INTRODUCTION
Physical function is a core outcome domain recommended for assessment in clinical trials of pain treatments by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) [12,50]. Indeed, surveys of chronic pain patients indicate that in addition to pain relief, improvement in physical function is an important treatment outcome [31,51]. In focus group studies of adults with chronic pain, participants report that their pain condition negatively impacts their overall physical functioning [6,51]. For example, out of 70 in-depth interviews of patients with painful diabetic peripheral neuropathy (DPN), 79% reported difficulty walking because of their pain-related symptoms [6]. Therefore, in addition to pain intensity, measures of physical function are often examined separately as secondary outcomes in analgesic clinical trials. However, there is likely a range of behavioral responses to pain with some patients restricting their daily activities, while others push through their pain to carry out functional tasks and activities. Thus, some patients with chronic pain might “titrate” their level of activity (physical function) in relation to their pain severity, while others maintain a certain level of activity or function regardless of their level of pain. It is conceivable then that therapeutic interventions may improve function in the former group without changing pain intensity ratings, while decreasing pain intensity in the latter group but without changing physical function. An important implication is that by focusing on pain reduction as the primary outcome, clinical trials of chronic pain may potentially miss clinically meaningful treatment responses that are highly valued by patients.
Integrating information on pain intensity and physical function into a composite outcome might provide a useful method for assessing treatment efficacy in chronic pain trials [13,52]. In clinical trials of rheumatoid arthritis, for example, the American College of Rheumatology’s ACR-20 is used as a “responder index” to identify patients who improve on multiple outcome domains, including pain and physical functioning [15]. In addition, several groups have developed responder indices for osteoarthritis (OA), chronic low back pain (CLBP), and fibromyalgia (FM) [1,5,9,25,33,34,42]. These investigators recognize that pain intensity alone does not adequately capture the entire patient experience with chronic pain, and that the goal of treatment lies beyond pain control alone and includes the reduction of functional limitations and disability. Accordingly, both pain intensity and physical function are consistently included in responder criteria for OA, CLBP, and FM.
We carried out a series of exploratory analyses on pain intensity and physical function outcomes in neuropathic pain patients enrolled in 15 trials of duloxetine, gabapentin, and pregabalin. For the first study aim, we sought to characterize the relationship of pain intensity with physical functioning in patients with DPN and in patients with postherpetic neuralgia (PHN). Second (Aim 2), we evaluated whether different composite responder outcomes of pain intensity and physical function improve the assay sensitivity of neuropathic pain trials across different medication treatments and conditions. Finally (Aim 3), to assess the potential clinical validity of the composite outcomes, we examined associations with patient ratings of change in overall health status.
METHODS
Study Population
Under the auspices of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the United States Food and Drug Administration (FDA), we accessed clinical trials data that were submitted by industry sponsors to the FDA and are stored on FDA’s Data Archiving, Reporting, and Regulatory Tracking System. Patient level data from 2 trials of duloxetine for DPN [17,59], 4 trials of gabapentin (2 for DPN [2] and 2 for PHN [36,39]), and 9 trials of pregabalin (5 for DPN [23,37,38,47] and 4 for PHN [11,40,55]) were analyzed. These trials are identified in Table 1 and were selected because they investigated efficacious first-line medications [16] and had similar inclusion/exclusion criteria per treatment indication, random treatment assignment, placebo control groups, double blind masking, a parallel study design, and a common set of outcome measures. Although 3 of the trials had statistically non-significant effects on the primary outcome of pain intensity (Study IDs 945–224, 1008–030, and 1008–040), these trials were retained to evaluate potential improvements in assay sensitivity with composite outcomes of pain intensity and physical function. However, participants randomized to treatment arms with subtherapeutic dosages (<60 mg/day for duloxetine, <1200 mg/day for gabapentin, and <150 mg/day for pregabalin) were excluded because they were not expected to separate from placebos. The Institutional Review Boards of the University of Washington and University of Rochester approved the analysis of these data.
Table 1.
Characteristics of randomized, controlled trials of gabapentin, pregabalin, and duloxetine for diabetic peripheral neuropathy and postherpetic neuralgia
| Study ID | Indication | Treatment length in weeks | Sample size | Age in years Mean (SD) | Women No. (%) | Baseline Pain Intensity Score Mean (SD) | Baseline Physical Function Score Mean (SD) | Publication |
|---|---|---|---|---|---|---|---|---|
| Gabapentin | ||||||||
| 945–210 | DPN | 8 | 165 | 53.0 (10.3) | 66 (40.0) | 6.5 (1.5) | 55.7 (27.6) | Backonja (1998)2 |
| 945–224 | DPN | 7 | 243* | 60.5 (10.2) | 103 (42.4) | 6.2 (1.6) | 46.6 (25.9) | Unpublished |
| 945–211 | PHN | 8 | 229 | 71.7 (10.3) | 110 (48.0) | 6.4 (1.7) | 41.3 (27.3) | Rowbotham (1998)39 |
| 945–295 | PHN | 7 | 334 | 73.4 (10.3) | 196 (58.7) | 6.5 (1.6) | 51.2 (29.1) | Rice (2001)36 |
| Pregabalin | ||||||||
| 1008–014 | DPN | 6 | 246 | 57.0 (9.7) | 97 (39.4) | 6.7 (1.5) | 54.7 (26.9) | Richter (2005)37 |
| 1008–029 | DPN | 5 | 260* | 59.5 (10.4) | 101 (38.9) | 6.3 (1.5) | 53.5 (26.8) | Lesser (2004)23 |
| 1008–040 | DPN | 8 | 167** | 61.3 (10.5) | 76 (45.5) | 6.6 (1.6) | 49.8 (25.4) | Unpublished |
| 1008–131 | DPN | 8 | 146 | 59.7 (11.4) | 64 (43.8) | 6.3 (1.6) | 52.1 (26.7) | Rosenstock (2004)38 |
| 1008–149 | DPN | 12 | 384 | 58.6 (11.6) | 174 (45.3) | 6.4 (1.4) | 54.3 (24.4) | Tölle (2008)47 |
| 1008–030 | PHN | 12 | 171* | 70.8 (9.8) | 83 (48.5) | 6.5 (1.6) | 63.4 (26.2) | Unpublished |
| 1008–045 | PHN | 8 | 238 | 72.2 (10.2) | 131 (55.0) | 6.8 (1.6) | 56.1 (27.2) | Sabatowski (2004)40 |
| 1008–127 | PHN | 8 | 173 | 71.5 (10.9) | 92 (53.2) | 6.3 (1.5) | 61.6 (27.0) | Dworkin (2003)11 |
| 1008–196 | PHN | 13 | 368 | 70.7 (10.6) | 200 (54.4) | 6.7 (1.5) | 55.5 (26.7) | van Seventer (2006)55 |
| Duloxetine | ||||||||
| HMAW | DPN | 12 | 342* | 60.1 (11.1) | 136 (39.8) | 5.9 (1.5) | 54.0 (26.1) | Goldstein (2005)17 |
| HMAVa | DPN | 12 | 334 | 60.7 (10.6) | 38.9 (130) | 6.0 (1.5) | 46.0 (26.1) | Wernicke (2006)59 |
Study subjects randomized to subtherapeutic dosages (<1200 mg/d of gabapentin, <150 mg/d of pregabalin, and <60 mg/d of duloxetine) were excluded
Study subjects randomized to the amitriptyline treatment arm were excluded
Outcome Measures
Pain intensity was measured on a 0–10 numeric rating scale (NRS) with higher scores indicating greater severity (0=No pain; 10=Worst possible pain). Participants were instructed to rate and record their pain intensity over the last 24 hours in a daily diary. The instructions varied somewhat across trials and are available upon request. Physical function was assessed with the 10-item Short Form-36 (SF36) subscale that ranges 0–100 with higher scores reflecting better function [58]. In trials of gabapentin and pregabalin, patients completed the Patient Global Impression of Change (PGIC) at the end of the trial, while the Patient Global Impression of Improvement (PGII) was used in the duloxetine trials [19]. Both measures provide a patient-centered assessment of overall change in health status on a 7-point scale (PGIC: 1=Very much worse and 7=Very much improved; PGII: 1=Very much worse and 7=Very much better).
Data Analysis
Data on the intention to treat populations were analyzed. The mean and standard deviation of age, pain intensity at baseline, and physical function at baseline as well as the proportion of women were computed to characterize the patients enrolled in each clinical trial (Table 1). Baseline and endpoint pain intensity scores were computed as the mean of the 7 diary entries prior to taking study medication and the last 7 diary entries while on study medication, respectively. For physical function, the baseline and endpoint scores were computed from data collected during clinic visits prior to taking study medication and after completing the course of treatment, respectively. Change in pain intensity and physical function was calculated by subtracting endpoint scores from baseline values; percent change was calculated by dividing change scores by baseline values and multiplying this quantity by 100.
Data across trials were pooled by neuropathic pain condition to examine the relationship between pain intensity and physical function. Scatter plots with locally weighted regression lines and 95% confidence interval bands were generated and Spearman rank correlations were computed (Figure 1; study aim 1). The proportion of patients achieving 30% and 50% reductions in pain intensity at study endpoint and 30% and 50% improvements in physical function were computed. Consistent with previous publications of these data, the last-observation carried forward (LOCF) was used to impute missing pain intensity data at study end point (see sensitivity analysis for imputation using baseline observation carried forward [BOCF]). Since physical function was assessed only at 2 time points, participants missing data at either baseline or study end point were considered non-responders. The effect of active treatment on responder outcomes was determined using random effects Poisson regression models with robust standard errors. These models account for potential within trial clustering effects in pooled analyses and estimate the risk ratio (RR) comparing the incidence of the responder outcome in the active treatment group relative to the placebo group (Tables 2–4; study aim 2) [24,43,60]. In addition, the number needed to treat (NNT) was computed with 95% confidence intervals (CI) calculated using Newcombe’s Method 10 [30], as recommended by Bender [3].
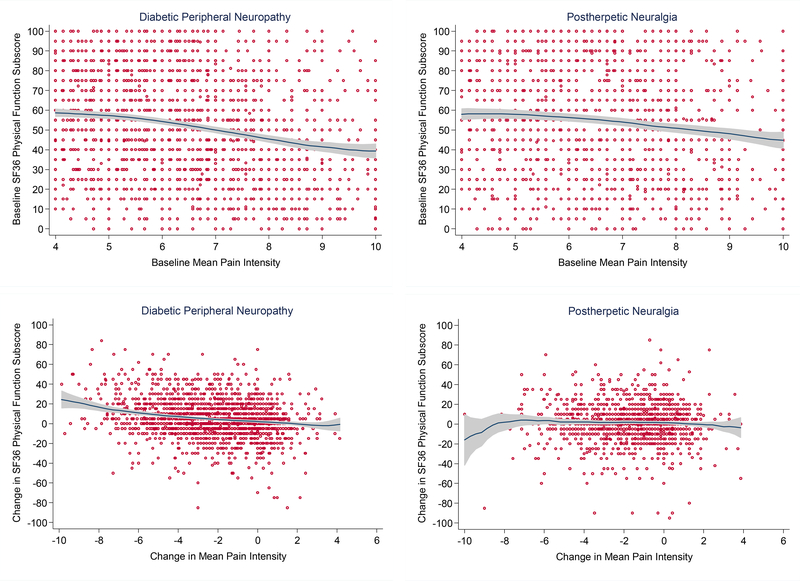
Figure 1.
Baseline and longitudinal (pre-to-post treatment) relationships between pain intensity and physical function in randomized, clinical trials of gabapentin, pregabalin, and duloxetine for diabetic peripheral neuropathy and postherpetic neuralgia
Note: Red circles represent observations of individual study participants; the locally weighted regression line is shown in navy blue; and the 95% confidence interval band is shaded gray.
Table 2.
Pain intensity and physical function responder outcomes (≥50% improvement) according to treatment group and neuropathic pain condition
| ≥50% reduction in Pain Intensity No. (%) | Risk Ratio(95% CI) | Number Needed to Treat*(95% CI) | ≥50% improvement in Physical Function No. (%) | Risk Ratio(95% CI) | Number Needed to Treat*(95% CI) | |
|---|---|---|---|---|---|---|
| Gabapentin | ||||||
| 2 DPN trials (N=408) | ||||||
| Placebo (n=158) | 36 (22.8) | 1.0 | 22 (13.9) | 1.0 | ||
| Gabapentin (n=250) | 101 (40.4) | 1.8 (1.1–2.8) | 5.7 (3.8–12.0) | 42 (16.8) | 1.2 (0.6–2.3) | Not calculated* |
| 2 PHN trials (N=563) | ||||||
| Placebo (n=227) | 30 (13.2) | 1.0 | 29 (12.8) | 1.0 | ||
| Gabapentin (n=336) | 106 (31.6) | 2.4 (1.6–3.6) | 5.5 (4.0–8.8) | 55 (16.4) | 1.3 (0.8–2.0) | Not calculated* |
| Pregabalin | ||||||
| 5 DPN trials (N=1,203) | ||||||
| Placebo (n=426) | 91 (21.4) | 1.0 | 66 (15.5) | 1.0 | ||
| Pregabalin (n=777) | 297 (38.2) | 1.8 (1.3–2.5) | 5.9 (4.6–8.7) | 145 (18.7) | 1.2 (0.9–1.6) | Not calculated* |
| 4 PHN trials (N=950) | ||||||
| Placebo (n=346) | 47 (13.6) | 1.0 | 55 (15.9) | 1.0 | ||
| Pregabalin (n=604) | 186 (30.8) | 2.4 (1.6–3.5) | 5.8 (4.5–8.4) | 89 (14.7) | 0.9 (0.7–1.3) | Not calculated* |
| Duloxetine | ||||||
| 2 DPN trials (N=676) | ||||||
| Placebo (n=223) | 58 (26.0) | 1.0 | 45 (20.2) | 1.0 | ||
| Duloxetine (n=453) | 218 (48.1) | 1.9 (1.7–2.1) | 4.5 (3.4–6.9) | 120 (26.5) | 1.3 (0.9–1.9) | Not calculated* |
Note: Pain intensity measured with 0–10 numeric rating scale; physical function measured with SF-36 10-item subscale with scores ranging 0–100
NNT was not calculated when the 95% CI for the risk ratio contained 1, indicating no significant benefit of active drug treatment over placebo
Table 4.
Validation of pain intensity and physical function composite outcomes in randomized, placebo-controlled trials of duloxetine, gabapentin, and pregabalin for diabetic peripheral neuropathy and postherpetic neuralgia
| No. of Drug Responders (%) | No. of Placebo Responders (%) | Risk Ratio (95% CI) | Number Needed to Treat (95% CI)* | |
|---|---|---|---|---|
| Diabetic Peripheral Neuropathy | ||||
| Pregabalin (5 trials; Validation subsample, n=601) | ||||
| Composite 1 | 281 (71.0) | 112 (54.6) | 1.3 (1.1–1.6) | 6.1 (4.1–12.2) |
| Composite 4 | 251 (63.4) | 97 (47.3) | 1.3 (1.0–1.7) | 6.2 (4.1–13.0) |
| Composite 6 | 221 (55.8) | 79 (38.5) | 1.4 (1.1–1.9) | 5.8 (4.0–11.3) |
| Composite 10 | 197 (49.8) | 58 (28.3) | 1.8 (1.3–2.4) | 4.7 (3.4–7.5) |
| Gabapentin (2 trials; N=408) | ||||
| Composite 1 | 174 (69.6) | 85 (53.8) | 1.3 (1.1–1.6) | 6.3 (4.0–16.2) |
| Composite 4 | 158 (63.2) | 70 (44.3) | 1.4 (1.0–2.1) | Not calculated* |
| Composite 6 | 137 (54.8) | 63 (39.9) | 1.4 (1.0–1.9) | Not calculated* |
| Composite 10 | 112 (44.8) | 54 (34.2) | 1.3 (0.9–1.8) | Not calculated* |
| Duloxetine (2 trials; N=676) | ||||
| Composite 1 | 344 (75.9) | 130 (58.3) | 1.3 (1.1–1.6) | 5.7 (4.0–9.9) |
| Composite 4 | 323 (71.3) | 115 (51.6) | 1.4 (1.1–1.7) | 5.1 (3.7–8.4) |
| Composite 6 | 294 (64.9) | 95 (42.6) | 1.5 (1.2–1.9) | 4.5 (3.3–7.0) |
| Composite 10 | 260 (57.4) | 76 (34.1) | 1.7 (1.3–2.2) | 4.3 (3.3–6.5) |
| Postherpetic Neuralgia | ||||
| Pregabalin (4 trials; Validation subsample, n=474) | ||||
| Composite 1 | 172 (56.4) | 65 (38.5) | 1.5 (1.1–1.9) | 5.6 (3.7–11.7) |
| Composite 4 | 151 (49.5) | 54 (32.0) | 1.5 (1.2–2.0) | 5.7 (3.8–12.0) |
| Composite 6 | 135 (44.3) | 48 (28.4) | 1.6 (1.1–2.1) | 6.3 (4.1–14.7) |
| Composite 10 | 112 (36.7) | 27 (16.0) | 2.3 (1.5–3.5) | 4.8 (3.6–7.9) |
| Gabapentin (2 trials; N=563) | ||||
| Composite 1 | 248 (73.8) | 118 (52.0) | 1.4 (1.1–1.8) | 4.6 (3.4–7.3) |
| Composite 4 | 211 (62.8) | 93 (41.0) | 1.5 (1.2–2.0) | 4.6 (3.4–7.4) |
| Composite 6 | 182 (54.2) | 86 (37.9) | 1.4 (1.1–1.8) | 6.1 (4.1–12.7) |
| Composite 10 | 141 (42.0) | 41 (18.1) | 2.3 (1.6–3.3) | 4.2 (3.2–6.1) |
Note: Pain intensity measured with 0–10 numeric rating scale; physical function measured with SF-36 10-item subscale with scores ranging 0–100
Composite 1 responder = ≥30% reduction in pain intensity or ≥30% improvement in physical function
Composite 4 responder = ≥40% reduction in pain intensity or ≥40% improvement in physical function
Composite 6 responder = ≥50% reduction in pain intensity or ≥50% improvement in physical function
Composite 10 responder = ≥50% reduction in pain intensity, or ≥20% reduction in pain intensity and ≥30% improvement in physical function
NNT was not calculated when the 95% CI for the risk ratio contained 1, indicating no significant benefit of active drug treatment over placebo
A multistage process was used to identify and evaluate potential composite responder outcomes of pain intensity and physical function. First, a literature search and discussion among co-authors yielded 10 potential composite outcomes for analgesic clinical trials. Second, an outcomes development cohort was assembled by randomly sampling half of the patients enrolled in each of the pregabalin trials for DPN and PHN. Pregabalin trials were selected because they had the largest number of patients available per treatment indication. Candidate outcomes that were significantly associated with pregabalin treatment effect in the development cohort (Table 3; study aim 2) were then tested in the validation cohort comprised of the second half sample of participants in the pregabalin trials (Table 4; study aim 2). Composite outcomes significantly associated with pregabalin versus placebo were then cross-validated in trials of duloxetine and gabapentin (Table 4; study aim 2). Risk ratios estimated from random effects Poisson regression models and NNT values were used to assess the statistical significance and potential clinical value of the candidate responder outcomes. In addition, we examined the percent distribution of the composite responder outcomes across PGIC/PGII ratings in data that were pooled according to pain condition (Figure 2; study aim 3), and we tested associations of the responder outcomes with PGIC/PGII ratings of “much improved” or “very much improved” using random effects Poisson regression (Table 5; study aim 3).
Table 3.
Performance of candidate pain intensity and physical function composite outcomes in a random subsample of subjects (exploratory data set) from randomized, placebo-controlled trials of pregabalin for diabetic peripheral neuropathy and postherpetic neuralgia
| Criteria for candidate composite outcomes | No. of Pregabalin Responders (%) | No. of Placebo Responders (%) | Risk Ratio (95% CI) |
|---|---|---|---|
| Diabetic Peripheral Neuropathy (5 trials; Subsample, n=602) | |||
| 1. ≥30% improvement in pain intensity or physical function | 252 (66.1) | 122 (55.2) | 1.2 (1.0–1.4)* |
| 2. ≥30% improvement in pain intensity and physical function | 64 (16.8) | 29 (13.1) | 1.6 (0.8–2.1) |
| 3. ≥30% improvement in pain intensity and physical function and 10 point improvement in the physical function raw score | 61 (16.0) | 28 (12.7) | 1.3 (0.9–1.9) |
| 4. ≥40% improvement in pain intensity or physical function | 210 (55.1) | 99 (44.8) | 1.2 (1.2–1.3)* |
| 5. ≥40% improvement in pain intensity and physical function | 54 (14.2) | 20 (9.1) | 1.6 (0.8–2.9) |
| 6. ≥50% improvement in pain intensity or physical function | 191 (50.1) | 79 (35.8) | 1.4 (1.2–1.6)* |
| 7. ≥50% improvement in pain intensity and physical function | 36 (9.5) | 17 (7.7) | 1.2 (0.6–2.4) |
| 8. ≥30% improvement in pain intensity and ≥20% improvement in physical function, or ≥20% improvement in pain intensity and ≥30% improvement in physical function | 90 (23.6) | 40 (18.1) | 1.3 (1.0–1.7) |
| 9. ≥50% improvement in pain intensity and ≥20% improvement in physical function, or ≥20% improvement in pain intensity and ≥50% improvement in physical function | 79 (20.7) | 35 (15.8) | 1.3 (0.9–2.0) |
| 10. ≥50% improvement in pain intensity, or ≥20% improvement in pain intensity and ≥30% improvement in physical function | 164 (43.0) | 64 (29.0) | 1.5 (1.2–1.8)* |
| Postherpetic Neuralgia (5 trials; Subsample, n=476) | |||
| 1. ≥30% improvement in pain intensity or physical function | 191 (63.9) | 75 (42.4) | 1.5 (1.2–1.9)* |
| 2. ≥30% improvement in pain intensity and physical function | 43 (14.4) | 16 (9.0) | 1.6 (1.1–2.4)* |
| 3. ≥30% improvement in pain intensity and physical function and ≥10 point improvement in the physical function raw score | 42 (14.1) | 15 (8.5) | 1.7 (1.1–2.5)* |
| 4. ≥40% improvement in pain intensity or physical function | 163 (54.5) | 68 (38.4) | 1.4 (1.1–1.8)* |
| 5. ≥40% improvement in pain intensity and physical function | 32 (10.7) | 9 (5.1) | 2.1 (1.1–4.0)* |
| 6. ≥50% improvement in pain intensity or physical function | 149 (49.8) | 61 (34.5) | 1.4 (1.2–1.8)* |
| 7. ≥50% improvement in pain intensity and physical function | 23 (7.7) | 7 (4.0) | 1.9 (0.9–4.3) |
| 8. ≥30% improvement in pain intensity and ≥20% improvement in physical function, or ≥20% improvement in pain intensity and ≥30% improvement in physical function | 58 (19.4) | 18 (10.2) | 1.9 (1.5–2.4)* |
| 9. ≥50% improvement in pain intensity and ≥20% improvement in physical function, or ≥20% improvement in pain intensity and ≥50% improvement in physical function | 50 (16.7) | 17 (9.6) | 1.7 (1.3–2.4)* |
| 10. ≥50% improvement in pain intensity, or ≥20% improvement in pain intensity and ≥30% improvement in physical function | 116 (38.8) | 35 (19.8) | 2.0 (1.8–2.3)* |
P value<0.05
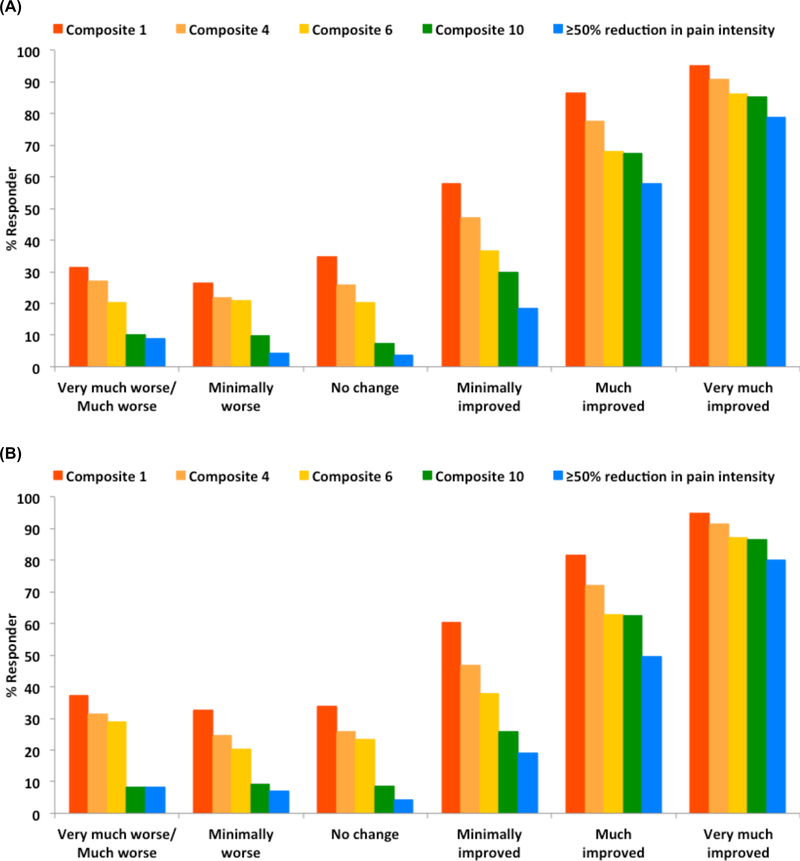
Figure 2.
Percent responder on different outcomes according to patient global impression of change/improvement in clinical trials of gabapentin, pregabalin, and duloxetine for diabetic peripheral neuropathy (A) and postherpetic neuralgia (B).
Table 5.
Association of responder outcomes with PGIC/PGII rating of “much improved” or “very much improved” in clinical trials of gabapentin, pregabalin, and duloxetine for diabetic peripheral neuropathy and postherpetic neuralgia
| Diabetic Peripheral Neuropathy | Postherpetic Neuralgia | |||
|---|---|---|---|---|
| Outcome measure | No. reporting “much improved” or “very much improved” on PGIC/PGII (%) | Risk Ratio (95% CI) | No. reporting “much improved” or “very much improved” on PGIC/PGII (%) | Risk Ratio (95% CI) |
| Composite 1 | ||||
| Non-responder | 100 (12.9) | 1.0 | 59 (9.2) | 1.0 |
| Responder | 886 (62.9) | 4.9 (4.3–5.5) | 365 (46.3) | 5.0 (3.7–6.8) |
| Composite 4 | ||||
| Non-responder | 169 (17.8) | 1.0 | 91 (11.9) | 1.0 |
| Responder | 817 (66.2) | 3.7 (3.3–4.2) | 333 (50.5) | 4.2 (3.6–5.1) |
| Composite 6 | ||||
| Non-responder | 244 (21.9) | 1.0 | 123 (14.5) | 1.0 |
| Responder | 742 (69.3) | 3.2 (2.9–3.5) | 301 (51.8) | 3.6 (2.8–4.6) |
| Composite 10 | ||||
| Non-responder | 253 (20.5) | 1.0 | 125 (12.6) | 1.0 |
| Responder | 733 (77.5) | 3.8 (3.4–4.2) | 299 (68.1) | 5.4 (4.3–6.7) |
| ≥50% reduction in pain intensity | ||||
| Non-responder | 334 (23.8) | 1.0 | 171 (15.8) | 1.0 |
| Responder | 652 (83.5) | 3.5 (3.1–4.0) | 253 (72.7) | 4.6 (3.8–5.6) |
Note: Composite 1 responder = ≥30% reduction in pain intensity or ≥30% improvement in physical function
Composite 4 responder = ≥40% reduction in pain intensity or ≥40% improvement in physical function
Composite 6 responder = ≥50% reduction in pain intensity or ≥50% improvement in physical function
Composite 10 responder = ≥50% reduction in pain intensity, or ≥20% reduction in pain intensity and ≥30% improvement in physical function
Two separate sensitivity analyses were conducted to confirm the robustness of the results. First, we excluded the 3 trials with non-significant treatment effects on pain intensity (Study IDs 945–224, 1008–030, and 1008–040) from the models evaluating the composite responder outcomes. Second, we classified participants who did not complete the trial (i.e., withdrawals) as non-responders for all of the outcomes and re-analyzed the data (Supplemental Tables 2–4). All data analyses were completed using Stata SE 13 (College Station, TX).
RESULTS
Table 1 presents the characteristics of the individual trials included in the current study. In total, there were 2,287 patients that participated in 9 trials for DPN and 1,513 patients that participated in 6 trials for PHN. Trial lengths for DPN and PHN ranged 5–12 weeks and 8–13 weeks, respectively. The mean age in trials for DPN (59.1±10.9 years) was lower than in trials for PHN (71.8±10.4 years), and the proportion of women was lower in the DPN trials (41.4%; n=947) than in the PHN trials (53.7%; n=812). Among patients in the DPN trials, the baseline mean pain intensity and physical function scores were 6.3 (±1.5) and 51.8 (±26.2), respectively, while in PHN trials the mean pain intensity and physical function scores at baseline were 6.6 (±1.6) and 54.1 (±28.2), respectively. The pooled outcome results are shown according to medication and neuropathic pain condition in Table 2 and Supplemental Table 1. For PHN, gabapentin and pregabalin reduced pain intensity significantly more than placebo, while duloxetine, gabapentin, and pregabalin decreased pain intensity significantly more than placebo among patients with DPN. Relative to those in the placebo group, patients who received an active medication were 1.8–2.4 times more likely to have a ≥50% reduction in pain intensity. However, none of the medication vs. placebo group comparisons were statistically significant for ≥50% improvement in physical function (Table 2). Similar results were observed in responder analyses examining ≥30% improvements in pain intensity and physical function (Supplemental Table 1).
Figure 1 illustrates that the relationship between pain intensity and physical function was weak in trials for both neuropathic pain conditions. In DPN and PHN trials, the correlations at baseline were −0.25 (P<0.001) and −0.15 (P<0.001), respectively. Correlation between change in pain intensity from baseline to post-treatment and change in physical function was small in DPN (ρ=−0.22; P<0.001) and non-significant in PHN (ρ=−0.05; P=0.08). Similarly, the percentage of DPN patients achieving a ≥30% improvement in physical function increased significantly with greater reduction in pain intensity, but not in those with PHN. Among patients with DPN that had <30%, 30–49%, and ≥50% reduction from baseline in pain intensity, the percentages with a ≥30% improvement in physical function were 20.8% (n=234), 27.8% (n=101), and 34.7% (n=278), respectively (P=<0.001; results not shown in tables). In PHN patients, the percentages achieving a ≥30% improvement in physical function were 19.3% (n=179), 21.2% (n=46), and 23.9% (n=88) among those with <30%, 30–49%, and ≥50% reduction in pain intensity, respectively (P=0.19).
In a random sample of half of the patients enrolled in pregabalin trials for DPN and PHN, the assay sensitivity of 10 composite outcomes of pain intensity and physical function were examined (Table 3). Of these candidate outcomes, pregabalin had significantly greater proportion of responders vs. placebo for Composites 1, 4, 6, and 10 in both neuropathic pain conditions. Table 4 shows the validation and cross-validation results for these 4 composite outcomes according to medication and neuropathic pain condition. All 4 of these outcomes had significant pregabalin vs. placebo differences in the validation subsamples for both neuropathic pain conditions, as well as for gabapentin in PHN and duloxetine in DPN; however, in contrast to Composite 1, Composites 4, 6, and 10 did not cross-validate with gabapentin for DPN. The NNTs for Composites 1, 4, and 6 were approximately 6, while Composite 10 had the lowest NNTs, ranging from 4–5 across the 3 medications and 2 neuropathic pain conditions.
To assess clinical validity, the associations of responder outcomes with PGIC/PGII rating of “much improved” or “very much improved” are presented in Table 5. All of the responder outcomes were statistically associated with the global ratings of improvement; Composites 1 and 10 had the strongest associations with improvement in patients with DPN and PHN, respectively. Importantly, however, Figure 2 illustrates that Composites 1, 4, and 6 had relatively high proportion of responders among patients who reported worsening health. In contrast, Composite 10 had lower proportions of responders with worsened health ratings and had a similar percent distribution of responders across PGIC/PGII categories as the responder outcome of ≥50% reduction in pain intensity.
In sensitivity analyses, the validation results did not change substantially when excluding the 3 trials with non-significant treatment effects for pain intensity. However, in contrast to the results shown in Table 4, the proportion of Composite 4 responders did not differ significantly between pregabalin and placebo for DPN (RR=1.3; 95% CI: 0.9–1.7) after excluding the pregabalin negative trials. In addition, study participants treated with gabapentin for DPN were significantly more likely to achieve Composite 4 (RR=1.8; 95% CI: 1.2–2.8) and Composite 6 (RR=1.7; 95% CI: 1.1–2.6) than those on placebo after excluding the negative gabapentin trial. The results of classifying participants who did not complete the trial (i.e. withdrawals) as non-responders are shown in Supplemental Tables 2–4. As expected with this type of imputation, the rates of treatment and placebo responders were lower relative to the results shown in Tables 2–4; however, the pattern of results were similar. Composite responders 1, 4, 6, and 10 were validated in trials of pregabalin for both pain conditions and cross-validated in trials of duloxetine for DPN and in trials of gabapentin for PHN. Composite 10 had more favorable NNT values relative to the other outcomes when withdrawals were classified as non-responders.
DISCUSSION
In pooled analyses of neuropathic pain trials, we evaluated the assay sensitivities of 10 potential composite outcomes that integrate data on pain intensity and physical functioning. A summary of the validation and cross-validation results is shown in Supplemental Table 5. The composite responder outcome of ≥50% reduction in pain intensity, or a ≥20% reduction in pain intensity and ≥30% improvement in physical function was validated for pregabalin in both neuropathic pain conditions and cross-validated in DPN patients for duloxetine and PHN patients for gabapentin. In addition, this outcome was favorably associated with PGIC/PGII ratings. To our knowledge, this is the first study to explore composite responder outcomes of pain intensity and physical function in clinical trials of DPN and PHN.
We applied a data driven approach to identify a composite responder outcome (from a pool of 10 candidates) that is sensitive to treatments for DPN and PHN. Multiple groups, including IMMPACT [12] and the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) [49], recommend responder outcomes to improve the reporting and informativeness of clinical trials. For example, based in part on a pooled analysis of pain intensity and PGIC ratings in analgesic clinical trials [14], IMMPACT recommends thresholds of ≥10–20%, ≥30%, and ≥50% reduction from baseline in pain intensity ratings to identify minimal, moderate, and substantial improvements, respectively [12]. Not only can the analysis of responder outcomes help clinicians translate trial results and inform patient’s expectation of treatment benefit [28], but they can also facilitate integration of multiple dimensions of treatment response into a single outcome. Indeed, composite responder outcomes have been recommended by FDA for use as primary outcome measures in a variety of therapeutic areas, such as irritable bowel syndrome and chronic obstructive pulmonary disease [7,35,53,54]. For these reasons, we applied responder analyses to develop criteria for a composite outcome instead of using other techniques, such as item response theory. However, as Senn and Julious have noted [41], there are limitations to dichotomizing outcome measures in clinical trials (i.e., responder analysis), including loss of statistical power and potential misclassification of responders/non-responders based on the cutoff value used.
In the current study, one of our goals was to identify a composite responder of pain intensity and physical functioning that detects treatment effects across different neuropathic pain conditions and medications. Composites 1, 4, 6, and 10 met this goal. As expected, the proportions of responders in active medication and placebo groups decreased as the criteria for defining the outcome become more conservative (Table 4). However, regardless of the level of improvement required to achieve composite responder outcomes 1, 4, and 6, the NNTs were approximately the same (~6) for these outcomes because the attributable risk difference between medication and placebo groups were similar. Although there are limitations to the clinical value of the NNT [22], it is a standardized metric to compare different definitions of responder outcomes. (In general, the NNTs observed in the current study were lower than the NNTs for 50% pain relief reported by Finnerup and colleagues in a meta-analysis of pharmacologic treatments for neuropathic pain [16]. This difference likely reflects that we analyzed data from pivotal trials that were submitted to FDA for approval of drug indications, while Finnerup et al. [16] computed NNTs from a more diverse pool of published and unpublished reports of trial results.) Notably, Composite 10 had the lowest NNT values for each medication and condition examined in the current study, except in trials of gabapentin for DPN. For this outcome, patients are considered a treatment responder if they have a substantial improvement in pain (≥50% reduction) or if they have a minimal improvement in pain (≥20% reduction) and a ≥30% improvement in physical function. Relative to a standard responder outcome of ≥50% reduction in pain intensity, the proportions of Composite 10 responders were higher in both the active and placebo treatment groups for all 3 medications and both pain conditions (see Tables 2 and 4), and the NNTs were slightly better for Composite 10. The limited benefit of Composite 10 for improving assay sensitivity is, in part, related to the proportion of patients (35% in DPN and 24% in PHN trials) that improved substantially on pain intensity also had improvements of ≥30% in physical functioning, thereby reducing the probability of capturing additional responders with the second component of Composite 10. Although requiring moderate-to-substantial improvements in both pain intensity and physical functioning may help to improve assay sensitivity by reducing placebo response (e.g., Composites 2, 3, 5, and 7 defined in Table 3), the proportion of patients achieving this type of outcome was limited in the current set of trials.
Interestingly, the correlation between pain intensity and physical function was low in DPN and PHN trials (Figure 1). Although previous studies have demonstrated the negative impact of DPN and PHN on physical function and health-related quality of life [8,10,18,20,21,45,46], few studies have reported the association between changes in pain intensity and physical function in response to efficacious treatment. As noted by Turk et al. [52], this information is needed because if the components of a composite outcome are too highly correlated, then a single primary endpoint would be sufficient. In contrast to the high correlations between pain and physical function reported in OA trials [4], the low correlations observed in the current study might reflect that patients with neuropathic pain do not incorporate functional impacts into their pain intensity ratings, neuropathic pain has less of an impact on physical activities, and/or the physical function scale used in the current study is inadequate, as discussed below.
The current study results should be interpreted in light of several strengths and limitations. First, in contrast to studies that developed responder outcomes for CLBP [42], FM [1], and OA [34] in which condition-specific measures of physical functioning were available, we were limited to the SF-36 physical function subscale, which might not adequately assess the functional impacts of DPN or PHN. In fact, this is a challenge for neuropathic pain research in general as there are few condition-specific measures of physical function that have adequate psychometric properties for clinical research [26,27]. Thus, there is a critical need for measures that capture functional limitations that are specific to the neuropathic pain population studied. Recommendations for the development of patient-centered physical function assessment tools were described by IMMPACT/OMERACT [44].
Another limitation of our study is the wide range of physical function scores at baseline in trials for DPN and PHN. Indeed, 20% and 26% of patients with DPN and PHN, respectively, had a baseline physical function score of ≥80 (results not shown in tables), a threshold above which patients could not achieve a 30% improvement in function (i.e., ceiling effect). One potential strategy for improving assay sensitivity to detect improvements in physical functioning is to require a minimum level of functional limitations as part of study eligibility in neuropathic pain trials, analogous to the inclusion criterion requirement for pain intensity (e.g., NRS≥4). Alternatively, researchers can consider using accelerometers to assess physical activity objectively, providing a potentially wider measurement range of a patient’s real world, daily activity pattern [32,44,48]. A third study limitation is that physical functioning was only measured at 2 time points (baseline and trial endpoint). Fourth, we used percent change in the pain intensity and physical function measures as outcome criteria for defining treatment responders, which can limit statistical power [41,56,57]. Fifth, we applied single imputation methods for missing data. Although we observed similar validation results using BOCF and LOCF, it would be valuable to examine alternative imputation approaches, including those that do not assume data are missing at random (e.g., pattern mixture analysis) [29]. Also, there were more missing data for physical functioning than for pain intensity. Patients missing physical function data were coded as non-responders for the physical function component of the composite outcomes, reducing statistical power to detect treatment benefit. A final caveat to note is that we did not examine the effect of treatment dosages on the composite outcomes.
Strengths of our study include access to individual patient data from 15 completed RCTs. These trials had a core set of outcome measures in common, similar study designs and inclusion/exclusion criteria for each neuropathic pain condition, and placebo controls. All of this facilitated pooling of data to attain reasonably large sample sizes to examine the relationship between pain and physical function in DPN and PHN patients, and to evaluate composite outcomes across 3 pharmacologic treatments and 2 neuropathic pain conditions.
In view of global population aging and the likely growth in the numbers of adults with DPN and PHN in the future, there is an urgent need to develop safe and efficacious treatments that improve outcomes that patients care about. A composite responder outcome of pain intensity and physical function may ultimately prove useful in better capturing the overall benefits of existing and future neuropathic pain treatments. However, additional prospective validation studies are needed, ideally with different treatments and condition-specific measures of physical function to establish the generalizability of a pain-physical function composite outcome.
Supplementary Material
ACKNOWLEDGEMENTS
Financial support for this project was provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public–private partnership, which has received research contracts (HHSF2232014001991C), grants, or other revenue from the United States Food and Drug Administration, multiple pharmaceutical and device companies, and other sources.
Footnotes
CONFLICT OF INTEREST
The views expressed in this article are those of the authors and no official endorsement by the Food and Drug Administration (FDA) or the pharmaceutical and device companies that provided unrestricted grants to support the activities of the ACTTION public-private partnership should be inferred. Drs. Allen, Burke, and Katz consult for pharmaceutical or device companies. Drs. Marshall and Resnick are employees of Pfizer Inc.; Dr. Vanhove is an employee of Jazz Pharmaceuticals; and Ms. Zhang is an employee of Eli Lilly and Company.
REFERENCES
- 1.Arnold LM, Williams DA, Hudson JI, Martin SA, Clauw DJ, Crofford LJ, Wang F, Emir B, Lai C, Zablocki R, Mease PJ. Development of responder definitions for fibromyalgia clinical trials. Arthritis Rheum. 2012;64(3):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831–36. [DOI] [PubMed] [Google Scholar]
- 3.Bender R Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22:102–110. [DOI] [PubMed] [Google Scholar]
- 4.Bingham CO, Bird SR, Smugar SS, Xu X, Tershakovec AM. Responder analysis and correlation of outcome measures: pooled results from two identical studies comparing etoricoxib, celecoxib, and placebo in osteoarthritis. Osteoarthritis Cartilage. 2008;16(11):1289–1293. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C, Evans CJ, Katz N, Mardekian J, Zlateva G, Simon LS. Further qualification of a therapeutic responder index for patients with chronic low back pain. J Rheumatol. 2011;38(2):362–369. [DOI] [PubMed] [Google Scholar]
- 6.Brod M, Pohlman B, Blum SI, Ramasamy A, Carson R. Burden of illness of diabetic peripheral neuropathic pain: a qualitative study. Patient. 2015;8(4):339–348. [DOI] [PubMed] [Google Scholar]
- 7.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA, Baird MJ, Schneier HA, Johnston JM. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107(11):1702–12. [DOI] [PubMed] [Google Scholar]
- 8.Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, Rice AS. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. Eur J Pain. 2008;12: 731–741. [DOI] [PubMed] [Google Scholar]
- 9.Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8(6):395–403. [DOI] [PubMed] [Google Scholar]
- 10.Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dworkin RH, Corbin AE, Young JP Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized placebo-controlled trial. Neurology. 2003;60:1274–1283. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Katz NP, Rowbotham MC, Peirce-Sandner S, Cerny I, Clingman CS, Eloff BC, Farrar JT, Kamp C, McDermott MP, Rappaport BA, Sanhai WR. Evidence-based clinical trial design for chronic pain pharmacotherapy: a blueprint for ACTION. Pain. 2011;152(3 Suppl):S107–115. [DOI] [PubMed] [Google Scholar]
- 14.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus H, Strand V, Tugwell P, Weinblatt M, Williams HJ, Wolfe F, Kieszak S. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–735. [DOI] [PubMed] [Google Scholar]
- 16.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–118. [DOI] [PubMed] [Google Scholar]
- 18.Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manag. 2005;30(4):374–85. [DOI] [PubMed] [Google Scholar]
- 19.Guy W (Public Health Service Alcohol, Drug Abuse, and Mental Health Administration). ECDEU Assessment manual for psychopharmacology, revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare, 1976: 217–222. DHEW Publication no. [ADM] 76–338. [Google Scholar]
- 20.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–82. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz N, Paillard FC, Van Inwegen R. A review of the use of the number needed to treat to evaluate the efficacy of analgesics. J Pain. 16(2):116–123. [DOI] [PubMed] [Google Scholar]
- 23.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–2110. [DOI] [PubMed] [Google Scholar]
- 24.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. [DOI] [PubMed] [Google Scholar]
- 25.Mease PJ, Clauw DJ, Christensen R, Crofford LJ, Gendreau RM, Martin SA, Simon LS, Strand V, Williams DA, Arnold LM. Toward development of a fibromyalgia responder index and disease activity score: OMERACT module update. J Rheumatol. 2011;38(7):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P, Claydon L, Hendrick P, Cook C, Baxter GD. Pain and physical functioning in neuropathic pain: a systematic review of psychometric propoerties of various outcome measures. Pain Prac. 2016;16(4):195–508. [DOI] [PubMed] [Google Scholar]
- 27.Mehta P, Claydon L, Hendrick P, Winser S, Baxter GD. Outcome measures in randomized controlled trials of neuropathic pain conditions: a systematic review of systematic reviews and recommendations for practice. Clin J Pain. 2015;31(2);169–176. [DOI] [PubMed] [Google Scholar]
- 28.Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Ann Rheum Dis. 2010;69(2):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council. The prevention and treatment of missing data in clinical trials. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 30.Newcombe R Improved confidence intervals for the difference between binomial proportions based on paired data. Statist Med. 1998;17:2635–2650. [PubMed] [Google Scholar]
- 31.O’Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, Price DD, Robinson ME. Patient-centered perspective on treatment outcomes in chronic pain. Pain Med. 2010;11(1):6–15. [DOI] [PubMed] [Google Scholar]
- 32.Patel KV, Dansie EJ, Turk DC. Impact of chronic musculoskeletal pain on objectively measured daily physical activity: a review of current findings. Pain Manage. 2013;3(6):467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham T, Van Der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30(7):1648–1654. [PubMed] [Google Scholar]
- 34.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. [DOI] [PubMed] [Google Scholar]
- 35.Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P, Fox SM, Schneier HA, Kurtz CB, Johnston JM. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107(11):1714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice ASC, Maton S. Gabapentin in posterpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. [DOI] [PubMed] [Google Scholar]
- 37.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253–260. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. [DOI] [PubMed] [Google Scholar]
- 39.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–42. [DOI] [PubMed] [Google Scholar]
- 40.Sabatowski R, Galvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, Versavel M. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109:26–35. [DOI] [PubMed] [Google Scholar]
- 41.Senn S, Julious S. Measurement in clinical trials: a neglected issue for statisticians? Stat Med. 2009;28(26):3189–3209. [DOI] [PubMed] [Google Scholar]
- 42.Simon LS, Evans C, Katz N, Bombardier C, West C, Robbins J, Copley-Merriman C, Markman J, Coombs JH. Preliminary development of a responder index for chronic low back pain. J Rheumatol. 2007;34(6):1386–1391. [PubMed] [Google Scholar]
- 43.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 44.Taylor AM, Philips K, Patel KV, Turk DC, Dworkin RH, Beaton D, Clauw DJ, Gignac MA, Markman JD, Williams DA, Bujanover S, Burke LB, Carr DB, Choy EH, Conaghan PG, Cowan P, Farrar JT, Freeman R, Gewandter J, Gilron I, Goli V, Gover TD, Haddox JD, Kerns RD, Kopecky EA, Lee DA, Malamut R, Mease P, Rappaport BA, Simon LS, Singh JA, Smith SM, Strand V, Tugwell P, Vanhove GF, Veasley C, Walco GA, Wasan AD, Witter J. Assessment of physical function and participation in chronic pain trials: IMMPACT/OMERACT recommendations. Pain. 2016;136(9):1836–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complicat. 2006;20:26–33. [DOI] [PubMed] [Google Scholar]
- 47.Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12: 203–213. [DOI] [PubMed] [Google Scholar]
- 48.Trudeau J, Van Inwegen R, Eaton T, Bhat G, Paillard F, Ng D, Tan K, Katz NP. Assessment of pain and activity using an electronic pain diary and actigraphy device in a randomized, placebo-controlled crossover trial of celecoxib in osteoarthritis of the knee. Pain Pract. 2015;15(3):247–55. [DOI] [PubMed] [Google Scholar]
- 49.Tubach F, Ravaud P, Beaton D, Boers M, Bombardier C, Felson DT, van der Heijde D, Wells G, Dougados M. Minimal clinically important improvement and patient subjective outcome measures in rheumatic disorders. J Rheumatol. 2007;34:1188–93. [PMC free article] [PubMed] [Google Scholar]
- 50.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. [DOI] [PubMed] [Google Scholar]
- 51.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 15 2008;137(2):276–285. [DOI] [PubMed] [Google Scholar]
- 52.Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, Cleeland CS, Cowan P, Dimitrova R, Farrar JT, Hertz S, Heyse JF, Iyengar S, Jadad AR, Jay GW, Jermano JA, Katz NP, Manning DC, Martin S, Max MB, McGrath P, McQuay HJ, Quessy S, Rappaport BA, Revicki DA, Rothman M, Stauffer JW, Svensson O, White RE, Witter J. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. 2008;139(3):485–493. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Chronic Obstructive Pulmonary Disease: Use of the St. George’s Respiratory Questionnaire as a PRO Assessment Tool. Guidance for Industry; March 2018. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm071575.pdf (accessed April 2018). [Google Scholar]
- 54.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Irritable Bowel Syndrome - Clinical Evaluation of Drugs for Treatment. 2012. Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf. (accessed April 2018).
- 55.van Seventer R, Feister HA, Young JP Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22:375–384. [DOI] [PubMed] [Google Scholar]
- 56.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: A simulation study. BMC Med Res Methodol. 2001;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow-up measurements. BMJ. 2001;323(7321):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ware JE, Snow KK, Kolinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 59.Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, Iyengar S, Raskin J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420. [DOI] [PubMed] [Google Scholar]
- 60.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.