Abstract
Background and Aim
Helicobacter pylori is one of the most common pathogenic bacteria in the human gut, and is also one of the most important factors that cause digestive disorders such as chronic inflammation, gastric ulcers, and even gastric cancer. Since the use of various antibiotics to treat H. pylori infection is associated with the development of resistance in this bacterium, the aim of this study was to determine the anti-H. pylori effects of Lactobacillus acidophilus, L. plantarum, and L. rhamnosus in the stomach tissue of C57BL/6 mice.
Materials and Methods
In this experimental study, 70 mice in ten groups were evaluated from July to September 2017 in the microbiology laboratory of the School of Medicine, Alborz University of Medical Sciences, Karaj, Iran. After induction of H. pylori infection in mice with the standard strain of H. pylori (ATCC 43504), the infected mice were treated with drug and Lactobacillus species in different groups. Then, the anti-H. pylori effects of lactobacilli were evaluated by stool antigen test and tissue staining.
Results
Based on ELISA results and histological findings, a reduction of inflammation was observed. The group which was only exposed to L. rhamnosus and the one which was exposed to all three strains of Lactobacillus showed the highest antimicrobial effect on H. pylori.
Conclusion
According to the results of this study, probiotic bacteria including L. acidophilus, L. plantarum, and L. rhamnosus could be useful in the reduction of H. pylori infection in the mouse model.
Keywords: Helicobacter pylori, Lactobacillus, Probiotic, Gastritis
Introduction
Helicobacter pylori is a gram-negative spiral-shaped or curved-rod, motile and microaerophilic bacterium which is the cause of several acute and chronic digestive tract infections and even malignancies [1, 2, 3]. Infection caused by this bacterium is spread throughout the world and is prevalent among all age groups such that the World Health Organization (WHO) classified H. pylori as a group 1 carcinogen with the ability to cause gastric cancer [4]. The prevalence of H. pylori differs in various countries and even in separate populations of a country and is related to financial and social conditions of the infected population and also to their lifestyle and type of nutrition [5, 6]. In many developing countries, H. pylori is widespread in 80–90% of the adult population, such that eradication of H. pylori infection is considered to be a public health priority in developing and also developed countries [1, 7]. Many virulence factors such as the shape of spiral bacteria, adhesion factors, and urease enzyme are thought to be involved in ailments caused by this bacterium; consequently, it is of the utmost importance to timely diagnose and get rid of this bacterium, especially during the onset of malignancy and before stomach tissue becomes cancerous [8, 9, 10]. Nowadays, various treatment regimens containing many antimicrobial agents are proposed to treat H. pylori, among which triplet therapy (including a bismuth or proton pump inhibitor and two antibiotics that are usually metronidazole, amoxicillin, tetracycline, or clarithromycin) is currently being used [11]. Most of the studies which have been conducted to treat gastritis and H. pylori infection up to now relied on antibiotic treatments and these studies indicate that by using triplet therapy, 85–90% of drug-sensitive bacteria could be eliminated in a span of 2 weeks [12, 13]. It must be noted that in addition to the drug resistance, there are many flaws and deficiencies in triplet and quadruplet treatments. Given the deficiencies of these treatments and the role of H. pyloriin the development of peptic ulcer, today, the role of probiotics in controlling H. pyloricolonization in the stomach has drawn the attention of many leading scientists [14, 15]. Based on conducted experiments, probiotics which have antimicrobial effects due to their mineral acids and bacteriocins could hinder the proliferation of H. pylori. One of the most important probiotic bacteria groups is Lactobacillus which has the ability to affect the healing process of diseases caused by H. pylori[16, 17]. Regarding the antibacterial property of probiotics, the aim of the present study is to determine the anti-H. pylori effects of Lactobacillus rhamnosus, L. acidophilus, and L. plantarum strains in the stomach tissue of C57BL/6 mice.
Materials and Methods
Bacterial Strain and Culture Condition
H. pylori ATCC43504 were purchased from England and were transferred to the microbiological laboratory of Alborz University of Medical Sciences (Karaj, Iran). They were then cultured on Brucella agar with 7.5% of sheep blood, 10% of fetal bovine serum, and containing antibiotics (amphotericin B at 2 mg/L, polymyxin B at 0.25 mg/L, and vancomycin at 10 mg/L. The cultures were incubated under microaerophilic conditions (5% O2, 10% CO2 and 85% N2) with a type C gas pack at 37°C for 5–7 days. Then, all the clear and spherical colonies were identified using Gram staining, catalase, oxidase, and rapid urease test. Standard L. acidophilus DSM (20079) and L. plantarum DSM (20174) strains were obtained from the Pasteur Institute (Tehran, Iran) and L. rhamnosus strain was isolated from organic honey in Mazandaran province (North of Iran) mountains. In the next step, bacteria were cultured on Man-Rogosa-Sharpe (MRS) agar and were incubated at 37°C for 48 h under microaerophilic conditions using a type C gas pack. Microbial suspensions were produced as 109 CFU/mL doses from cultured bacteria using PBS solution.
Animal Preparation
Seventy male C57BL/6 mice (pathogen-free, 6–8 weeks old), weighing about 20–40 g at the beginning of evaluation, were purchased from the Razi institute of Karaj (Iran). All the animals were housed at the animal facility of Alborz University of Medical Sciences under standard conditions (food and water ad libitum at room temperature, 21 ± 3°C, 12:12-h light/dark cycle, and 55% humidity).
Induction of H. pylori Infection in C57BL/6 Mice
All the animals were randomly divided into ten experimental groups. Nine groups were considered as treatment groups and one group as a control group (7 mice in each group) (Table 1). Suspension containing H. pylori (5 × 1010 CFU/mL) and PBS (1 mL) was made and was inoculated into the stomach using polyethylene tubes once daily for 1 month. Each animal received 1 mL of the prepared suspension.
Table 1.
Drugs and Lactobacillus compounds used in different animal groups
| Animal groups | Drug and microbial compounds |
|---|---|
| Control group | None |
| Treatment groups | |
| First | Bismuth + omeprazole during the first week + L. acidophilus during the second week |
| Second | Bismuth + omeprazole during the first week + L. plantarum during the second week |
| Third | Bismuth + omeprazole during the first week + L. rhamnosus during the second week |
| Fourth | Bismuth + omeprazole during the first week + L. acidophilus and L. plantarum during the second week |
| Fifth | Bismuth + omeprazole during the first week + L. acidophilus and L. rhamnosus during the second week |
| Sixth | Bismuth + omeprazole during the first week + L. rhamnosus and L. plantarum during the second week |
| Seventh | Bismuth + omeprazole during the first week + all three Lactobacillus strains during the second week |
| Eighth | Bismuth + omeprazole during the first week + clarithromycin during the second week |
| Ninth | Bismuth + omeprazole during the first week |
Inoculation of Drugs and Lactobacilli to Animals
In all of the 9 treated groups, bismuth and omeprazole were inoculated during the first week after H. pylori inoculation. Then, in the second week, the mice were treated by gavage with the following: L. acidophilus (group 1), L. plantarum (group 2), L. rhamnosus (group 3), L. acidophilus and L. plantarum (group 4), L. acidophilus and L. rhamnosus (group 5), L. rhamnosus and L. plantarum(group 6), all three Lactobacillus strains (group 7), and clarithromycin (group 8). In group 9, only bismuth and omeprazole were inoculated.
H. pylori Stool Antigen Test
H. pylori stool antigen test (RIA test kit) was performed on mice in the control and infected groups 4, 8, and 12 weeks after infection with H. pylori. At 4 weeks after the last inoculation, 30 mg of animal stool were collected in extraction tubes and 1 mL of extraction solution was added to the tubes containing stool. For stool antigen identification, some of the animals in the control and infected groups were sacrificed, the gut section was cut open, and a few biopsies were performed on the body and pylorus of the stomach for histological analysis. ELISA was performed and the results were analyzed. Also, in weeks 8 and 12 one animal was sacrificed in both the control and infected groups and histological analysis was performed. ELISA results were evaluated based on the measured standards, and the interpretation of positive and negative results was done based on titration (titration <0.05 was considered as negative and >0.05 as positive results).
Assessment of the Presence of H. pyloriafter Infection with H. pylori by PCR Method
In this study, after extraction of DNA using a DNA extraction kit (QIAamp; Cat. No. 51304), 16SrRNA and ureC genes of H. pylori were amplified by using specific primers (Table 2) under specific thermal conditions as follows: initial denaturation at 95°C (3 min), second denaturation at 93°C (45 s), primer annealing at 56°C (30 s), extension at 72°C (45 s), and final DNA extension at 95°C (5 min), during 35 cycles.
Table 2.
Specific primers for amplification of 16SrRNA and ureC genes
| Primers | Sequences | Size of PCR product (bp) | Ref. No. | |
|---|---|---|---|---|
| 16SrRNA | forward | ATAGACGGGGACCCGCACAAG | 120 | 32 |
| reverse | TGGCAAGCCAGACACTCCA | |||
| ureC | forward | AAGCTTTTAGGGGTGTTAGGGGTTT | 294 | 33 |
| reverse | AAGCTTACTTTCTAACACTAACGC |
Assessment of H. pylori Infection and Histopathology Staining of Gastric Tissue Samples
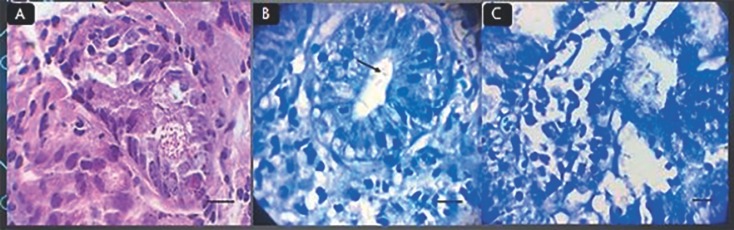
In order to evaluate H. pylori infection in the mice, histopathological examination was conducted by a pathologist. One mouse from each group was randomly selected and sacrificed 12 weeks after inoculation. After obtaining longitudinal cross-sections of the animals' stomachs, samples were fixed in 10% formalin and embedded in paraffin. Tissue samples were sent to the laboratory for pathological analysis. Tissues were stained using hematoxylin and eosin and Giemsa staining, and alterations in epithelial cells of the stomach were investigated in the control and infected groups following induction of infection and inflammation (Fig. 1).
Fig. 1.
A Stomach epithelium in control group stained with hematoxylin and eosin. Original magnification, ×40. B Stomach epithelium after infection in 8th week stained using Giemsa stain. Arrow points to bacterium. Original magnification, ×40. C Stomach epithelium after infection in first week stained with Giemsa stain. Original magnification, ×40.
Statistical Analysis
All data were analyzed with the Kruskal-Wallis test using SPSS version 21. p < 0.05 was considered statistically significant.
Results
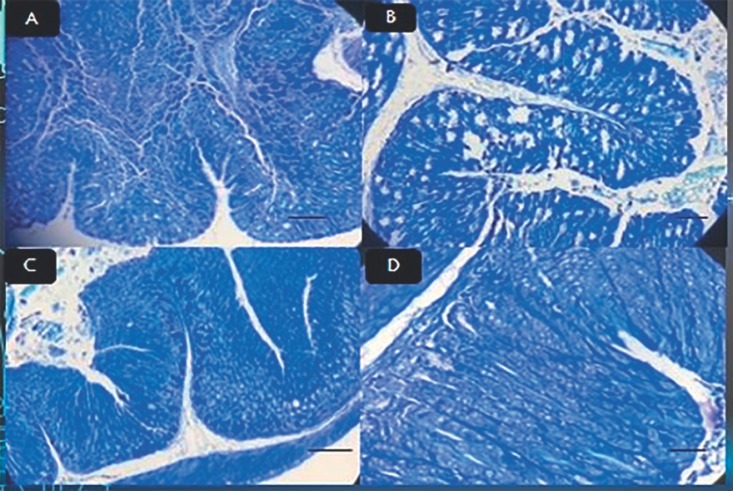
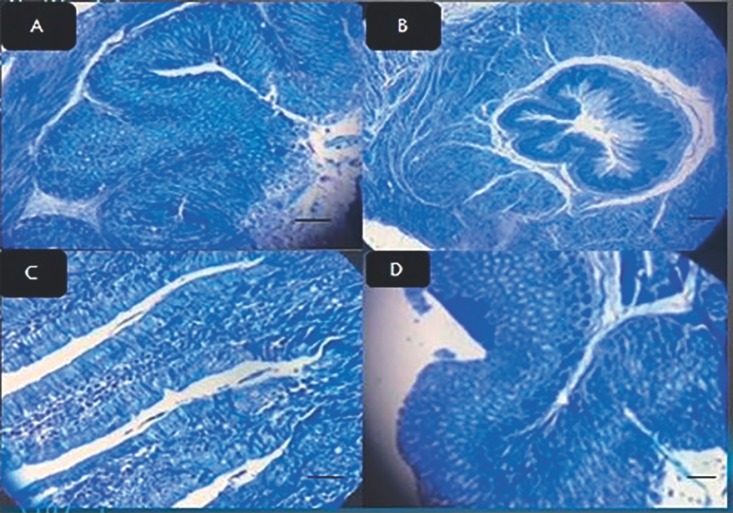
Two weeks after exposure of mouse stomachs to drugs and bacteria, stool samples were obtained; the stool antigen test was performed and the results were compared to those collected before the exposure. The results were confirmed using PCR. The final results indicated that H. pylori infection improved after being exposed to the aforementioned compounds in the treatment groups and control group (Table 3). In the first treatment group, after exposing the cells to L. acidophilus, followed by Giemsa tissue staining (Fig. 2), irregular cell dressing, epithelial mucus maturation, and inflammation under the mucosa was observed under the microscope. According to the results of the tissue study in this group, the therapeutic group had the lowest anti-H. pylori effect on the elimination of H. pylori in gastritis (Table 1). The results of the PCR in this group indicated that the applied treatment method was not able to remove bacteria. In the second group, after being exposed to L. plantarum, the cells had a more regular appearance (Fig. 2). Also, the epithelial cells were decreased compared to the first group and the percentage of recovery was close to the third treatment group (Table 1). In the third treatment group, before the treatment and during the infection, cellular edema, swelling of the cell, cellular loosening, and chromatin accumulation as well as the presence of bacteria in the tissue was observed. The results indicated that the treatment was more effective in this group than in the other treatment groups, and the results of the ELISA represented a complete anti-H. pylori effect on gastritis caused by H. pylori compared with other groups (Table 1). Also, the confirmation of anti-H. pylori effect in the stomach tissue of the mouse was investigated by PCR method. In the fourth treatment group, after L. acidophilus and L. plantarumexposure, edema, swelling, cellular loosening, and chromatin accumulation in epithelial cells were observed and a slight improvement was achieved (Table 1; Fig. 3). A moderate recovery was achieved in the fifth treatment group. In the sixth treatment group, which was treated with L. rhamnosus and L. plantarum, there was a better recovery than in the fourth and fifth treatment groups (Table 1). Treatment with all three lactobacilli, which was performed in the seventh group, was shown to be the most effective treatment, right after the treatment group which was exposed to L. rhamnosus (Table 1; Fig. 2). Exposure to bismuth and omeprazole without any probiotics also had a moderate and lower recovery rate than the Lactobacillus groups (Table 1). In summary, all of the groups which were treated with L. rhamnosus had the highest rate of H. pylori eradication and subsequent peptic ulcer recovery.
Table 3.
ELISA titration results (µg/mL) of mice in the nine treatment groups and the control group
| Mouse No. 1 | Mouse No. 2 | Mouse No. 3 | Mouse No. 4 | Mouse No. 5 | Mouse No. 6 | Mouse No. 7 | |
|---|---|---|---|---|---|---|---|
| After infection (control group) | 0.225 | 0.230 | 0.525 | 0.852 | 1.101 | 1.011 | 1.012 |
| After treatment with L. acidophilus | 1.002 | 0.950 | 1.005 | 0.083 | 0.302 | 0.903 | 1.005 |
| After treatment with L. plantarum | 0.042 | 0.0351 | 0.048 | 0.040 | 0.032 | 0.001 | 0.00 |
| After treatment with L. rhamnosus | 0.006 | 0.002 | 0.001 | 0.005 | 0.002 | 0.00 | 0.00 |
| After treatment with L. acidophilus and L. plantarum | 0.051 | 0.062 | 0.031 | 0.022 | 0.055 | 0.00 | 0.052 |
| After treatment with L. acidophilus and L. rhamnosus | 0.042 | 0.041 | 0.035 | 0.028 | 0.025 | 0.041 | 0.015 |
| After treatment with L. plantarum and L. rhamnosus | 0.028 | 0.015 | 0.018 | 0.005 | 0.019 | 0.00 | 0.00 |
| After treatment with clarithromycin | 0.025 | 0.052 | 0.016 | 0.059 | 0.010 | 0.012 | 0.030 |
| After treatment with all three lactobacilli | 0.008 | 0.005 | 0.00 | 0.014 | 0.008 | 0.006 | 0.00 |
| After treatment with bismuth + omeprazole | 0.800 | 0.078 | 0.856 | 1.020 | 1.015 | 1.013 | 0.090 |
Fig. 2.
A Stomach epithelium of mice after treatment with L. rhamnosus stained with Giemsa stain. Original magnification, ×40. B Stomach epithelium of mice after treatment with L. acidophilus stained with Giemsa stain. Original magnification, ×40. C Stomach epithelium of mice after treatment with L. plantarum stained with Giemsa stain. Original magnification, ×40. D Stomach epithelium of mice after treatment with all three lactobacilli stained with Giemsa stain. Original magnification, ×40.
Fig. 3.
A stomach epithelium of mice after treatment with L. plantarum + L. acidophilus stained with Giemsa stain. Original magnification, ×40. B Stomach epithelium of mice after treatment with L. rhamnosus + L. plantarum stained with Giemsa stain. Original magnification, ×40. C Stomach epithelium of mice after treatment with L. rhamnosus + L. acidophilus stained with Giemsa stain. Original magnification, ×40. D Stomach epithelium of mice after treatment with clarithromycin (control group) stained with Giemsa stain. Original magnification, ×40.
Discussion
The high rate of H. pylori resistance to antibiotics which are commonly used in treatment is a great challenge for treating and overcoming infections caused by this microorganism. Most studies which have been conducted concerning gastritis and H. pylori infections have used a drug combination consisting of proton pump inhibitors or bismuth and antibiotics, including metronidazole, amoxicillin, tetracycline, and clarithromycin [18]. In order to achieve effective results, the medications should be administered for 7–14 days. The results of these studies indicate that most often there is an 85–90% chance of eliminating this bacterium by triplet therapy (if the bacterium is sensitive to antibiotics), but the efficacy of these regimens decreases if the bacterium develops antibiotic resistance [19]. No doubt, as with many other combination therapies, there are numerous side effects with H. pylori treatment. Due to the treatment complications and the increased antibiotic resistance, there is a great tendency to develop new alternative therapies. To limit side effects, using the probiotics in H. pylori management is taken into consideration. Although a large number of studies focused on treatment and eradication of H. pylori in gastric diseases, the effect of probiotic strains including Lactobacillus received far less attention. Considering the global requirement for the treatment of gastrointestinal diseases, particularly gastritis, caused by H. pylori, our goal was to investigate the antimicrobial effects of L. plantarum, L. acidophilus, and L. rhamnosus along with bismuth and omeprazole on gastritis caused by H. pylori infection in C57BL/6 mice. According to our results, Lactobacillus compounds were able to improve the gastritis process and decrease H. pylori infection. The group which received L. rhamnosus had the highest rate of reduction of H. pylori-induced inflammation. In addition, complete recovery of gastritis was observed. After that, the group which received all three Lactobacillus strains showed the highest anti-inflammatory effect, and the group which received L. acidophilus had the weakest outcome in microbial amelioration. Other groups exhibited an average anti-H. pylori effect and did not achieve complete recovery. Nevertheless, in all the tested groups, some improvements were observed in intestinal epithelial cells. Various studies have investigated the effect of probiotics in gastrointestinal infections [20, 21, 22, 23] and most of them confirm the results that we have obtained regarding the effect of probiotics in gastrointestinal diseases, namely, gastritis. Sgouras et al. [24] (2004), investigated the potential antimicrobial effect of L. casei in C57BL/6 mice infected with H. pylori which was collected from patients suffering from gastritis and peptic ulcer. The group which received L. casei had a much higher H. pylori eradication rate. Sgouras et al. [25] (2005) also explored the antimicrobial and anti-inflammatory effect of L. johnsonii on gastritis in C57BL/6 mice. Their result indicated that in the primary stages of infection, L. johnsoniiwas able to mitigate gastritis induced by H. pylori by lowering chemical signals produced by inflammatory proteins which mobilize lymphocytes and neutrophils in lamina propria. Another study conducted by Cui et al. [26] (2010) also demonstrated that Lactobacillus strains could generate a marked reduction in H. pylori colonization in BALB/c mice. According to their results, mice which were infected with H. pylori and exposed to Lactobacillus strains showed a significant reduction in H. pylori compared to the control group that received saline. In addition, Sunanliganon et al. [16] (2012) investigated the antimicrobial effect of L. plantarum in mice that suffered from H. pylori-induced gastritis, and concluded that L. plantarum hinders the growth of H. pylori. Many other experiments have been conducted concerning antimicrobial and anti-inflammatory effects of Lactobacillus in infections caused by H. pylori[27, 28]. In some of these studies, it has been pointed out that Lactobacillus produces anti-inflammatory effects through balancing inflammatory and anti-inflammatory cytokines, increasing expression of interleukin 10, and reducing TNFα [29, 30, 31]. These results validate our findings in the present study.
Conclusion
Based on the findings of the current study, significant anti-H. pylori effects of L. acidophilus, L. plantarum, and L. rhamnosus can be had by introducing them as a supplement against H. pylori infection. Using these Lactobacillus species as pharmaceutical supplements along with proton pump inhibitors may have more beneficial effects on improvement of gastritis induced by H. pylori. In addition, the Lactobacillus species which have been used in our study may be able to reduce resistance to antibiotics that are commonly used in the treatment of H. pylori-induced gastritis, and therefore could have a tremendous role in treating gastritis in humans in the future.
Statement of Ethics
The experimental protocol was approved by the Animal Care Committee of Alborz University of Medical Sciences, Karaj, Iran.
Disclosure Statement
The authors declare that they have no competing interest. No funding was received for this study.
Acknowledgments
The authors would like to thank the staff of the Department of Microbiology at Alborz University of Medical Sciences, Karaj, Iran, for their sincere assistance and efforts to make this project happen.
References
- 1.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. World Gastroenterology Organization. World Gastroenterology Organisation Global Guideline Helicobacter pylori in developing countries. J Gastrointestin Liver Dis. 2011 Sep;20((3)):299–304. [PubMed] [Google Scholar]
- 2.Barati M, Talebi Taher M, Hashemi M, Boghratian A. NaserEslami P. FREQUENCY OF H. PILORI INFECTION AND GASTRIC AND DUODENAL LESIONS IN PATIENTS. Majallah-i Ulum-i Pizishki-i Razi. 2003;10((35)):347–53. [Google Scholar]
- 3.Nekouian R, Rasouli BS. Implementation of cancer prevention control program in private sector in Iran. J Cancer Policy. 2017;13:70–4. [Google Scholar]
- 4.Owens SR, Smith LB. Molecular aspects of H. pylori-related MALT lymphoma. Pathology research international. 2011:2011. doi: 10.4061/2011/193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor A, O'Morain CA, Ford AC. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. 2017 Apr;14((4)):230–40. doi: 10.1038/nrgastro.2016.195. [DOI] [PubMed] [Google Scholar]
- 6.Satarug S, Vesey DA, Gobe GC. Current health risk assessment practice for dietary cadmium: data from different countries. Food Chem Toxicol. 2017 Aug;106(Pt A):430–45. doi: 10.1016/j.fct.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Chiang TH, Liou JM, Chen HH, Wu MS, Graham DY. Mass eradication of Helicobacter pylori to prevent gastric cancer: theoretical and practical considerations. Gut Liver. 2016 Jan;10((1)):12–26. doi: 10.5009/gnl15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakoob J, Abbas Z, Ahmad Z, Tariq K, Awan S, Mustafa K, et al. Gastric lymphoma: association with Helicobacter pylori outer membrane protein Q (HopQ) and cytotoxic-pathogenicity activity island (CPAI) genes. Epidemiol Infect. 2017 Dec;145((16)):3468–76. doi: 10.1017/S0950268817002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher LD, den Hartog G, Ernst PB, Crowe SE. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell Mol Gastroenterol Hepatol. 2017 Feb;3((3)):316–22. doi: 10.1016/j.jcmgh.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias J, Lebens M, Wai SN, Holmgren J, Svennerholm AM. Surface expression of Helicobacter pylori HpaA adhesion antigen on Vibrio cholerae, enhanced by co-expressed enterotoxigenic Escherichia coli fimbrial antigens. Microb Pathog. 2017 Apr;105:177–84. doi: 10.1016/j.micpath.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Huang Y. Effect of Lactobacillus acidophilus and Bifidobacterium bifidum supplementation to standard triple therapy on Helicobacter pylori eradication and dynamic changes in intestinal flora. World J Microbiol Biotechnol. 2014 Mar;30((3)):847–53. doi: 10.1007/s11274-013-1490-2. [DOI] [PubMed] [Google Scholar]
- 12.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016 May;65((5)):870–8. doi: 10.1136/gutjnl-2015-311019. [DOI] [PubMed] [Google Scholar]
- 13.Nazifii A. EFFECT OF OMEPRAZOLE PLUS CIPROFLOXACIN ON HEALING OF DUODENAL ULCER AND ERADICATION OF HELICOBACTER PYLORI. Majallah-i Ulum-i Pizishki-i Razi. 2001;8((25)):334–8. [Google Scholar]
- 14.Patel A, Shah N, Prajapati JB. Clinical application of probiotics in the treatment of Helicobacter pylori infection—a brief review. J Microbiol Immunol Infect. 2014 Oct;47((5)):429–37. doi: 10.1016/j.jmii.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Mousavi F, Rahnema M, Heydarieh N, Tajabadi Ebrahimi M. The Effect Of Iranian Native Lactobacillus Pentosus On Healing Of Gastric Ulcers In Male Wistar Rats. Arak MedUniv J. 2013;16:81–90. [Google Scholar]
- 16.Sunanliganon C, Thong-Ngam D, Tumwasorn S, Klaikeaw N. Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J Gastroenterol. 2012 May;18((20)):2472–80. doi: 10.3748/wjg.v18.i20.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojetti V, Bruno G, Ainora ME, Gigante G, Rizzo G, Roccarina D, et al. Impact of Lactobacillus reuteri supplementation on anti-Helicobacter pylori levofloxacin-based second-line therapy. Gastroenterology research and practice. 2012:2012. doi: 10.1155/2012/740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gené E, Calvet X, Azagra R, Gisbert JP. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Aliment Pharmacol Ther. 2003 May;17((9)):1137–43. doi: 10.1046/j.1365-2036.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- 19.Bell GD, Powell K, Burridge SM, Pallecaros A, Jones PH, Gant PW, et al. Experience with ‘triple’ anti-Helicobacter pylori eradication therapy: side effects and the importance of testing the pre-treatment bacterial isolate for metronidazole resistance. Aliment Pharmacol Ther. 1992 Aug;6((4)):427–35. doi: 10.1111/j.1365-2036.1992.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 20.Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap Adv Gastroenterol. 2014 Jan;7((1)):4–13. doi: 10.1177/1756283X13503514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World Journal of Gastrointestinal Pathophysiology. 2014;5((4)):384–391. doi: 10.4291/wjgp.v5.i4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo C-H, Wang SS, Lu C-Y, Hu H-M, Kuo F-C, Weng B-C, et al. Long-term use of probiotic-containing yogurts is a safe way to prevent Helicobacter pylori: based on a Mongolian gerbil's model. Biochemistry research international. 2013:2013. doi: 10.1155/2013/594561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asgari B, Kermanian F, Derakhshan N, Asna-Ashari M, Sadat ZR, Yaslianifard S. Honey-Derived Lactobacillus Rhamnosus Alleviates Helicobacter Pylori-Induced Gastro-Intestinal Infection And Gastric Inflammation In C57Bl/6 Mice: An Immuno-Histologic Study. Arq Gastroenterol. 2018 Jul-Sep;55((3)):279–82. doi: 10.1590/S0004-2803.201800000-70. [DOI] [PubMed] [Google Scholar]
- 24.Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, et al. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004 Jan;70((1)):518–26. doi: 10.1128/AEM.70.1.518-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol. 2005 Dec;12((12)):1378–86. doi: 10.1128/CDLI.12.12.1378-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui Y, Wang CL, Liu XW, Wang XH, Chen LL, Zhao X, et al. Two stomach-originated Lactobacillus strains improve Helicobacter pylori infected murine gastritis. World J Gastroenterol. 2010 Jan;16((4)):445–52. doi: 10.3748/wjg.v16.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manfredi M, Bizzarri B, Sacchero RI, Maccari S, Calabrese L, Fabbian F, et al. Helicobacter pylori infection in clinical practice: probiotics and a combination of probiotics + lactoferrin improve compliance, but not eradication, in sequential therapy. Helicobacter. 2012 Aug;17((4)):254–63. doi: 10.1111/j.1523-5378.2012.00944.x. [DOI] [PubMed] [Google Scholar]
- 28.Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, et al. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008 Apr;13((2)):127–34. doi: 10.1111/j.1523-5378.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 29.Lionetti E, Miniello VL, Castellaneta SP, Magistá AM, de Canio A, Maurogiovanni G, et al. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006 Nov;24((10)):1461–8. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014 May-Jun;48((5)):407–13. doi: 10.1097/MCG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 31.Wendakoon CN, Thomson AB, Ozimek L. Lack of therapeutic effect of a specially designed yogurt for the eradication of Helicobacter pylori infection. Digestion. 2002;65((1)):16–20. doi: 10.1159/000051926. [DOI] [PubMed] [Google Scholar]
- 32.Dadashzadeh K, Milani M, Rahmati M, Akbarzadeh A. Real-time PCR detection of 16S rRNA novel mutations associated with Helicobacter pylori tetracycline resistance in Iran. Asian Pac J Cancer Prev. 2014;15((20)):8883–6. doi: 10.7314/apjcp.2014.15.20.8883. [DOI] [PubMed] [Google Scholar]
- 33.de Negreiros Bessa PP, Barbosa FC, do Carmo AP, Furtado GB, Barroso FC, Rabenhosrt SH. Presence of the Genes cagA, cagE, virB11 and Allelic Variation of vacA of Helicobacter pylori Are Associated with the Activity of Gastritis. Open Journal of Gastroenterology. 2014;4((11)):347–55. [Google Scholar]