feasible • Technically challenging |
|
| Mucus exosome sampling | ||
| • Allows for study of exosomal contents (lipids, proteins, nucleic acids) • Noninvasive • Repetitive sampling feasible |
• UCF is time-consuming • Requires specific equipment • Other microvesicles may be co-purified • UCF is used best for larger sample sizes; for a small sample size, the nanowire-on-micropillar technique and immunoaffinity-based isolation strategies may be best |
TABLE 2.
List of commonly reported applications for each technique
| Technique | Common sample targets/indications |
|---|---|
| Bulk and focal surface fluid collection | • Extracellular proteins, mediators, and biomarkers: Prostaglandins, ECP, EPX, leukotrienes, cytokines/interleukins, chemokines, tryptase, MMP, elastase, exosomes • Measurement of exudative response: As determined via α2-macroglobulin levels or total protein • Cytology of mucus: Eosinophils, basophils, mast cells, neutrophils, leukocytes • Microbiology: Gram stain, fungal stains, culture studies, DNA sequencing • Nucleic acid studies: Isolation, extraction, and sequencing for transcriptome analysis or microbiota characterization |
| Nasal brushing/scraping | • Cytology of epithelial surface: Eosinophils, basophils, mast cells, neutrophils, leukocytes, epithelial cells, goblet cells • Cell culture |
| Nasal biopsy | • Immunohistochemistry: Tissue remodeling, eosinophil count, olfactory epithelium identification • Hematoxylin-and-eosin staining: Tissue architecture, characterization of inflammatory infiltrate • In-situ hybridization studies: For specific mRNA characterization • Cell culture • Microbiology: Gram stain, fungal stains, culture studies, DNA sequencing • Nucleic acid studies: Isolation, extraction, and sequencing for transcriptome analysis or microbiota characterization |
ECP = eosinophilic cationic protein; EPX = eosinophilic peroxidase; MMP = matrix metalloproteinase.
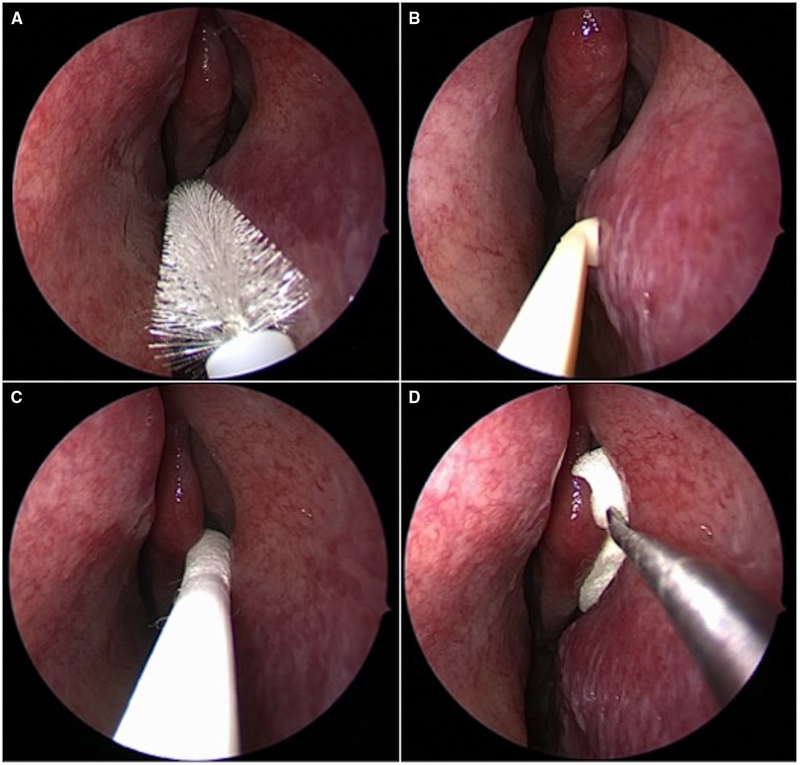
FIGURE 1.
Endoscopic images of select techniques, including nasal cytology brush (A) and nasal curette (B) sampling of inferior turbinate, as well as middle meatal placement of a cotton-tipped swab (C) and foam sponge (D).
Techniques for collection of nasal secretions
Nasal secretions may contain several biomarkers of interest.Mucusservesanumberofcriticalfunctionsintheupper airway, including roles in airway filtration, olfaction, and as a key component of the host defense system. In addition, barrier dysfunction occurring in chronic inflammatory states may allow for representation of stromal molecular or cellular components to translocate to the airway surface liquid.4 Nasal mucus contains ions and proteins in an aqueous base, and is composed of 2 distinct layers–the apical mucus layer, which traps inhaled particulate matter and pathogens, and the basal periciliary layer, which acts as a lubricant for ciliary beating.5 The ion concentration in mucus is variable with a sodium concentration ranging from 102 to 150 mEq/L, and a chloride concentration of 41 to 46 mEq/L.6 The active absorption and secretions of these ions across the apical membrane of airway epithelium regulates the water content of mucus. Much of the molecular weight of mucus is composed of mucin, a glycoprotein secreted by goblet cells. The oligosaccharides in mucin bind water, thus making this protein responsible for the viscous properties of mucus.7 Mucus also contains lysozyme, lactoferrin, and peroxidase, all key components of the innate immune system that can be quantified in nasal secretions.8 Other immune-related proteins in mucus include complement factors, immunoglobulins, interleukins, leukotrienes, macrophage inflammatory protein, eosinophilic cationic protein, and matrix metalloproteinases. Importantly, certain cytokines obtained from mucus may be representative of those derived from sinus tissue.9 Previous work has also established the association between secreted inflammatory markers and objective levels of olfactory dysfunction,10 and transcriptomic and proteomic analysis of secretions has been used to better characterize the molecular basis of inflammatory disease.11 Finally, the exudative response, defined as the transmigration of plasma or leukocytes to the apical surface of an epithelium often in response to inflammatory or allergic stimuli, can be measured via total protein or α2-macroglobulin levels.12
In addition to the study of inflammatory markers, nasal secretions may also be collected to characterize secreted microsomes that are representative of the nasal microbial environment.13 Bacteria, fungal organisms, and viruses can all be easily recovered from nasal secretions. Microbial collection is most commonly performed via nasal swab14 or lavage,15 the former of which has been shown to be representative of mucosal tissue sampling.16
Methods of obtaining nasal secretions may fall into either 1 of 2 categories: bulk surface fluid collection and focal surface fluid collection. Bulk surface fluid collection relies on recovering secretions from throughout the nasal cavity, usually with the aid of a normal saline diluent. Techniques include: (1) blowing of nasal secretions; (2) nasal aspiration; and (3) nasal lavage or washings. Focal surface fluid collection is one that is primarily absorptive in nature, making use of filter paper, cotton wool, foam, or sponges to recover nasal secretions. These materials can be directed to certain areas within the nasal cavity or nasopharynx for more precise sample procurement, if desired.
Bulk surface fluid collection
Blowing of nasal secretions
This technique involves having the subject force air through the nasal passages to mobilize secretions that may be present. No widely cited reference for this method exists, although a protocol has been presented by Klimek et al.17 Blowing of nasal secretions as a collection technique has largely fallen out of favor due to inconsistency in sampling and feasibility of alternative methods.
Nasal aspiration
Methods for introducing a suction device to collect nasal secretions seem to vary widely. Flexible or rigid suction may be employed, usually connected to a mucus specimen collector such as the Lukens Trap (McKesson, San Francisco, CA). The location of directed suctioning may depend on the process being studied. For example, the nasal cavity or nasopharynx may be of interest for the study of rhinitis or nasopharyngeal microbial colonization, respectively. The middle meatus is an ideal single site for obtaining samples for microbiologic analysis, as this mucus correlates closely with specimens obtained through invasive maxillary sinus puncture.18,19 However, no study has comprehensively compared the characteristics of secretions between specific anatomic locations.
Aspiration of mucus under endoscopic guidance reduces the risk of iatrogenic trauma caused by blind aspiration, and therefore may reduce blood contamination of acquired samples. Successful sample collection is also contingent on mucus being present in the nasal cavity. This may be overcome by spraying the nasal cavity first with normal saline, as described by Fujimoto et al.20
Nasal lavage
Nasal lavage involves instilling a volume of solution into the nasal cavities followed by its collection after a predetermined dwell time. The most widely cited protocol was presented by Naclerio et al.21 Nasal lavage fluid has been used to identify biomarkers used in determining the inflammatory endotype of chronic rhinosinusitus (CRS).22,23 Given the inherent dilution that occurs in this technique, it may be helpful in qualitative assessment (ie, presence or absence of target), or when subsequent laboratory assays are highly sensitive or are able to concentrate the compound of interest.
Focal surface fluid collection
Cotton wool
Kramer et al24 used cotton wool strips to obtain nasal secretions for analysis of tryptase and eosinophilic cationic protein. They described insertion of a strip of cotton measuring 4 × 0.6 cm into the middle meatus under anterior rhinoscopy. The strip is then removed after 10 minutes and centrifuged to extract secretions. Cotton wool may also be used in swab form, particularly in cases of microbial sampling. If the middle meatus is to be sampled, endoscopic guidance should be used so as to minimize contact with vibrissae or other nasal surfaces.25
Foam rubber
Lü and Esch26 compared various approaches with polyurethane foam to a nasal lavage method in subjects with seasonal allergic rhinitis,assaying the relative amounts of various inflammatory cytokines, immunoglobulins, and allergen-specific autoantibodies collected from the 2 methods (the authors employed the nasal lavage technique, as described by Naclerio et al21). They found that foam collectors were able to procure 8 times the amount of key proteins, such as tryptase and eosinophilic cationic protein, when compared with the nasal lavage method. Methods were not compared within-subject, however, and the authors did acknowledge a large intersubject variability in biomarker levels with both techniques. They further noted that the samples collected via polyurethane foam were stable for at least 1 month at 4°C or colder. Like other focal surface fluid collection techniques, foam sponges can be directed at different anatomic regions of interest, such as the internal nasal valve27 or ethmoid bulla,11 among others.
Filter paper
Alam et al28 developed a method using strips of filter paper for recovering cytokines after allergen challenge, prompted by an inability to obtain significant mucus cytokine levels using a nasal lavage technique. The authors used strips measuring 7 × 30 mm that were placed on the inferior turbinate, and noted that 3 separate strips could be placed on each side of the nose.
Absorbent fibrous matrix
A proprietary, paper-like material developed for isolating leukocytes from whole blood (Leukosorb; Pall Scientific, Port Washington, NY) has been used endonasally to extract nasal secretions.29 Fluids obtained via this method were then used to study a number of biomarkers, including interleukin (IL)-1β, IL-6, and IL-8, as well as detect the presence of respiratory pathogens, such as influenza virus.
Techniques for nasal cytology
In this section we focus primarily on techniques used to harvest cells. Analysis of cells recovered from the epithelial surface can facilitate investigation of the mechanisms underlying sinonasal inflammation. Eosinophils and mast cells, for example, are associated with the presence of inflammatory conditions such as CRS with nasal polyps, and allergic rhinitis.30 Neutrophils, generally considered an indicator of acute inflammation, may also play a role in the pathophysiology of CRS.31 In addition, nasal respiratory epithelial cells may also be obtained for a variety of purposes, including cell culture or transcriptomic analysis. It should be noted here that, although nasal mucus obtained via bulk or focal fluid collection may be used for cytologic analysis, this may represent a different cell population than that collected by the techniques listed in this section.12 Many cell types can be found in the airway surface liquid, and may be a result of active inflammatory processes, barrier dysfunction facilitating passive relocation of cells, or direct sampling of cells within the epithelium through applied mechanical force.
Nasal brush
Pipkorn et al32 described a method for obtaining nasal cells using a nylon brush. With their protocol, the authors recovered samples from asymptomatic individuals containing epithelial cells (45%), granulocytes (38%), monocytes (16%), and eosinophils (1.3%). Samples were subsequently analyzed via light and electron microscopy, and chemical assays were performed for histamine content. This method is commonly used to obtain samples for the establishment of primary epithelial cell cultures for study of both upper and lower airway physiology.
Nasal scraping
Plastic disposable nasal curettes (Rhino-pro; Arlington Scientific, Springville, UT) have been developed to obtain cytologic samples for both diagnostic and research purposes. Meltzer et al33 described a protocol using these curettes for cytologic purposes in patients with allergic rhinitis. Compared with nasal brushing, nasal scraping may optimize the yield of nasal epithelial cells with improved reproducibility.34 Inferior turbinate scraping specimens have demonstrated yields of approximately 1 to 2 × 106 cells, of which >90% are of epithelial type.35
Techniques for nasal histology: mucosal biopsy
Nasal mucosal biopsy is the only technique that allows for analysis of tissue architecture and reliable incorporation of subepithelial components into the sample. Histologic techniques may then be used to study epithelial morphology, ciliary activity, lymphocytic infiltrates, and goblet cell density. Biopsies for general study of nasal epithelium are often taken fromthe anterior portion of the inferior turbinate due to easy access and exposure to nasal airflow. Biopsy may also be used to examine tissue characteristics of certain regions of the nasal cavity and sinuses that cannot otherwise be studied using surface sampling techniques. For example, biopsy of olfactory epithelium is necessary to obtain sufficient olfactory receptor neurons.
Although mucosal biopsies for research purposes may be easily obtained from subjects who are receiving general anesthesia for an endonasal procedure, in-office mucosal biopsy has also been shown to be feasible. However, some of these early protocols36 make use of topical cocaine, which, despite its excellent anesthetic and vasoconstrictive properties, has fallen out of favor by many investigators due to its classification as a controlled substance. An alternative method using topical co-phenylcaine (5% lidocaine with 0.5% phenylephrine) has been described.37 The authors argued that a biopsy site anterior to the inferior turbinate may be more easily obtained than biopsy of the head of the turbinate, while still providing sufficient tissue that is generally representative of nasal mucosa. A similar strategy can be used to target other areas of the sinonasal cavity, such as the middle turbinate, uncinate process, or nasal polyp. It should be mentioned here that regardless of the topical anesthetic used, these medications generally inhibit the ciliary function of respiratory epithelial cells.38
Although technically more challenging, in-office biopsy of olfactory epithelium has also been described.39 The authors reported obtaining successful biopsies in 8 of 10 subjects; however, tissue yield is generally low and negative biopsies are not uncommon.
Techniques for nasal mucus exosome concentration and isolation
Exosomes are 30- to 150-nm membrane-bound vesicles that are secreted by virtually all cell types,40,41 and may convey more of an overall functional sense of associated tissue rather than gene products classically ascribed to a single cell type.42 They consist of a lipid bilayer (cholesterol, sphingomyelin, ceramide, saturated fatty acids, and phosphatidylserine) and contain lipids, proteins, DNA, and RNA specific to their cell of origin.43–45 Consequently, exosomes may be used as a biomarker that shows great potential for noninvasive diagnostics and conducting “liquid biopsies.”46 As exosomes represent only a small fraction of the cell’s secretome, exosome isolation techniques allow for further analysis of the exosome independent of other cell contents. Exosomes are isolated from nasal mucus, which is often gathered via focal surface fluid collection techniques, as described earlier (eg, polyvinyl acetate sponge application27). Once isolated, exosomal samples may be used to study physiologic and pathophysiologic mechanisms, including sinonasal inflammation or malignancy. Multiple methods for isolation and purification of exosomes have been described47; however, most methods of exosome processing only concentrate exosomes and do not isolate them.
The most common technique for concentrating exosomes is ultracentrifugation (UCF). The separation technique is based on size and buoyant density. In sequenced centrifugation steps, exosomes can be successively concentrated while removing cellular and protein contaminants. Théry et al48 described an ultracentrifugation protocol for exosome concentration.
Due to the low specificity of ultracentrifugation, adding in a sucrose gradient centrifugation step leads to higher purity of the extracted exosomes.49 Alternative methods include immunoaffinity-based isolation strategies that sort biologic components by the expression of specific proteins on their surfaces,50–53 commercial precipitation kits,53 non-filter systems that use acoustic energy to exert forces on exosomes,54,55 and the nanowire-on-micropillar technique that physically traps exosomes.56
The benefits of using exosomes as a substrate for the investigation of CRS are increasingly evident. First, they may be harvested noninvasively, thereby enabling serial and prospective sampling allowing for early detection of both disease progression and treatment response. Second, while studying mucus offers similar attributes, exosomes have been shown to have an improved signal-to-noise ratio over whole mucus, thereby allowing for more sensitive biomarker and biosignature assessment. Third, rather than functioning as passive markers of disease, exosomes participate in interepithelial transport of pro-inflammatory proteins. This suggests their study may help to unravel the mechanisms underlying field inflammatory effects observed in CRS, for instance.
Discussion
Proper procurement of biologic specimens can be critical to the execution of biomedical research. Once the hypothesis has been defined, the investigator should be familiar with various techniques for sample collection, processing, and analysis. In this work we aimed to catalog a number of different techniques that have been developed to objectively study the nasal airway and paranasal sinuses.
The optimal assay used and location of sampling is based on the question to be investigated; that is, one technique or sample site is not inherently better than the next. Nasal secretions may be harvested in a number of different ways, either via bulk surface fluid collection or focal collection methods. Techniques that aim primarily to obtain cells include nasal brushing or curettage. Biopsy is generally required for studies in which evaluation of tissue histology or subepithelial cells is needed.
This work has not attempted to systematically review or critically compare all available research methods. However, it is apparent that collection technique influences sample yield and, critically, influences the experiments that can be performed and subsequent conclusions. To illustrate, Klimek and Rasp17 performed a comparison study examining the influence of nasal secretion sampling techniques on the yield of eosinophilic cation protein (ECP). Using 839 subjects, they compared blowing of nasal secretions, simple nasal aspiration, a nasal microsuction technique, absorption via cotton wool, absorption via foam rubber, nasal lavage, and nasal spray washings. They found that the techniques that employed washing or absorption performed well, and that yields for the aspiration techniques were generally lower.The authors commented that sample yield may be influenced by retrieved sample volume, dilution factor, sample desiccation, and mucosal damage. Although not discussed in their work, absorptive materials may retain or release certain proteins or biomarkers to varying degrees, thus influencing yield. However, this phenomenon has not been specifically characterized in the literature.
The Klimek and Rasp study shows that a wide variety of techniques can be used to collect the same biomarker, albeit to varying degrees. This is an important point for the researcher to consider when choosing a collection technique. However, a number of other factors must be taken into account in addition to yield, including the time and effort needed to collect the specimen, subject tolerability of various techniques, tester variability, reproducibility, and potential costs of equipment. These concerns are all highly relevant to study design, recruitment and retention of enrolled subjects, and funding concerns.
These and other important aspects must be considered by investigators before study initiation, and, if a method is not clearly most appropriate, a small pilot study comparing different methods is recommended. Another parallel condition that must be carefully decided upon is subject selection. Specifically, how does one select for a “healthy” control sample in the study of paranasal sinus disease? Should this be an internal or external control? Clear diagnostic criteria have been agreed upon for diseases such as CRS and allergic rhinitis, but defining characteristics have not been established for control subjects. Can “healthy” subjects be defined based on history alone, or is objective testing, such as skin-prick testing and/or computed tomography (CT) imaging, required to exclude cases with subclinical disease? In addition, the most appropriate control tissue for nasal polyps is yet to be defined, with great heterogeneity in current research practice.
Location of tissue sampling is also a controversial aspect of data collection, particularly with respect to focal sampling. Most published protocols strive to obtain a representative sample of the nasal milieu while minimizing technical challenges and subject intolerance. No consensus exists regarding the ideal representative sampling area. It has been documented that inflammatory cell profile and cytokine expression can vary depending on the anatomic location within the nose and paranasal sinuses.57 However, other investigators have shown that the cytokine profile of mucus collected from the middle meatus mirrors that collected from the olfactory cleft in patients with olfactory dysfunction, perhaps obviating the need for site-specific sampling in difficult-to-access regions.10 Ideally, we recommend surface sampling of the disease-specific location under study. Unless a specific anatomic region is of interest, ease of access seems to override most other considerations when choosing the sampling site.
Finally, sample stability during cold storage has not been well characterized. Most samples are not analyzed immediately upon collection and are usually frozen at −20°C or −80°C so that they can be batch analyzed for efficiency. Frozen storage of nasal secretions, cells, or mucosal tissue is often necessary for the creation of biorepositories. An international consortium maintains a guide of best practices for the creation and maintenance of biobanks for research purposes.58 Respiratory cell media, RNA-stabilizing solution, or other buffers may be used depending on the anticipated assays. Many protocols have not published stability tests after storage of samples, but presumably these experiments have been performed. For example, when a polyurethane foam nasal secretion collection apparatus was assayed, Lü and Esch demonstrated statistically nonsignificant differences with sample evaporation at 1, 2, and 4 weeks at various temperatures (4°C, −20°C, and −80°C).26 However, time period of storage or number of freeze-thaw cycles for nasal secretions is not definite, and quality control experiments are recommended for anything beyond the bare minimum.
Conclusion
Fortunately, many techniques are available for the procurement of sinonasal biologic samples, and the area is extremely valuable for translational respiratory research given its accessibility. Study investigators must take many factors into consideration when selecting a technique for specimen collection. As evidenced by the various methods collected herein, no ideal technique exists. Investigators must weigh the benefits and drawbacks of each protocol and how each may best achieve a research goal while fitting within the constraints of a particular study design. Pilot studies are recommended before initiation of a new study in order to compare and contrast possible collection methods.
Acknowledgments
Funding sources for the study: National Institutes of Deafness and Communication Disorders of the National Institutes of Health (NIH) (K23DC014747 to V.R.R. and K23DC014747-S1 to C.J.M.); Flight Attendants Medical Research (CIA130066 to V.R.R. and F.D.d.V.). The content is solely representative of the authors and does not necessarily represent the official views of the NIH or the Flight Attendants Medical Research Institute.
Appendix
Cited protocols
Bulk surface fluid collection
Blowing of nasal secretions17:
Subjects are asked to blow into an aluminum dish attheir convenience.
Each side is sampled by having the subject close 1 nostrilmanually.
Samples are transferred into a plastic tube and diluted1:1 with phosphate-buffered saline.
Nasal aspiration20:
Ten sprays of normal saline from a disposable nasalspray bottle are applied to each nostril.
The lavage fluid is then collected with a flexible siliconetube connected to a vacuum. This is done by moving the tubing 2 to 4 cm back and forth inside the nasal cavity for 1 minute on each side.
The inside of the tubing is rinsed with 1.0 mL saline to obtain the lavage fluid.
Nasal lavage21:
The volume instilled within each nostril is 2.5 to5.0 mL. A physiologic solution, generally 0.9% NaCl prewarmed to 37°C, is instilled within each nostril with an 80% recovery (range, 65%−90%). An agent to disrupt the disulfide bonds of the mucus polypeptide chains can be included.
Subjects, in a sitting position, extend their neck gentlybackward to 30 degrees from the horizontal, such that the nasal cavity is pointing upward and instilled fluid will not be lost anteriorly because of gravity.
Posterior loss is limited by having subjects close thirsoft palate, hold their breath during the period of nasal lavage retention, and hold their mouth slightly open.
The fluid is usually left within the nasal cavity for 10 seconds. The subject then leans forward and expels the fluid from the nostrils by gently exhaling into a collecting funnel that drains into a container.
In studies in which nasal challenge induces nasal blockage, the obstruction of the nasal lumen will limit the amount of fluid that can be retained within the nasal cavity, and smaller lavage volumes may be used. Intranasal α-agonist administration has been used to facilitate the undertaking of nasal lavage by decongesting the nose. This has been shown not to influence the recovery of mediators or markers of vascular permeability within the nasal lavage fluid under challenge circumstances.
Focal surface fluid collection
Cotton wool24:
Cotton wool strips with a length of 4 cm and a width of 6 mm are placed into the middle meatus with bayonet forceps under direct rhinoscopic view and are left in place for 10 minutes.
This is followed by centrifugation of the strips at 2000g for 10 minutes. The samples are then stored at −20°C and prediluted 1:5 before testing.
Foam rubber26:
A cylindrical polyurethane foam roll (measuring 12 mm × 24 mm) is inserted about 1 inch into the nostril along the floor of the nasal cavity, in between the septum and inferior turbinate for at least 5 minutes.
The foam is then removed and inserted into a centrifugetube, where it should be stored at −20°C or −80°C until the sample is ready to be centrifuged.
The sample is then thawed and can be centrifuged at 3000g for 20 minutes to recover the fluid. The fluid can be stored at −80°C for up to 1 month with minimal fluid loss.
Filter paper28:
Small strips of Whatman No. 42 filter paper (7 × 30 mm) are cut, sterilized, and placed on the anterior portion of the inferior turbinate. A total of 6 filter strips can be placed, 3 on each side of the nose.
Filter strips are left for 10 minutes and then removed and air-dried.
Individual strips are stored in a sterile tube at −70°C until further use.
To elute the cytokines, 2 filter strips from the same patient are placed in a tube with 0.55 mL of 0.1 mol/L Tris buffer, pH 7.4, in normal saline containing 0.3% human serum albumin (HSA), 0.01% sodium azide, and 0.002% Tween.
The tube is then placed on a rocker overnight at 4°C, after which cytokine assays can be performed.
Absorbent fibrous matrix (Leukosorb)29:
Strips are cut from sheets of Leukosorb medium to adimension of roughly 4 × 40 mm.
Before the strips were inserted, each nostril was briefly moistened with 100 μL of 0.9% sterile, normal saline solution.
Leukosorb strips were then inserted into each nostrilonto the anterior part of the inferior turbinate.
After insertion, nostrils were clamped shut using apadded nose clip for 2 minutes.
Strips were then removed from the nostril and collectedin 1.5-mL collection tubes, and stored at −20°C until fluid elution and analysis.
Nasal cytology
Nasal brush method32:
The brush is introduced into the nose under direct visual guidance and placed between the nasal septum and the inferior turbinate. No anesthesia is used. The brush is introduced to its full length and then removed with a slight rotating movement.
The brush is then placed immediately into a 3 mL plastic tube containing 2 mL buffered salt solution.
The brush is then shaken vigorously in the salt solution and carefully brushed off on the wall of the tube.
Nasal scraping33:
Samples were obtained after having the subject blow their nose to clear excess secretions.
The tip of the probe is then passed gently along the medial surface of the inferior turbinate under direct visualization. Two or 3 short scrapes are used to obtain the sample.
The sample is then spread onto a microscope slide and immediately fixed with 95% ethyl alcohol.
Techniques for mucosal histology
General mucosal biopsy37:
Local decongestion and anesthesia is achieved by topical administration on cotton wool pledgets placed in the nasal cavities for 10 minutes.
Biopsies are taken under direct visualization from a mucosal fold on the lateral nasal wall anterior to the inferior turbinate using an up-cutting 45° Blakesley forceps.
Gauze is placed in the nasal cavity for hemostasis for 10 minutes.
Olfactory epithelium biopsy39:
The nasal cavity is sprayed with a mixture of 2% lidocaine and 0.025% oxymetazoline.
After several minutes, rigid nasal endoscopy with a 2.7-mm 0-degree nasal endoscope identifies the side with the greatest olfactory cleft space.
Further anesthetic is applied with the subject in the Mygind position, supine, and with the head hanging and neck extended off the edge of the examination table. A flexible angiocath is then used to direct 0.5 to 1 mL of anesthetic solution into the superior nasal cavity and olfactory cleft. Subjects remain in this position for 2 to 3 minutes and are then returned to an upright sitting position.
A sickle knife is then used to create a 5 to 7 mm posteriorto anterior diagonal incision along the septum approximately 5 to 8 mm below the cribriform plate and >5 mm posterior to the attachment of the middle turbinate.
The sickle knife is used to carefully elevate a superiorly based flap of mucosa.
A 3-mm cup forceps is used to pinch off a portion of the superiorly raised flap.
Anesthetic soaked cotton wisps are placed into the olfactory cleft for hemostasis.
Technique for nasal secretion exosome concentration and isolation48:
Nasal secretions are harvested by placing compressed polyvinyl alcohol sponges (PVA; Medtronic, Minneapolis, MN) against the middle meatus for 5 minutes taking care not to abrade the mucosa or contaminate the sponge with blood. The sponges are then removed.
Mucus samples are extracted from the PVA sponges by centrifugation (1500g at 4°C for 30 minutes).
Mucus is then diluted in 150 μL of 1× phosphate-buffered saline (PBS; Life Technologies, Carlsbad, CA) with Protease Inhibitor Cocktail (1:100; Sigma, St Louis, MO).
Cellular debris is pelleted by centrifugation at 45 minutes at 12,000g at 4°C.
Supernatant is then suspended in 4.5 mL of PBS in polypropylene tubes (5.0 mL, 13 × 51 mm; Thinwall; Beckman Coulter, Indianapolis, IN) and ultracentrifuged for 2 hours at 110,000g, at 4°C.
The supernatant is then collected and the pellet resuspended in 4.5 mL 1× PBS.
Next, the suspension is filtered through a 0.22-μm filter (Fisher Scientific, Pittsburgh, PA) and collected in a fresh ultracentrifuge tube.
For the second ultracentrifugation step, the filtered suspension is centrifuged for 70 minutes at 110,000g at 4°C.
Again, the supernatant is collected and the pellet resuspended in 175 μL PBS M-PER™ Mammalian Protein Extraction Reagent (Thermo Scientific, Waltham, MA) with 1× protease inhibitor (Halt™ Protease Inhibitor Cocktail [100×]; Thermo Scientific) for further protein analysis.
Footnotes
Potential conflicts of interest: None disclosed.
References
- 1.Levy JM, Smith SS, Varshney R, et al. Trends in sinusitis research: a systematic review of extramural funding. Int Forum Allergy Rhinol. 2017;7:1104–1107. [DOI] [PubMed] [Google Scholar]
- 2.Poole A, Urbanek C, Eng C, et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol. 2014;133:670–678.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster MJ, Tarran R. Slippery when wet: airway surface liquid homeostasis and mucus hydration In: Levitane I, Delpire E, Rasgado-Flores H, eds. Current Topics in Membranes. Cell Volume Regulation. Vol. 81 Cambridge, MA: Academic Press; 2018:293–335. [DOI] [PubMed] [Google Scholar]
- 6.Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994;150:271–281. [DOI] [PubMed] [Google Scholar]
- 7.Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007;117:932–938. [DOI] [PubMed] [Google Scholar]
- 8.Kaliner MA. Human nasal respiratory secretions and host defense. Am Rev Respir Dis. 1991;144(Suppl 3): S52–56. [DOI] [PubMed] [Google Scholar]
- 9.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123:E72–78. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Chandra RK, Li P, Hull BP, Turner JH. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope. 2018;128:E304–E310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman AD, Nocera AL, Mueller SK, Otu HH, Libermann TA, Bleier BS. Translating transcription: proteomics in chronic rhinosinusitis with nasal polyps reveals significant discordance with messenger RNA expression. Int Forum Allergy Rhinol. 2019;9:776–786. [DOI] [PubMed] [Google Scholar]
- 12.Howarth PH, Persson CGA, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 2005;115(Suppl 1):S414–441. [DOI] [PubMed] [Google Scholar]
- 13.Nocera AL, Miyake MM, Seifert P, Han X, Bleier BS. Exosomes mediate interepithelial transfer of functional P-glycoprotein in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2017;127:E295–E300. [DOI] [PubMed] [Google Scholar]
- 14.Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurora R, Chatterjee D, Hentzleman J, Prasad G,Sindwani R, Sanford T. Contrasting the microbiomes from healthy volunteers and patients with chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2013;139:1328–1338. [DOI] [PubMed] [Google Scholar]
- 16.Bassiouni A, Cleland EJ, Psaltis AJ, Vreugde S, Wormald P-J. Sinonasal microbiome sampling: a comparison of techniques. PLoS One. 2015;10:e0123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimek L, Rasp G. Norm values for eosinophil cationic protein in nasal secretions: influence of specimen collection. Clin Exp Allergy. 1999;29:367–374. [DOI] [PubMed] [Google Scholar]
- 18.Vogan JC, Bolger WE, Keyes AS. Endoscopically guided sinonasal cultures: a direct comparison with maxillary sinus aspirate cultures. Otolaryngol Head Neck Surg. 2000;122:370–373. [DOI] [PubMed] [Google Scholar]
- 19.Benninger MS, Payne SC, Ferguson BJ, Hadley JA, Ahmad N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:3–9. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto C, Kido H, Sawabuchi T, et al. Evaluation of nasal IgA secretion in normal subjects by nasal spray and aspiration. Auris Nasus Larynx. 2009;36:300–304. [DOI] [PubMed] [Google Scholar]
- 21.Naclerio RM, Meier HL, Kagey-Sobotka A, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128:597–602. [DOI] [PubMed] [Google Scholar]
- 22.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogasawara N, Klingler AI, Tan BK, et al. Epithelial activators of type 2 inflammation: elevation of thymic stromal lymphopoietin, but not IL-25 or IL-33, in chronic rhinosinusitis with nasal polyps in Chicago, Illinois. Allergy. 2018;73:2251–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer MF, Burow G, Pfrogner E, Rasp G. In vitro diagnosis of chronic nasal inflammation. Clin Exp Allergy. 2004;34:1086–1092. [DOI] [PubMed] [Google Scholar]
- 25.Javer AR, Genoway K, Tsaparas Y. Comparison of swabs versus suction traps for endoscopically guided sinus cultures. J Otolaryngol Head Neck Surg. 2008;37:185–191. [PubMed] [Google Scholar]
- 26.Lü FX, Esch RE. Novel nasal secretion collection method for the analysis of allergen specific antibodies and inflammatory biomarkers. J Immunol Methods. 2010;356:6–17. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SK, Nocera AL, Dillon ST, et al. Noninvasive exosomal proteomic biosignatures, including cystatin SN, peroxiredoxin-5, and glycoprotein VI, accurately predict chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2019;9:177–186. [DOI] [PubMed] [Google Scholar]
- 28.Alam R, Sim TC, Hilsmeier K, Andrew Grant J. Development of a new technique for recovery of cytokines from inflammatory sites in situ. J Immunol Methods. 1992;155:25–29. [DOI] [PubMed] [Google Scholar]
- 29.Rebuli ME, Speen AM, Clapp PW, Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol. 2017;312:L288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gröger M, Bernt A, Wolf M, et al. Eosinophils and mast cells: a comparison of nasal mucosa histology and cytology to markers in nasal discharge in patients with chronic sino-nasal diseases. Eur Arch Otorhinolaryngol. 2013;270:2667–2676. [DOI] [PubMed] [Google Scholar]
- 31.Besançon-Watelet C, Béné MC, Montagne P, Faure GC, Jankowski R. Eosinophilia and cell activation mediators in nasal secretions. Laryngoscope. 2002; 112:43–46. [DOI] [PubMed] [Google Scholar]
- 32.Pipkorn U, Karlsson G, Enerbäck L. A brush method to harvest cells from the nasal mucosa for microscopic and biochemical analysis. J Immunol Methods. 1988;112:37–42. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer EO, Orgel HA, Rogenes PR, Field EA. Nasal cytology in patients with allergic rhinitis: effects of intranasal fluticasone propionate. J Allergy Clin Immunol. 1994;94:708–715. [DOI] [PubMed] [Google Scholar]
- 34.Pipolo C, Bianchini S, Barberi S, et al. Nasal cytology in children: scraping or swabbing? Rhinology. 2017;55:242–250. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Myers AC, Chen L, et al. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fokkens WJ, Vroom TM, Gerritsma V, Rijntjes E. A biopsy method to obtain high quality specimens of nasal mucosa. Rhinology. 1988;26:293–295. [PubMed] [Google Scholar]
- 37.Thornton MA, Walshe P, Costello RW, McConn-Walsh R, Walsh MA. An alternative technique for nasal biopsy. Laryngoscope. 2004;114:1060–1062. [DOI] [PubMed] [Google Scholar]
- 38.Ingels KJ, Nijziel MR, Graamans K, Huizing EH. Influence of cocaine and lidocaine on human nasal cilia. Beat frequency and harmony in vitro. Arch Otolaryngol Head Neck Surg. 1994;120:197–201. [DOI] [PubMed] [Google Scholar]
- 39.Holbrook EH, Rebeiz L, Schwob JE. Office-based olfactory mucosa biopsies. Int Forum Allergy Rhinol. 2016;6:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. [DOI] [PubMed] [Google Scholar]
- 42.Théry C, Zitvogel L, Amigorena S. Exosomes: com- position, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. [DOI] [PubMed] [Google Scholar]
- 43.Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006;5:1760–1771. [DOI] [PubMed] [Google Scholar]
- 44.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 45.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 46.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. [DOI] [PubMed] [Google Scholar]
- 47.Stremersch S, De Smedt SC, Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244:167–183. [DOI] [PubMed] [Google Scholar]
- 48.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 49.Liga A, Vliegenthart ADB, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15:2388–2394. [DOI] [PubMed] [Google Scholar]
- 50.Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database Issue):D1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tauro BJ, Greening DW, Mathias RA, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. [DOI] [PubMed] [Google Scholar]
- 53.van Deun J, Mestdagh P, Sormunen R, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;3:24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko J, Carpenter E, Issadore D. Detection and isolation of circulating exosomes and microvesicles for cancer monitoring and diagnostics using micro-/nano-based devices. Analyst. 2016;141:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Wu H, Fine D, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamil A, Ghaffar O, Lavigne F, Taha R, Renzi PM, Hamid Q. Comparison of inflammatory cell profile and Th2 cytokine expression in the ethmoid sinuses, maxillary sinuses, and turbinates of atopic subjects with chronic sinusitis. Otolaryngol Head Neck Surg. 1998;118:804–809. [DOI] [PubMed] [Google Scholar]
- 58.Campbell LD, Astrin JJ, DeSouza Y, et al. The 2018 revision of the ISBER best practices: summary of changes and the editorial team’s development process. Biopreserv Biobank. 2018;16:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]