Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an autosomal recessive and fatal multisystem metabolic disorder. It presents with wide-ranging gastrointestinal and neurologic symptoms. It is caused by a mutation in the TYMP gene which impairs thymidine phosphorylase (TP) activity, therefore leading to the accumulation of thymidine and deoxyuridine in plasma and tissues. Thus, MNGIE can be diagnosed by findings of high levels of thymidine and deoxyuridine. Herein, we present the case of a 40-year-old male who presented with diarrhea, vomiting, and abdominal pain, severe weight loss, neurologic deficits, and distal motor weakness progressing over a period of 13 years. The combination of this broad clinical picture along with results of magnetic resonance imaging, electromyography, colonic biopsies, genetic testing, and elevated plasma and tissue thymidine and deoxyuridine levels confirmed the diagnosis of MNGIE. TYMP gene mutation impairs TP function. TP mutations in the nuclear DNA lead to mitochondrial DNA deletions causing mitochondrial failure and ultimately cell death. Treatment modalities are targeting the restoration of TP activity or aiming to decrease the high levels of thymidine and pyrimide. However, diagnosing this disease is still a challenge and often overdue. This patient's 13-year delay in diagnosis shows the importance of a complete neurological exam and muscle strength testing in patients with gastrointestinal symptoms. The diagnosis of MNGIE requires interdepartmental collaborative work for diagnosis delay prevention and for optimal patient care.
Keywords: Mitochondrial neurogastrointestinal encephalomyopathy, Genetic mutation, Mitochondrial dysfunction, Gastroenterology, Neurogastroenterology
Introduction
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an extremely rare and fatal metabolic disorder with an autosomal recessive mode of inheritance. MNGIE is caused by mutations at the level of a nuclear gene involved in the pyrimidine deoxyribonucleosides metabolism, thus indirectly affecting the replication and the expression of genes in the mitochondria [1, 2, 3]. The prevalence of MNGIE worldwide is estimated to be <10 in a million [4] with around 200 patients with MNGIE identified in the world [5]. MNGIE is a progressive and degenerative disorder in which several organ systems are involved, and it is associated with high morbidity [3]. Although the clinical picture is usually complex, the main symptoms are gastrointestinal (GI) dysmotility, peripheral neuropathy, progressive external ophthalmoplegia, cachexia, diffuse leukoencephalopathy seen on magnetic resonance imaging (MRI) of the brain, and evidence of dysfunctionality of the mitochondria [6]. Patients typically present with these clinical symptoms during their 20s and 30s, while the average age of onset is 19 years [7]. The most prominent features in MNGIE are the GI symptoms which involve vomiting, constipation/diarrhea, and episodes of pseudo-obstruction. These symptoms lead to weight loss and cachexia in a progressive fashion. Moreover, and as implied by the name of the disorder, patients with MNGIE present with neurologic symptoms, with peripheral neuropathy, ophthalmoparesis, and hearing loss being the most common [2, 7]. The neurologic symptoms are mild and less severe than the GI symptoms [7]. The extremely low prevalence of MNGIE and the multiple organ systems involvement paint a complex clinical picture leading to a delay in the diagnosis. Certain authors in the literature estimate a delay in the diagnosis between 5 and 10 years [8]. Moreover, the patient may be subjected to invasive medical interventions, such as exploratory abdominal laparotomies. The diagnosis requires an interdepartmental approach due to the multiple organ systems involved and the wide clinical picture. A delayed diagnosis is associated with a worse prognosis. MNGIE should be suspected when the patient presents with both neurologic and GI symptoms; especially when leukoencephalopathy is found on MRI and there is involvement of the ocular system. Once MNGIE is suspected, the confirmatory diagnosis is made via the combination of Sanger sequencing of TYMP gene and checking for urine and plasma levels of thymidine and deoxyuridine [9].
MNGIE is caused by mutations in the nuclear gene TYMP which encodes the enzyme thymidine phosphorylase (TP) [2]. Mutations affecting the TYMP gene lead to the accumulation of thymidine and deoxyuridine in the plasma and tissue; hence its use in developing the diagnosis of MNGIE. As such, MNGIE can be described as a result of an intergenomic communication defect in which mutations in a nuclear gene affect the expression of mitochondrial genes [7]. Other diagnostic methods for MNGIE include clinical examination, TP activity, MRI, electrodiagnostic procedures, and biochemical findings [3]. There are no specific modes of therapies for MNGIE; rather, patients are managed symptomatically while considering each patient as an individual [3]. Laforce et al. [10] reported that the average age of death for patients with MNGIE is 37 years.
Herein, we present the case of a 40-year-old male patient presenting with neurogastrointestinal symptoms and diagnosed with MNGIE.
Case Report
A 40-year-old male patient born to nonconsanguineous parents presented to our hospital for more than 15 episodes of nonbloody, nonmucoid diarrhea associated with severe diffuse abdominal pain and vomiting of 30 days duration. The patient concurrently complained of severe weight loss, visual problems, progressive bilateral hearing loss, and progressive distal muscle weakness more prominent in the lower extremities of 2 years duration.
He recalls neurological symptoms beginning at the age of 27 years with episodes of intermittent bilateral blurry vision which would later resolve on their own. At the age of 32 years, the visual symptoms reemerged with worsened blurry vision and diplopia. After presenting to an ophthalmologist, a brain MRI was ordered with no significant findings at that time.
Aged 40 years, the patient presented to our hospital for the aforementioned complaints. He reported a significant weight loss of 18.5 kg representing a 34.4% decrease of his initial weight (∼55.4 kg) over the course of 2 years despite normal food intake. He also described postprandial episodes of nonbloody, nonbilious vomiting associated with diffuse abdominal pain for the last 8 months. The patient denied any symptoms of body dysmorphia or laxative abuse. On physical examination, the patient looked cachectic with a current body mass index (BMI) of 13.06 kg/m2. The patient had mild bilateral ptosis and ophthalmoplegia along with decreased sensations and proprioception in the lower extremities. The patient was also experiencing mild-to-moderate weakness of the upper and lower extremities and showed hyporeflexia in the upper extremities, while the reflexes were absent in the lower extremities. The motor weakness was more prominent in the lower extremities, leading to the patient's inability to ambulate without the assistance of a cane. Moreover, no weakness was to be reported upon flexion and extension of the neck, with absent pathological reflexes. The abdominal examination was negative for any tenderness or organomegaly.
On laboratory workup, the patient was found to have a mild increase in the hepatic enzyme AST with a value of 91 IU/mL. ALT was 33 IU/mL, and GGT was 341 IU/mL. The lipid panel revealed cholesterol levels of 201 mg/dL and triglyceride levels of 401 mg/dL.
The patient was found to have elevated levels of blood lactate (4.82 mmol/L) and elevated blood pyruvate levels (0.28 mmol/L). The complete blood count, thyroid function tests, arterial blood gases, metabolic tests (urine organic acids and blood-urine amino acids), and creatine kinase levels all returned normal. The results of the pulmonary function test, done to assess the function of the patient's lungs, were in favor of a mild restrictive lung disease. An audiogram was also performed to assess the patient's complaints of hearing loss, and it showed bilateral sensorineural hearing loss, while the electromyography showed severe neuropathy with demyelination suggestive of associated myopathy.
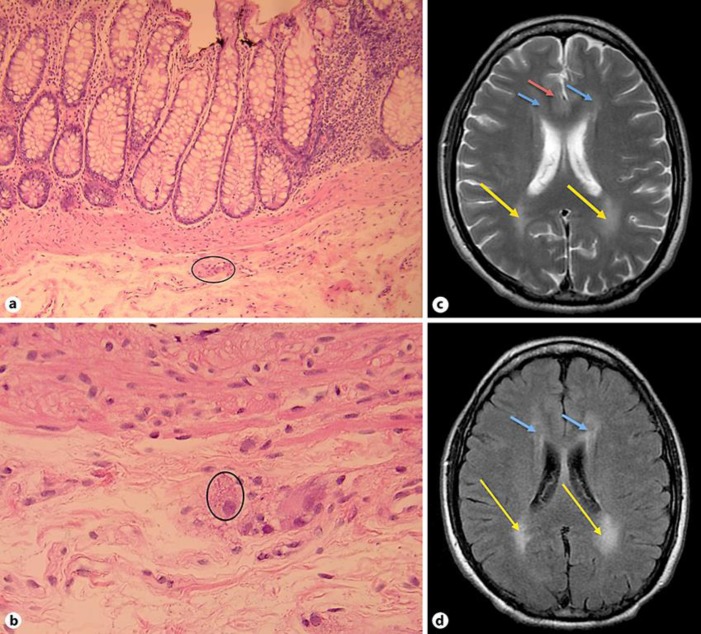
For the evaluation of the GI symptoms, the patient underwent stool analysis and an esophagogastroduodenoscopy with gastric, duodenal, and ileal biopsies, all of which returned with negative results with the biopsies showing no evidence of active inflammation, mucosal changes of chronicity, specific granulomas, or parasite. Moreover, a colonoscopy with superficial colonic biopsies was performed showing no evidence of thickening of the subepithelial basement membrane or evidence of intraepithelial lymphocytosis. The patient underwent a computed tomography scan of the abdominopelvic area revealing diffuse dilated fluid filling the small bowel loops without any evidence of bowel obstruction. Due to persistent and severe GI symptoms, the patient underwent a second colonoscopy with mucosectomy for deeper tissue studies. The pathology sections showed colonic tissue with normal mucosa, intact muscularis mucosa, and mildly cauterized submucosa. Ganglion cells were seen in the submucosal plexus showing features of intracytoplasmic eosinophilic inclusions consistent with megamitochondria (Fig. 1a, b). The appearances were consistent with mitochondrial inclusions. Clinical and genetic studies were advised for the diagnosis.
Fig. 1.
Colonic histology and brain MRI. a Colonic biopsy showing active inflammation and neuroganglion cell nests in rectal submucosa. HE stain, magnification ×40. b Colonic biopsy showing multiple round eosinophilic intracytoplasmic inclusions of megalomitochondria in submucosal ganglionic cells. HE stain, magnification ×200. c Axial T2 FSE brain MRI showing hyperintensity within the periventricular white matter anteriorly (blue arrows) but more so posteriorly (orange arrows) with minimal and faint extension to the subcortical areas in both frontal lobes (red arrow). d Axial T2 FLAIR brain MRI showing hyperintensity within the periventricular white matter anteriorly (blue arrows) but more so posteriorly (orange arrows).
Although no previous brain MRIs were available for comparison, the brain MR images demonstrated T2 FLAIR hyperintensity within the periventricular white matter, more so posteriorly, with minimal faint extension to the subcortical areas in both frontal lobes and in both temporoparietal areas (Fig. 1c, d). There were no findings of brainstem or posterior fossa involvement. The ventricles and the subarachnoid spaces were normal.
Taking into consideration the clinical presentation and the workup findings, subsequent metabolic evaluation showed elevated plasma and urine thymidine and deoxyuridine; these findings are consistent with TP deficiency. Genetic testing was performed revealing 2 heterozygous TYMP variants. The 1st variant was c.401C>A p.(Ala134Glu), which was reported by Garone et al. [11]. The 2nd variant was c.1142T>G p.Val256Phe, which was reported by Etienne et al. [12]. Thus, the diagnosis of MNGIE was confirmed.
Discussion
We report a 40-year-old Lebanese man demonstrating the typical laboratory findings and clinical symptomatology of MNGIE disease with heterozygous mutations of the TYMP gene. Our patient displayed GI symptoms of chronic diarrhea, postprandial vomiting, and progressive weight loss with a BMI of 13.06 kg/m2 at the time of diagnosis. Biopsies obtained with colonoscopy revealed intracytoplasmic eosinophilic inclusions in the ganglion cells of the submucosal plexus consistent with megamitochondria (Fig. 1b), providing an important diagnostic clue towards MNGIE. Neurological symptomatology included self-resolving diplopia, ptosis, bilateral sensorineural hearing loss, peripheral neuropathy and hyporeflexia in the upper and lower extremities more prominent in the lower extremities. This correlated with MRI findings of T2 FLAIR hyperintensity in the periventricular white matter with extension into the subcortical areas in both frontal lobes and in both temporoparietal areas (Fig. 1c, d). The patient also exhibited myopathic symptoms, including muscular atrophy and marked weakness of the upper and lower extremity musculature, with electromyography consistent with severe neuropathy with demyelination suggestive of associated myopathy. GI biopsies were preferred to muscle biopsies as they are more reliable in MNGIE with muscle biopsies displaying inconsistent pathological changes [13]. This constellation of symptoms and findings led to a comprehensive metabolic evaluation which displayed elevated plasma and urine thymidine and deoxyuridine consistent with a defect in the TP enzyme. This was followed up with genetic testing where the patient was found to have heterozygous TYMP variants, confirming the diagnosis of MNGIE. Although our patient demonstrated features of MNGIE at the age of 27 years, there was a 13-year delay in being diagnosed, higher than the average reported delay in diagnosis of 5–10 years in the literature. Furthermore, the average age at death for MNGIE patients is 37 years usually secondary to their perilous nutritional status [10] with our patient being 40 years old at the time of diagnosis. This may be due to heterozygous TYMP variants manifesting at a later age and in milder forms secondary to residual TP activity [14]. This delay in diagnosis shows the importance of a complete neurological exam and testing muscle strength in patients presenting with GI dysmotility.
TP is a catabolic enzyme that plays an important role in pyrimide salvage and catalyzes phosphorolysis of the nucleosides thymidine or deoxyuridine to their respective bases thymine or uracil [2]. Mutations leading to loss of function or reduced activity of TP lead to increases in systemic levels of thymidine and deoxyuridine, sometimes reaching levels 100-fold higher than controls. While TP is highly expressed in various tissues, such as the liver, lungs, spleen, platelets, and lymphocytes, it is only expressed in very low levels in skeletal muscle. However, skeletal muscle is one of the tissues primarily affected by MNGIE; this is due to toxic accumulation of thymidine and deoxyuridine. This elevated thymidine and reduced pyrimidine salvage also indirectly causes the other features of MNGIE [10]. Mitochondrial DNA depends on enzymes encoded by nuclear DNA for its transcription and replication, an example of intergenomic communication. TP mutations in the nuclear DNA lead to altered levels of nucleosides in mitochondria, ultimately causing mitochondrial DNA deletions, depletion, and somatic point mutations causing mitochondrial failure and ultimately cell death [2].
Many treatment modalities have been suggested for the management of MNGIE, such as platelet transfusions, which have been successfully used to transiently restore TP activity and reduce thymidine and deoxyuridine levels. Hemodialysis has been shown to decrease the levels of these toxic metabolites in the serum and urine, but not in the cerebrospinal fluid, with no improvement in neurological function. Allogenic stem cell transplantation, although a well-documented treatment modality for MNGIE, has many limitations, such as finding compatible donors, high rates of graft failure, as well as the risk of graft-versus-host disease. It is also associated with high morbidity and mortality secondary to most MNGIE patients being in a critical medical condition when diagnosed. Another treatment modality that has shown promising results is liver transplantation, with the liver exhibiting high levels of TP expression and normalization of thymidine levels after transplantation [15]. Hence, a collaborative effort between physicians and scientific researchers is needed to improve the quality of life for MNGIE patients. It is up to the former to reduce the excessive delay in diagnosing the disease, while the latter must continue to improve and optimize current treatment modalities as well as discovering new ones.
Statement of Ethics
The patient provided written informed consent for the analysis and publication of the case-related information.
Disclosure Statement
The authors declare that they have no conflicts of interest related to this publication.
Funding Sources
No funding was received for this study.
Author Contributions
Study concept: Antonios Tawk, Mohammed Hussein Kamarreddine, Said Farhat. Supervision: Antonios Tawk, Mohammed Hussein Kamarreddine, Said Farhat. Data acquisition and methodology: Ghadi Abboud, Mona Dagher, Mohamad Chams, Fatmeh Ghandour-Hajj, Mounir Khoury. Formal analysis and investigation: Antonios Tawk, Said Farhat, Mounir Khoury, Fatmeh Ghandour-Hajj. Writing − original draft preparation: Mona Dagher, Mohamad Chams. Writing − review and editing: Antonios Tawk, Mohammed Hussein Kamarreddine, Said Farhat.
References
- 1.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999 Jan;283((5402)):689–92. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 2.Hirano M, Nishigaki Y, Martí R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes. Neurologist. 2004 Jan;10((1)):8–17. doi: 10.1097/01.nrl.0000106919.06469.04. [DOI] [PubMed] [Google Scholar]
- 3.Pacitti D, Levene M, Garone C, Nirmalananthan N, Bax BE. Mitochondrial Neurogastrointestinal Encephalomyopathy: Into the Fourth Decade, What We Have Learned So Far. Front Genet. 2018 Dec;9:669. doi: 10.3389/fgene.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orphanet (2019). Prevalence and Incidence of Rare Diseases: Bibliographic Data. Orphanet Report Series; Number 1, January 2019. Available online at: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf.
- 5.Halter JP, Michael W, Schüpbach M, Mandel H, Casali C, Orchard K, et al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2015 Oct;138((Pt 10)):2847–58. doi: 10.1093/brain/awv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bariş Z, Eminoğlu T, Dalgiç B, Tümer L, Hasanoğlu A. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): case report with a new mutation. Eur J Pediatr. 2010 Nov;169((11)):1375–8. doi: 10.1007/s00431-010-1237-0. [DOI] [PubMed] [Google Scholar]
- 7.Nishigaki Y, Martí R, Copeland WC, Hirano M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J Clin Invest. 2003 Jun;111((12)):1913–21. doi: 10.1172/JCI17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filosto M, Scarpelli M, Tonin P, Testi S, Cotelli MS, Rossi M, et al. Pitfalls in diagnosing mitochondrial neurogastrointestinal encephalomyopathy. J Inherit Metab Dis. 2011 Dec;34((6)):1199–203. doi: 10.1007/s10545-011-9332-6. [DOI] [PubMed] [Google Scholar]
- 9.Scarpelli M, Russignan A, Zombor M, Bereczki C, Zappini F, Buono R, et al. Poor outcome in a mitochondrial neurogastrointestinal encephalomyopathy patient with a novel TYMP mutation: the need for early diagnosis. Case Rep Neurol. 2012 Sep;4((3)):248–53. doi: 10.1159/000346260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laforce R, Jr, Valdmanis PN, Dupré N, Rouleau GA, Turgeon AF, Savard M. A novel TYMP mutation in a French Canadian patient with mitochondrial neurogastrointestinal encephalomyopathy. Clin Neurol Neurosurg. 2009 Oct;111((8)):691–4. doi: 10.1016/j.clineuro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011 Nov;134((Pt 11)):3326–32. doi: 10.1093/brain/awr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne G, Shamseddine K, Pulley M, Milfred F. Two new gene mutations for late onset mitochondrial neurogastrointestinal encephalopathy (MNGIE) Transl Neurosci. 2012 Dec;3((4)):413–4. [Google Scholar]
- 13.Szigeti K, Wong LJ, Perng CL, Saifi GM, Eldin K, Adesina AM, et al. MNGIE with lack of skeletal muscle involvement and a novel TP splice site mutation. J Med Genet. 2004 Feb;41((2)):125–9. doi: 10.1136/jmg.2003.013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel R, Coulter LL, Rimmer J, Parkes M, Chinnery PF, Swift O. Mitochondrial neurogastrointestinal encephalopathy: a clinicopathological mimic of Crohn's disease. BMC Gastroenterol. 2019 Jan;19((1)):11. doi: 10.1186/s12876-018-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filosto M, Cotti Piccinelli S, Caria F, Gallo Cassarino S, Baldelli E, Galvagni A, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE-MTDPS1) J Clin Med. 2018 Oct;7((11)):389. doi: 10.3390/jcm7110389. [DOI] [PMC free article] [PubMed] [Google Scholar]