Hispanic breast cancer survivors found a smartphone-based intervention aimed at increasing health-related quality of life to be acceptable and feasible.
Keywords: Hispanic, Breast cancer survivor, eHealth, Health-related quality of life, Feasibility
Abstract
Hispanic breast cancer survivors (BCS) are at high risk for experiencing poor health-related quality of life (HRQoL) after completion of active breast cancer treatment. Therefore, there is a need to develop culturally tailored interventions for Hispanic BCS. To date, there have been limited interventions that have demonstrated that increasing cancer-related knowledge, self-efficacy in communication, and self-management skills can improve HRQoL among Hispanic BCS. These interventions have been delivered in person or by phone, which may be burdensome for Hispanic BCS. To facilitate intervention delivery, we developed My Guide, a Smartphone application aimed at improving HRQoL among Hispanic BCS. The purpose of the current study is to describe the feasibility results of a 4-week pilot trial testing My Guide among Hispanic BCS. Twenty-five women enrolled in the study (75% recruitment rate) and 22 women were retained (91.6% retention rate). Mean time spent using My Guide across the 4 weeks was 9.25 hr, and mean score on the satisfaction survey was 65.91 (range 42–70), in which higher scores reflect greater satisfaction. Participants’ scores on the Breast Cancer Knowledge Questionnaire significantly improved from study baseline (M = 9.50, SD = 2.92) to the postintervention assessment (M = 11.14, SD = 2.66), d = 0.59. Participants’ HRQoL scores improved over the course of 4 weeks, but these improvements were not statistically significant. Overall, My Guide was feasible and acceptable. Future studies will assess the preliminary efficacy of My Guide in improving HRQoL in a larger, randomized trial of Hispanic BCS.
Implications
Practice: Health care providers may consider using technology-delivered interventions to improve and monitor health-related quality of life among Hispanic breast cancer survivors to improve treatment outcomes.
Policy: Policymakers may consider supporting health care legislation that includes funding allocations to reimburse for evidence-based interventions delivered through mHealth technologies.
Research: Further research is needed to test the efficacy of mHealth interventions geared toward improving health-related quality of life among Hispanic breast cancer survivors in a larger sample of women.
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among Hispanic women and accounts for 29% of all Hispanic cancer diagnoses; it is also the leading cause of cancer-related death among Hispanic women [1]. Compared with non-Hispanic White women, Hispanic breast cancer survivors (BCS) are more likely to report poorer health-related quality of life (HRQoL) and greater cancer-related psychosocial needs [2], even after adjusting for socioeconomic status [3, 4]. Thus, interventions specifically tailored to Hispanic BCS that address diminished HRQoL are important to improving adherence to post-treatment care and ensuring favorable long-term health outcomes [5–7]. However, few interventions to date have been developed to specifically address the supportive care needs of Hispanic BCS [2].
The available observational and intervention studies of Hispanic BCS show that increasing patients’ cancer-related knowledge, self-efficacy in patient–provider communication, and self-management skills can improve HRQoL [8–14]. Thus, it is important for interventions to provide Hispanic BCS with education about their symptoms and the tools to manage their cancer-related symptoms and stress. Such interventions have the potential to promote significant improvements in HRQoL, and potentially improve long-term health outcomes. However, evidence among Hispanic BCS is currently limited to in-person or telephone-based intervention implementation, which can be burdensome for patients in terms of both time and cost. Therefore, there is a need for more scalable and culturally informed eHealth interventions [15], which provide particularly innovative opportunities among Hispanics, who seek online health information at similar or higher rates than other racial and ethnic groups in the USA [16].
This article describes the feasibility findings from the My Guide 4-week pilot trial. My Guide is an evidence-based, culturally informed Smartphone-based application developed to improve HRQoL among Hispanic BCS. Consistent with Bowen and colleagues [17], we defined feasibility as participant engagement (i.e., recruitment, retention, application use), acceptability (i.e., satisfaction), knowledge (i.e., intervention target of breast cancer knowledge), and preliminary intended effects of the trial (i.e., HRQoL) [17]. The aims of this pilot study were to (i) evaluate the enrollment, retention, and usage of the Smartphone-based My Guide trial for Hispanic BCS; (ii) assess participant satisfaction with My Guide; and (iii) report findings on the knowledge (i.e., intervention target of breast cancer knowledge) and preliminary intended effects (i.e., HRQoL) of My Guide. We hypothesized that recruitment would be comparable with that of prior psychosocial studies with racially diverse cancer patients (≥70%), and that retention would be acceptable (≥80%). We hypothesized that women would use the application for a minimum of 8 hr during the 4-week program. Finally, we explored changes in breast cancer knowledge and HRQoL after completing the program. Results from this pilot trial will be used to inform the next phase of testing for My Guide, which will be a larger randomized controlled trial.
METHODS
Participants
Participants were 25 Hispanic BCS who were recruited from the Robert H. Lurie Comprehensive Cancer Center at Northwestern University, the University of Illinois at Chicago, and community-based support groups for Hispanic BCS in the Chicagoland area. Eligible participants were identified via electronic medical record screening, physician referral, and through recruitment flyers in support groups. Women were eligible if they self-identified as Hispanic/Latina, had Stage 0–IIIA breast cancer, completed active treatment for breast cancer, were within 3–24 months post-active treatment, demonstrated lower HRQoL on the Functional Assessment of Cancer Therapy–Breast measure when compared with general population scores, had no prior history of cancers, and had no unmanaged severe mental illness.
Procedures
The institutional review board (IRB) approved this study. Participants’ clinical information was collected via self-report and through medical chart extraction. All participants were provided training to use the My Guide application during the study baseline assessment. Participants were encouraged to use the application for approximately 3 hr per week for the duration of the 4-week trial. Participants were instructed to complete the weekly Functional Assessment of Cancer Therapy–General Seven (FACT-G7) questionnaire that was available in the My Guide application. Trained, bilingual research staff conducted weekly 15-min telecoaching calls in Weeks 2–4 of the trial to increase adherence to the recommended time of application use and to resolve any technological issues when accessing the application.
The telecoaching protocol for this study was adapted from a model of supportive accountability to enhance adherence to eHealth interventions [18]. Participants received telecoaching calls in Weeks 2–4 of the trial, for a maximum of three telecoaching calls. Telecoaches were trained in motivational interviewing, goal setting, and sensitivity to issues relevant for Hispanic BCS. All telecoaching sessions were recorded, reviewed, and discussed during weekly supervision with a licensed clinical psychologist trained to ensure adherence to each aspect of the telecoaching protocol (e.g., reinforcing use of the application and facilitating problem solving related to participant-identified barriers to using My Guide).
All participants were given an option to use their own Smartphone or to borrow one for the duration of the study. A $100 incentive was provided, with an additional $40 data usage reimbursement for participants using personal phones.
My Guide application and telecoaching content development
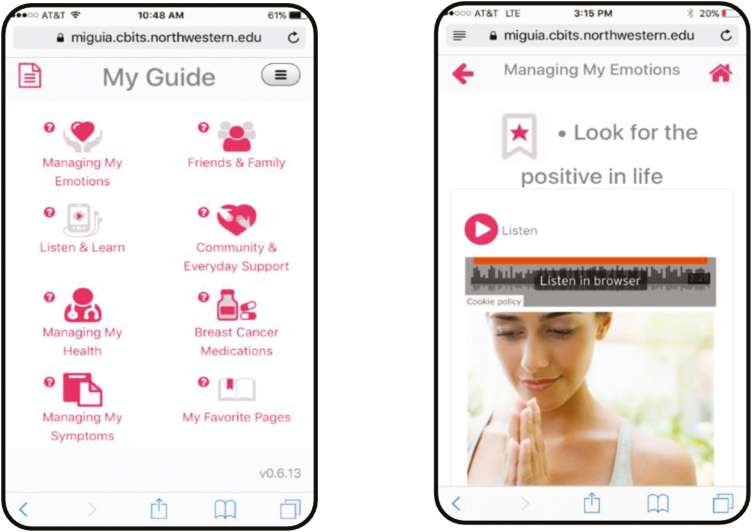
The content in the My Guide application was informed by models of stress and coping, cognitive behavioral stress management [19–21], the literature on psychosocial adaptation during and after breast cancer [11, 13], and studies indicating that cancer knowledge, stress awareness and management, social support, and enhanced patient–provider communication and intimacy can improve HRQoL outcomes in Hispanic BCS [8–14]. The application was developed in collaboration with a community-based partner (the Latina Breast Cancer Association) who provided feedback across all stages of the project including study design, culturally informing the application content, and recruitment and retention strategies. The My Guide application content focused on enhancement of psychosocial adaptation after breast cancer, cancer knowledge, stress awareness and management, social support, and communication with friends, family, and oncology providers (see Table 1). Written content was supplemented by video and audio recordings, which were developed specifically for My Guide by the co-authors to provide expert explanations of side effects after treatment, including hormonal therapies, as well as stress management skills training (see Fig. 1).
Table 1.
My Guide application content and features
| Content or feature | Description |
|---|---|
| Managing my symptoms | Physical and psychological symptoms, late effects and concerns commonly experienced after breast cancer treatment, and relevant management strategies |
| Managing my health | General information on breast cancer diagnosis, treatment, and management strategies following treatment |
| Friends and family | Relationships with family, friends, and other acquaintances that might have been affected by a cancer diagnosis and strategies to address these changes |
| Managing my emotions | Emotions most commonly experienced after cancer treatment and stress management skills training |
| Breast cancer medications | Information on the different types of hormonal therapies for breast cancer treatment, associated side effects, management strategies, and adherence strategies |
| Community and everyday support | Resources for breast cancer survivors and cancer-specific, community-based organizations |
| Videos and audio programs | Videos and audio-based content includes expert testimonials on breast cancer, survivorship topics, and stress management training |
| Bookmarks | Allows participants to bookmark their preferred sections for easy reference |
Fig 1.
Screenshots of My Guide.
A unique aspect of this application is that it aligns with values and beliefs that many Hispanics hold such as familism and fatalism, and it addresses challenges that disproportionally affect Hispanics such as language barriers [12, 22, 23]. To address fatalistic beliefs and stigma about cancer, My Guide emphasizes the high 5-year survival rates for Hispanic women diagnosed with nonmetastatic disease, encourages continued surveillance and follow-up care with their oncology provider, contains information to discredit fatalistic beliefs about cancer (e.g., cancer is a punishment or death sentence), and provides hopeful survivorship stories. Given the emphasis of Hispanic culture on family values and support, My Guide contains strategies for balancing family and caregiver needs with cancer-related concerns and acknowledges the important role of family support while recognizing that Hispanics families in the USA may be dispersed due to immigration. My Guide also contains information on local community agencies for Hispanics in the Chicagoland area for additional support (e.g., support groups in Spanish, childcare, transportation assistance, and financial assistance). The application contains strategies for overcoming barriers to communicating with providers such as requesting translator services or bringing a bilingual family member to medical appointments to translate and take notes during the appointment. My Guide includes information on health promotion after cancer including suggestions for increasing physical activity (e.g., salsa classes) and recipe substitutions relevant to a Hispanic/Latino diet. To address low literacy concerns, the My Guide application is accessible through an audio format that is embedded within the application. All images in the My Guide application were purposefully selected to reflect the diversity of Hispanic women. My Guide content was translated into Spanish by IRB-certified translators who were bilingual native Spanish-speakers. In addition to psychosocial content, the My Guide application contained a validated HRQoL assessment for participants to complete on a weekly basis. Participants were able to access all of the My Guide content at once. The application was developed in conjunction with the Center for Behavioral Intervention Technologies (CBITs) at the Northwestern University Feinberg School of Medicine.
Telecoaching content was translated into Spanish by bilingual native Spanish-speakers. The telecoaching calls were brief (15 min) and focused on encouraging adherence to the My Guide application and overcoming barriers to using the application. It is important to note that the goal of telecoaching was not to deliver intervention content, but rather to facilitate adherence to the My Guide application. Telecoaching calls were tailored based on the past-week’s application usage and unique barriers to adherence reported by individual participants. For example, if a participant reached her weekly goal, then telecoaches reinforced this behavior. Alternatively, if a participant was short of her goal, then telecoaches used problem solving strategies to increase adherence in the following week.
Measures
Engagement
We assessed engagement with the My Guide application through study recruitment, retention, and participant use of My Guide (e.g., logins, time spent on the application, number of completed telecoaching calls). Based on previous eHealth psychosocial and behavioral studies in oncology, a minimum of a 70% recruitment rate [24, 25], 80% retention rate, 80% telecoaching call rate, and an average of 8 hr of application use were considered adequate levels of engagement [26].
Acceptability
To assess acceptability, all participants were asked to complete a satisfaction survey including usefulness, satisfaction, learnability, and usability of the application. This author-constructed satisfaction questionnaire was synthesized from validated measures assessing eHealth intervention feasibility, and it was adapted for our study to better fit our target population and application design [27–30]. Above average scores on the questionnaire were considered acceptable. In addition, we solicited open-ended feedback from participants on how to improve the My Guide application content and tabulated the most common responses to guide future iterations of the application. The satisfaction questionnaire was administered in person during the post-trial assessment.
Knowledge
All participants completed the Knowledge about Breast Cancer Questionnaire [31]. This questionnaire consists of 16 true or false questions regarding general breast cancer knowledge. In addition to the general questions about diagnosis and treatment in the original questionnaire, we added questions related to endocrine therapy. Higher scores indicated better knowledge. The questionnaire was administered at study baseline and during the in-person post-trial assessment. At neither time point were participants informed of the correct responses to the questions.
Preliminary intended effects
The FACT-G7 was used to assess the preliminary intended effects of the trial on improving HRQoL among participants [32]. Higher scores represent better HRQoL. Participants accessed the FACT-G7 within the My Guide application and were instructed to complete the assessment once per week. Internal consistencies for the FACT-G7 scales in this study were acceptable and ranged from 0.69 to 0.88.
Data analysis
All analyses were performed using IBM SPSS Statistics software, version 24. Descriptive statistics were used to characterize the sample and to elucidate patterns of participant engagement with the application. Paired t-tests were used to detect statistically significant differences in mean scores across time. Effect sizes were computed by calculating the mean difference between two scores, and then dividing the result by the pooled SD.
RESULTS
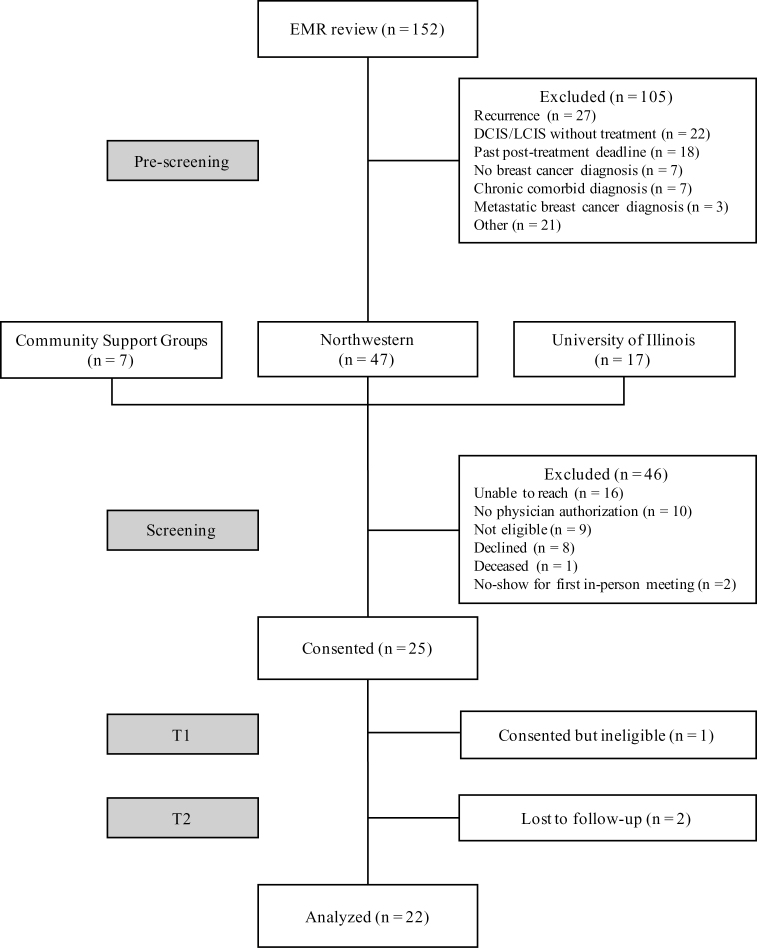
Table 2 shows participant demographics, and Fig. 2 is a consort diagram. We enrolled 25 participants; however, one participant was later found to be ineligible, and was excluded from analyses.
Table 2.
Participant characteristics
| Demographic characteristics by language preference (N = 22) | ||
|---|---|---|
| Spanish (n = 15) | English (n = 7) | |
| Mean age (years) | 54.59 | 48.69 |
| Nativity | ||
| Foreign-born | 14 (93.3%) | 1 (14.3%) |
| U.S.-born | 1 (6.7%) | 6 (85.7%) |
| Marital status | ||
| Married/relationship/partnered | 7 (46.7%) | 4 (57.1%) |
| Single | 2 (13.3%) | 1 (14.3%) |
| Separated | 2 (13.3%) | 1 (14.3%) |
| Divorced | 2 (13.3%) | 1 (14.3%) |
| Widowed | 2 (13.3%) | 0 (0%) |
| Employment status | ||
| Employed | 8 (53.3%) | 3 (42.9%) |
| Homemaker | 3 (20%) | 1 (14.3%) |
| Not employed, not looking for work | 1 (6.7%) | 2 (28.6%) |
| Not employed, but looking for work | 2 (13.3%) | 0 (0%) |
| Retired | 1 (6.7%) | 1 (14.3%) |
| Household income | ||
| <$11,000 | 8 (53.3%) | 0 (0%) |
| $11,000–$25,000 | 3 (20%) | 1 (14.2%) |
| $25,000–$50,000 | 2 (13.3%) | 4 (57.1%) |
| $50,000–$75,000 | 1 (6.7%) | 1 (14.3%) |
| >$75,000 | 1 (6.7%) | 1 (14.3%) |
| Cancer stage | ||
| 0 | 1 (6.7%) | 1 (14.3%) |
| I | 0 (0%) | 2 (28.6%) |
| II | 9 (60%) | 4 (57.1%) |
| III | 2 (13.3%) | 0 (0%) |
| Treatment modality | ||
| Hormone therapy | 11 (73.3%) | 5 (71.4%) |
| Radiation therapy | 10 (66.7%) | 5 (71.4%) |
| Chemotherapy | 9 (60%) | 5 (71.4%) |
Fig. 2.
Participant flow through My Guide feasibility trial. EMR = electronic medical record, DCIS = ductal carcinoma in situ, LCIS = lobular carcinoma in situ.
Engagement
The recruitment rate was 75% (24 of 32 eligible patients were enrolled) and the retention rate was 91.7% (22 of 24 eligible participants were retained and included in analyses). Mean number of hours spent using the application across the 4 weeks was 9.25 hr (SD = 7.83), and the telecoaching rate was 93% (mean 2.8 calls out of 3 possible, range 1–3 calls). Fifteen participants completed the program in Spanish and seven participants completed the program in English.
Acceptability
The mean satisfaction score was 65.91 (range 42–70, SD = 5.89), in which higher scores reflect greater satisfaction with the My Guide application. Participants most commonly expressed a desire for (i) detailed information on survivorship recommendations for diet and physical activity (45% of participants), (ii) additional videos to break up the text (32% of participants), (iii) additional relaxation exercises (32% of participants), and (iv) more content on cancer recurrence (27% of participants).
Knowledge
Scores on the Breast Cancer Knowledge Questionnaire significantly improved from study baseline (M = 9.50, SD = 2.92) to the post-trial assessment (M = 11.14. SD = 2.66); t(21) = −3.12, p = .002, d = 0.59 (medium effect size).
Preliminary intended effects
Participants’ scores on the FACT-G7 improved throughout from Week 1 (M = 18.58, SD = 4.54), to Week 2 (M = 18.33, SD = 4.21), Week 3 (M = 19.36, SD = 3.67), and finally to Week 4 (M = 19.40, SD = 4.55). However, there were no statistically significant changes in the FACT-G7 scores across time.
DISCUSSION
These findings support the preliminary feasibility of a novel 4-week Smartphone-based trial to improve HRQoL among Hispanic BCS. To the best of our knowledge, this is the first trial to demonstrate the preliminary feasibility of a Smartphone-based, supportive oncology trial for Hispanic BCS. Overall, My Guide was feasible and acceptable. Participants were engaged in their usage and were satisfied with the application. In addition, their breast cancer knowledge and HRQoL increased over the course of 4 weeks.
Our recruitment rate was promising, especially in the context of logistical challenges that are often associated with the recruitment of underserved minorities into research trials (e.g., lack of time, lack of childcare, transportation provision, limited flexibility at work) [33]. Our successful recruitment and retention may be attributed to the limited obstacles our participants faced to enroll and participate in the study. For example, the My Guide application was easily downloadable onto a phone and was self-guided, and therefore it did not require participants to travel to hospital clinics for study participation. In addition, participants were paid for their time, which could have facilitated enrollment and retention. Finally, participants’ weekly use of the application was very good, and weekly telecoaching calls may have facilitated adherence to the application.
Findings from the exit surveys revealed that participants viewed the My Guide application very favorably. However, because exit surveys were administered in person, it is possible that satisfaction responses were influenced by the desire to yield socially desirable responses. The lowest ratings on the satisfaction survey related to the usability of My Guide, which suggests that additional participant training and improvements to make the My Guide application more user-friendly should be considered in a future version of the application. The highest ratings on the satisfaction survey related to the application appearance, the understandability of the information included, overall satisfaction with the program, and overall usefulness of My Guide. Participants also provided useful feedback related to My Guide application content that will enhance future versions of the application (e.g., more information related to diet and exercise, additional video content).
Our findings revealed a statistically significant improvement in breast cancer knowledge. Although the mean scores in the FACT-G7 trial improved across time, these changes were not statistically significant. However, it is important to keep in mind that this pilot trial was not powered to detect statistically significant changes.
This study focused on a small sample of Hispanic women and is therefore not generalizable to the entire population of BCS. However, notable strengths of the application were its availability in English and Spanish, its audio accessible features, and its integration of cultural values for Hispanic women. Our findings suggest that a Smartphone-based supportive oncology application was feasible in this patient population, and it could have important implications for practice and policy (pending further validation of results in a larger trial). Health care providers may consider using technology-delivered interventions to monitor and improve HRQoL among Hispanic BCS. Furthermore, policymakers may consider supporting health care legislation that includes funding allocations to reimburse for evidence-based interventions delivered through communication technologies. Findings from this pilot feasibility trial will be used to inform a subsequent version of the My Guide application. For example, given the high study retention rate and good weekly use of the My Guide application in the field trial, the next version of the application will be paired with a more easily scalable, stepped-care telecoaching protocol in which only participants with low use of the My Guide application will have continued telecoaching calls. The next version of the My Guide application will be tested in a randomized clinical trial to establish the efficacy of the application in improving HRQoL with a larger sample of Hispanic BCS.
Acknowledgments
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Cancer Institute, Grant Numbers U54CA202995, U54CA202997, and U54CA203000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Compliance with Ethical Standards
Primary Data: Findings reported in this manuscript have not been previously published, and this manuscript is not being simultaneously submitted elsewhere. The authors have not previously reported any data reported in this manuscript. Authors have full control of all primary data and they agree to allow the journal to review their data if requested.
Conflict of Interest: Authors have confirmed that they have no conflicts of interest to disclose.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. American C. Cancer Facts & Figures for Hispanics/Latinos 2015–2017 Atlanta, GA: American Cancer Society; 2015. Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-034778.pdf. Accessibility verified October 2, 2015. [Google Scholar]
- 2. Moadel AB, Morgan C, Dutcher J. Psychosocial needs assessment among an underserved, ethnically diverse cancer patient population. Cancer. 2007;109(suppl 2):446–454. [DOI] [PubMed] [Google Scholar]
- 3. Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. J Cancer Surviv. 2011;5(2):191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luckett T, Goldstein D, Butow PN, et al. . Psychological morbidity and quality of life of ethnic minority patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12(13):1240–1248. [DOI] [PubMed] [Google Scholar]
- 5. Andersen BL, Yang HC, Farrar WB, et al. . Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113(12):3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersen BL, Thornton LM, Shapiro CL, et al. . Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010;16(12):3270–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGregor BA, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: a review of stress pathways and biological mediators. Brain Behav Immun. 2009;23(2):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413–428. [DOI] [PubMed] [Google Scholar]
- 9. Ashing-Giwa KT. The contextual model of HRQoL: a paradigm for expanding the HRQoL framework. Qual Life Res. 2005;14(2):297–307. [DOI] [PubMed] [Google Scholar]
- 10. Ashing-Giwa KT, Padilla GV, Bohórquez DE, Tejero JS, Garcia M. Understanding the breast cancer experience of Latina women. J Psychosoc Oncol. 2006;24(3):19–52. [DOI] [PubMed] [Google Scholar]
- 11. Graves KD, Jensen RE, Cañar J, et al. . Through the lens of culture: quality of life among Latina breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nápoles-Springer AM, Ortíz C, O’Brien H, Díaz-Méndez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yanez B, Maggard Gibbons M, Moreno PI, Jorge A, Stanton AL. Predictors of psychological outcomes in a longitudinal study of Latina breast cancer survivors. Psychol Health. 2016;31(11):1359–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prochaska JJ, Coughlin SS, Lyons EJ. Social Media and Mobile Technology for Cancer Prevention and Treatment. Am Soc Clin Oncol Educ Book. 2017;37:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez MH, Gonzalez-Barrera A, Patten E. Closing the digital divide: Latinos and technology adoption 2013. Available at http://www.pewhispanic.org/2013/03/07/closing-the-digital-divide-latinos-and-technology-adoption/. Accessibility verified October 1, 2014.
- 17. Bowen DJ, Kreuter M, Spring B, et al. . How we design feasibility studies. Am J Prev Med. 2009;36(5):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazarus RS, Folkman S. Transactional theory and research on emotions and coping. Eur J Pers. 1987;1(3):141–169. [Google Scholar]
- 20. Yanez B, McGinty HL, Buitrago D, Ramirez AG, Penedo FJ. Cancer outcomes in Hispanics/Latinos in the United States: an integrative review and conceptual model of determinants of health. J Lat Psychol. 2016;4(2):114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antoni MH, Lechner S, Diaz A, et al. . Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23(5):580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes?J Pers. 2009;77(6):1707–1746. [DOI] [PubMed] [Google Scholar]
- 23. Interian A, Díaz-Martínez AM. Considerations for culturally competent cognitive-behavioral therapy for depression with Hispanic patients. Cogn Behav Pract. 2007;14(1):84–97. [Google Scholar]
- 24. Kissane DW, Grabsch B, Clarke DM, et al. . Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277–286. [DOI] [PubMed] [Google Scholar]
- 25. Breitbart W, Rosenfeld B, Pessin H, Applebaum A, Kulikowski J, Lichtenthal WG. Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol. 2015;33(7):749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yanez B, McGinty HL, Mohr DC, et al. . Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer. 2015;121(24):4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venkatesh V, Davis FD. A theoretical extension of the technology acceptance model: four longitudinal field studies. Manag Sci. 2000;46(2):186–204. [Google Scholar]
- 28. Lewis JR. Psychometric evaluation of the post-study system usability questionnaire: the PSSUQ. Proc Hum Factors Ergon Soc Annu Meet. 1992;36(16):1259–1260. [Google Scholar]
- 29. Brooke J. SUS-A quick and dirty usability scale. Usability Evaluation in Industry. 1996;189(194):4–7. [Google Scholar]
- 30. Lund AM. Measuring usability with the USE questionnaire12. Usability Interface. 2001;8(2):3–6. [Google Scholar]
- 31. Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer. 2008;112(5):1153–1161. [DOI] [PubMed] [Google Scholar]
- 32. Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. [DOI] [PubMed] [Google Scholar]
- 33. Waheed W, Hughes-Morley A, Woodham A, Allen G, Bower P. Overcoming barriers to recruiting ethnic minorities to mental health research: a typology of recruitment strategies. BMC Psychiatry. 2015;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]