Abstract
Purpose:
To characterize chronic disease, health-related quality of life (HRQOL), emotional distress and social attainment among long-term survivors of neuroblastoma.
Methods:
Chronic health conditions among 137 ≥10-year neuroblastoma survivors (median age: 31.9 [range: 20.2–54.6] years) and 272 community controls (median age: 34.7 [range: 18.3–70.2] years) were graded using a modified version of the Common Terminology Criteria of Adverse Events version 4.03. HRQOL and emotional distress were assessed using the Medical Outcomes Study 36-Item Short Form and Brief Symptom Inventory-18. Log-binomial and logistic regression were used to compare the prevalence of chronic conditions and frequency of reduced HRQOL, distress, and social attainment between survivors and controls. The cumulative burden approach was used to estimate multimorbidity.
Results:
By age 35 years survivors experienced, on average, 8.5 (95%CI=7.6–9.3) grade 1–4 conditions, which was higher than controls (3.3 [95%CI=2.9–3.7]). Compared to controls, survivors had a higher prevalence of any pulmonary (p=0.003), auditory (p<0.001), gastrointestinal (p<0.001), neurological (p=0.003), and renal condition (p<0.001), were more likely to report poor physical HRQOL (p=0.01), symptoms of anxiety (p=0.01) and somatization (p=0.01), and were less likely to live independently (p=0.01) or marry (p=0.01). In analyses limited to survivors, those with ≥1 grade 3–4 conditions were more likely to report reduced general health (OR=6.6, 95%CI=1.6–26.9), greater bodily pain (OR=4.2, 95%CI=1.0–17.0), and unemployment (OR=3.2, 95%CI=1.2–8.5).
Conclusion:
Given the high burden of chronic diseases and the associations of these morbidities with reduced HRQOL and social attainment, screening and interventions that provide opportunities to optimize health are important among neuroblastoma survivors.
Keywords: neuroblastoma, cancer survivor, quality of life
Precis:
Long-term survivors of neuroblastoma are at increased risk of pulmonary, auditory, gastrointestinal, neurological and renal conditions and are more likely to report poor physical health-related quality of life, and symptoms of anxiety and somatization when compared to community controls. Chronic conditions such as hearing loss, spinal disorders and short stature are associated with poorer health-related quality of life among survivors.
INTRODUCTION
Neuroblastoma is the most common extra-cranial solid tumor among children accounting for approximately 6% of all cancers in children.1 Previous reports of neuroblastoma survivors have documented multimorbidity impacting a spectrum of organ systems.2–8 In the largest study to date consisting of 954 neuroblastoma survivors in the Childhood Cancer Survivor Study (CCSS) found increased risks of musculoskeletal, neurological, endocrine and sensory conditions compared to siblings.9 However, a limitation of these data was that health outcomes were based on self-report, and thus subject to both ascertainment and recall bias. Although other studies have evaluated late health outcomes in neuroblastoma survivors, these studies have been limited by one or more factors including small sample size,8,10 retrospective study design with inconsistent ascertainment of outcomes,2,5,6 focus on subgroups of neuroblastoma (i.e., diagnosis during infancy or with high risk disease),3,6,7,11 lack of extended follow-up into adulthood,3,6,7 and lack of a suitable non-cancer group for comparison. Nevertheless, the high frequency of chronic illness is of concern because conditions such as pulmonary disease, peripheral neuropathy, and endocrinopathies have been associated with adverse psychological outcomes in child and adolescent survivors of neuroblastoma12 while hearing loss has been associated with psychosocial and academic problems in school-aged survivors.13 The extent to which chronic health conditions are associated with quality of life, emotional distress, and social attainment in adult survivors of neuroblastoma has not previously been reported.
The purpose of this study was to characterize the occurrence of chronic health conditions among a cohort of long-term (10+ year), adult survivors of neuroblastoma who underwent comprehensive, standardized screening of multiple organ systems. Additionally, among our neuroblastoma cohort we examined associations between selected chronic health conditions and health-related quality of life (HRQOL), emotional distress, and adult social attainment.
METHODS
Survivors of neuroblastoma included in this study are participants in the St. Jude Lifetime Cohort (SJLIFE) study.14 SJLIFE is a retrospective cohort study with prospective follow-up and ongoing data accrual.15,16 To be included in this analysis, survivors had to: (1) be treated at St. Jude Children’s Research Hospital (SJCRH) for neuroblastoma during childhood; (2) be 18 years of age or older and 10 or more years from diagnosis; and, (3) have completed a comprehensive clinical assessment at SJCRH as of June 30, 2015. A group of 272 controls with no history of childhood cancer who completed a comparable evaluation were also included in analyses. These individuals were recruited from the community or non-first-degree relatives or friends of SJCRH patients and were frequency-matched on age, sex and race/ethnicity.
Comprehensive clinical evaluations at SJCRH included a history and physical exam by a health practitioner, laboratory testing, and standardized evaluation of physical function. In addition, survivors underwent risk-based evaluations (echocardiography, semen analysis, and cataract testing) as per guidelines endorsed by the Children’s Oncology Group.17 Controls underwent the same clinical assessments as survivors, with the exception of semen analysis, cataract testing, hearing loss testing and dual X-ray absorptiometry (DXA). Hearing loss and vision difficulties were self-reported by controls. Chronic conditions were graded using a modified version of the Common Terminology Criteria of Adverse Events (CTCAE), version 4.03.18 One hundred and sixty conditions were graded as (mild [grade 1], moderate [grade 2], severe/disabling [grade 3], or life-threatening [grade 4]). Controls underwent the same testing as survivors except for DEXA, semen analysis and cataract testing.
HRQOL was assessed using the Medical Outcomes Study 36-Item Short Form (SF-36).19 The SF-36 includes eight subscales measuring general health, physical function, role limitations caused by physical factors, bodily pain, social function, mental health, role limitations cause by emotional factors, and vitality. Emotional distress (anxiety, depression, and somatization) was assessed using the Brief Symptom Inventory-18.20 Both the SF-36 and BSI-18 have a mean of 50 and standard deviation of 10. SF-36 scores ≤40 and BSI-18 scores ≥63 were considered to represent poor HRQOL and clinically significant emotional distress, respectively. Social attainment was assessed using indicators of education (≤high school graduate vs. training after high school, some college, and ≥college graduate), employment (not currently working [unemployed, disabled, retired] vs. employed [working part-time or full-time], marital status (not currently married [single, never married] vs. currently married, living with a partner as married or history of marriage [divorced, widowed]), and independent living (yes [living with spouse/partner, roommates or alone] vs. no [living with parents, siblings, or other relatives]).
Log-binomial regression was used to compare the prevalence of chronic conditions (grade 1–4) between survivors and controls using prevalence ratios (PR) and 95% confidence intervals (CI). Models were adjusted for attained age and sex. As previously described. the mean cumulative count of chronic conditions by age was used to estimate cumulative burden, that is, the average number of recurrent/multiple health events.21 The overall cumulative burden of disease was calculated for both survivors and controls; models were also stratified by sex and stage among survivors. Log-binomial regression was used to compare the frequency of reduced HRQOL, emotional distress, and social attainment between survivors and controls, and to assess associations between frequently occurring chronic conditions (hearing loss, short stature, and spinal disorders) with these outcomes. All models were adjusted for attained age, age at diagnosis, sex, and race/ethnicity.
RESULTS
We identified 235 eligible survivors of neuroblastoma a median of 31.9 (range: 18.6–55.2) years after diagnosis (Table 1). Among these 136 (57%) underwent comprehensive clinical assessments (survivor participants; Supplementary Figure 1). Compared to non-participants, survivor participants were younger at diagnosis (mean [SD] 1.6 [2.1] vs. 2.3 [2.8] years, p=0.042); however, participants and non-participants did not differ by attained age, race/ethnicity, or treatment exposures (P>0.5). Nineteen patients underwent autologous stem cell transplant (ASCT). Eligible survivors did not differ from community controls based on sex or race/ethnicity but were younger at study evaluation (mean [SD], 32.4 [8.2] vs. 34.7 [9.9] years, p=0.006).
Table 1:
Characteristics of Neuroblastoma Survivors and SJLIFE Community Controls
| Survivor Participants1 N (%) |
Survivor Non-Participants2 N (%) |
p-value | Eligible Survivors N (%) |
Community Controls N (%) |
p-value | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 69 (51) | 56 (57) | 0.38 | 125 (53) | 129 (48) | 0.26 |
| Female | 67 (51) | 43 (43) | 110 (47) | 139 (52) | ||
| Age at diagnosis | ||||||
| Median (Range) | 0.9 (0.0–14.4) | 1.0 (0–12.4) | 0.042 | 0.9 (0–14.4) | ||
| Attained age3 | ||||||
| Median (Range) | 31.9 (20.2–54.6) | 32.0 (18.6–55.2) | 0.19 | 31.9 (18.6–55.2) | 34.4 (18.3–59.8) | 0.006 |
| Race/Ethnicity | ||||||
| White | 115 (85) | 81 (82) | 0.58 | 186 (83) | 234 (87) | 0.21 |
| Other | 21 (15) | 18 (18) | 39 (17) | 34 (13) | ||
| Treatment Era | ||||||
| Before 1980 | 43 (32) | 26 (26) | 0.014 | 69 (29) | ||
| 1980–1994 | 83 (61) | 53 (54) | 136 (58) | |||
| After 1995 | 10 (7) | 20 (20) | 30 (13) | |||
| Stage (INSS) | ||||||
| 1 | 32 (24) | 26 (26) | 0.18 | 58 (25) | ||
| 2A | 15 (11) | 18 (18) | 33 (14) | |||
| 2B | 26 (19) | 16 (16) | 42 (18) | |||
| 3 | 18 (13) | 7 (7) | 25 (11) | |||
| 4 | 30 (22) | 27 (27) | 57 (24) | |||
| 4S | 15 (11) | 5 (5) | 20 (9) | |||
| Site | ||||||
| Cervical | 9 (7) | 7 (7) | 16 (7) | |||
| Thorax | 44 (32) | 30 (30) | 74 (32) | |||
| Abdomen | 73 (54) | 58 (59) | 131 (56) | |||
| Pelvis | 9 (7) | 4 (4) | 13 (6) | |||
| Unknown | 1 (1) | 0 (0) | 1 (1) | |||
| Chemotherapy | ||||||
| Any | ||||||
| Cyclophosphamide | 98 (72) | 66 (67) | 0.37 | 164 (70) | ||
| Ifosfamide | 8 (6) | 7 (7) | 0.71 | 15 (6) | ||
| Doxorubicin | 90 (66) | 55 (56) | 0.10 | 145 (62) | ||
| Carboplatin | 15 (11) | 14 (14) | 0.48 | 29 (12) | ||
| Cisplatin | 41 (30) | 32 (32) | 0.72 | 73 (31) | ||
| Etoposide | 23 (17) | 22 (22) | 0.31 | 45 (19) | ||
| Teniposide | 25 (18) | 16 (16) | 0.66 | 41 (17) | ||
| Vincristine | 14 (10) | 13 (13) | 0.50 | 27 (11) | ||
| Radiotherapy | ||||||
| Any | ||||||
| Neck/thyroid | 18 (13) | 10 (10) | 0.46 | 28 (12) | ||
| Chest | 26 (19) | 19 (19) | 0.99 | 45 (19) | ||
| Abdomen | 16 (12) | 15 (15) | 0.45 | 31 (13) | ||
| Pelvis | 12 (9) | 13 (13) | 0.29 | 25 (11) |
Neuroblastoma survivors who underwent clinical evaluation and were included in analyses
Non-participants included those survivors eligible to participate but were lost to follow-up, had refused consent, or had consented but were awaiting campus evaluation
Age at follow-up for survivor non-participants was June 30th, 2015.
SJLIFE – St. Jude Lifetime Cohort Study; INSS -, International Neuroblastoma Staging System
Chronic Health Conditions
At 35 years of age, the cumulative incidence of any chronic health condition among survivors was 95% (95% CI=90–98%) and the cumulative incidence of at least one grade 3–4 condition was 67% (95% CI=58–76). Compared to controls, survivors had a statistically significant higher prevalence of any pulmonary (PR=1.7, 95% CI=1.3–2.3), auditory (PR=10.2, 95% CI=4.9–21.0), gastrointestinal (PR=1.9, 95% CI=1.4–2.5), neurological (PR=1.9, 95% CI=1.5–2.3), and renal conditions (PR=2.7, 95% CI=1.6–4.6), as well as an increased prevalence of subsequent neoplasm (PR=6.4, 95% CI=2.1–20.2). The severity of chronic conditions by CTCAE grade are provided in Supplementary Table 1.
As shown in Figure 1, the most prevalent conditions among survivors included obesity (58.1%), hearing loss (36.6%), low bone mineral density (36%), peripheral sensory neuropathy (34.1%), hypercholesterolemia (26.5%), and hypertriglyceridemia (25.0%). However, when compared to controls, the prevalence of obesity (p=0.78), hypercholesterolemia (p=0.89), and hypertriglyceridemia (p=0.13) were not significantly different. Hearing loss occurred predominantly among those survivors who had received either cisplatin or carboplatin (86.7% vs. 4.4%, p<0.001). The mean dose of cisplatin among survivors with and without sensory peripheral neuropathy was 200.3 (±321.6) mg/m2 and 154.4 (±282.1) mg/m2, p=0.39, respectively, while the mean dose of carboplatin in survivors with and without sensory peripheral neuropathy was 236.6 (±959.4) mg/m2 and 239.6 (±879.4) mg/m2, p=0.98. Hypertension, hypercholesterolemia, and hypertriglyceridemia were not increased among survivors who had received platinum-based chemotherapy (data not shown).
Figure 1:
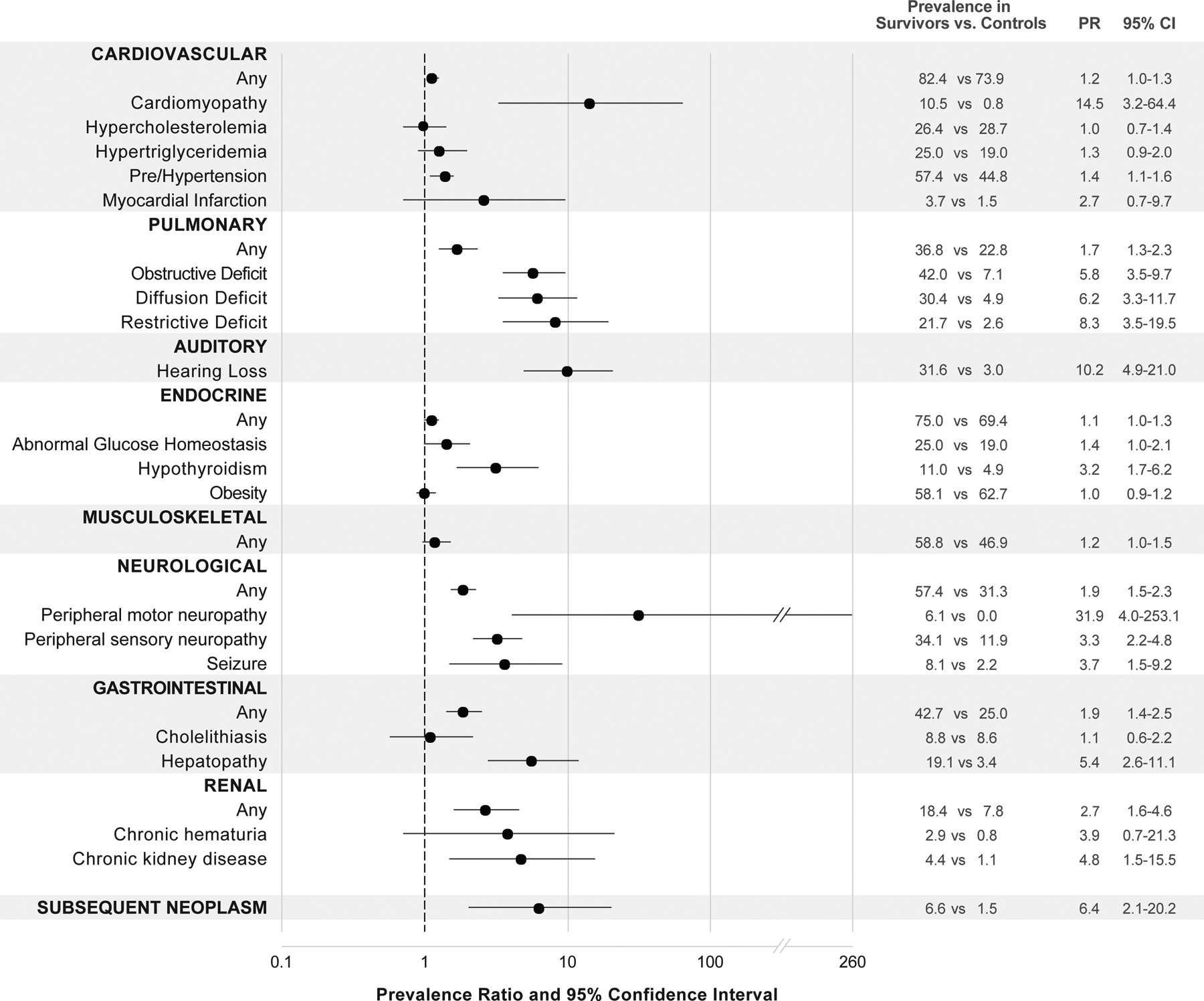
Prevalence ratios for selected chronic conditions in neuroblastoma survivors and community controls.
Hematological (7.4%), immunological (0.7%), and ocular conditions (1.5%) were uncommon among neuroblastoma survivors. Growth hormone deficiency and hypogonadism also occurred at low frequency, with four survivors developing growth hormone deficiency, three females and two males developing primary hypogonadism, and three males developing central hypogonadism. Hypothyroidism was increased among patients who received chest radiation (23.1% vs. 8.3%, p=0.029). Mean height Z-score was −0.29 [±1.21] and −0.30 [±1.44] SD for male and female, survivors respectively. Twenty-two percent of survivors had an adult height Z-score more than 1.3 SD below expected values. Although short stature was more frequent among survivors who had received radiation involving the abdomen and/or chest compared to those who had not received these therapies (34.6% vs. 19.1%), the difference did not meet statistical significance (p=0.09). Mean whole body and lumbar spine Z-scores were 0.12 [±1.35] and −0.70 [±1.05], respectively. Among patients with scoliosis, 14.7% had received radiotherapy, 29.4% had undergone laminectomy and/or thoracotomy, and 20.6% had received a combination of radiotherapy and laminectomy/thoracotomy.
Fifteen subsequent neoplasms occurred among nine survivors including eight basal cell carcinomas (six occurred within the radiation field, two outside the radiation field), three carcinomas (one in field, two patients did not receive radiation), two Hodgkin lymphomas (one in field, one patient did not receive radiation), one Ewing sarcoma (in field), and one malignant peripheral nerve sheath tumor (outside the radiation field). The percentage of second primary neoplasms was elevated in survivors compared to primary neoplasms among controls (6.6% vs. 1.5%, PR=6.4, 95% CI=2.1–20.2). None of the nine survivors identified as carrying pathogenic or likely pathogenic genetic variants in FANCC (two survivors), FANCA, FH, POLH, SDHA, SDHB, SMARCA4, TP53, and CDKN1B developed a subsequent neoplasm.
Cumulative burden of chronic conditions in survivors and controls.
By age 40 years survivors experienced, on average, 11.5 (95%CI=10.2–12.8) grade 1–4 and 2.9 (95%CI=2.4–3.5) grade 3–4 conditions, which was higher than the burden of grade 1–4 (4.9 [95%CI=4.4–5.5]) and grade 3–4 (1.3 [95%CI=1.1–1.5]) conditions among controls (Figure 2a). As seen in Figure 2b and 2c, burden of grade 3–4 disease was higher in females than males (3.2 vs. 2.5, p=0.82) and higher among those diagnosed with stage 3 or 4 neuroblastoma (3.6 vs. 2.5, p=0.77) across the age span, although these differences did not reach statistical significance. The organ system contributing the highest cumulative burden of grade 1–4 conditions among survivors (at age 50 years) was cardiovascular (4.4, 95%CI=3.7–5.3, Supplementary Table 2), while the systems with the highest burden of grade 3–4 conditions were endocrine (0.82, 95%CI=0.59–1.12) and gastrointestinal (0.51, 95%CI=0.16–0.92, Supplementary Table 3).
Figure 2:
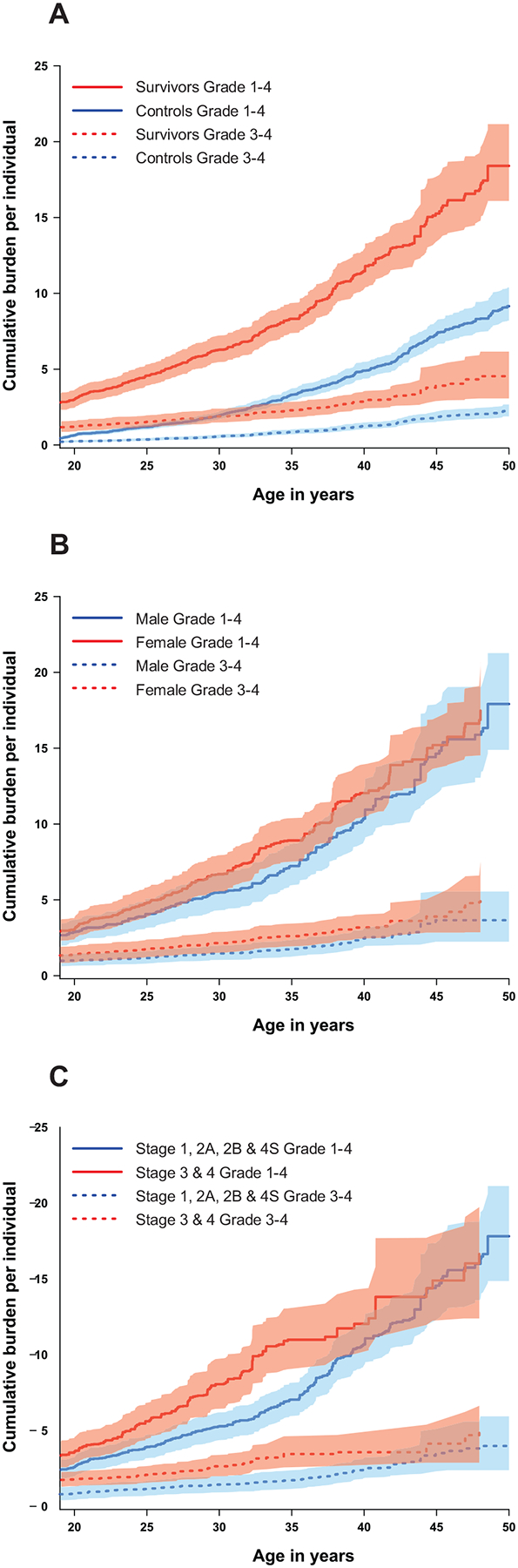
The cumulative burden of morbidity per individual in A) survivors of neuroblastoma compared to controls, B) by sex, and C) by stage.
HRQOL, emotional distress, and social attainment
As shown in Table 2, the frequency of poor HRQOL in the domains of general health (OR=2.2, 95%CI=1.4–3.7) and physical functioning (OR=3.1, 95%CI=1.4–7.2) was higher in survivors compared to controls. Survivors were 2- to 3- times more likely than controls to report symptoms of anxiety (OR=2.5, 95% CI=1.2–5.1) and somatization (OR=2.2, 95% CI=1.2–4.2). Additionally, survivors were more likely to report not living independently (OR=1.8, 95%CI=1.1–2.8), and not being married (OR=1.5, 95%CI=1.1–1.9) than controls.
Table 2:
Prevalence of poor HRQOL, emotional distress, and social attainment
| Survivor | Control1 | ||||
|---|---|---|---|---|---|
| Frequency | Frequency | PR2 | 95%CI | p-value | |
| HRQOL | |||||
| Physical function | 14.2% | 4.2% | 3.1 | 1.4–7.2 | 0.01 |
| Role physical | 12.6% | 6.5% | 1.2 | 0.7–2.1 | 0.57 |
| General health | 25.9% | 9.2% | 2.2 | 1.3–3.7 | <0.01 |
| Social function | 13.3% | 9.9% | 1.0 | 0.5–1.8 | 0.92 |
| Bodily pain | 16.3% | 10.4% | 1.3 | 0.8–2.3 | 0.25 |
| Role emotional | 13.4% | 9.5% | 1.1 | 0.6–1.9 | 0.87 |
| Mental health | 24.4% | 16.8% | 1.1 | 0.7–1.8 | 0.55 |
| Vitality | 21.5% | 15.3% | 1.2 | 0.8–2.0 | 0.38 |
| Emotional distress | |||||
| Anxiety | 16.2% | 5.4% | 2.5 | 1.2–5.1 | 0.01 |
| Depression | 13.1% | 6.5% | 1.4 | 0.6–2.9 | 0.41 |
| Somatization | 19.1% | 7.3% | 2.2 | 1.2–4.2 | 0.01 |
| Social Attainment | |||||
| Non-independent living | 29.1% | 14.5% | 1.8 | 1.1–2.8 | 0.01 |
| Never married | 52.9% | 30.9% | 1.5 | 1.1–1.9 | 0.01 |
| Not currently working3 | 23.5% | 16.0% | 1.3 | 0.8–2.1 | 0.26 |
| Less than college degree4 | 37.5% | 19.5% | 1.2 | 0.8–1.8 | 0.31 |
262 controls were included in analyses.
Models adjusted for age at follow-up, sex, education, employment and household income.
Model not adjusted for employment status.
Model not adjusted for education level. Educational attainment dichotomized as being ≤high school graduate vs. having some training after high school, some college, or being ≥college graduate.
HRQOL – health-related quality of life; PR – prevalence ratio; CI – confidence interval
In analyses limited to survivors, hearing loss was associated with 2- to 2.5-fold increased odds of survivors reporting poor general health (OR=2.2, 95% CI=1.2–4.1), greater bodily pain (OR=2.6, 95% CI=1.2–5.9), and problems with social functioning (OR=2.6, 95% CI=1.1–6.6) (Table 3). Obesity was associated with an increased odds of reporting bodily pain (OR=3.0, 95%CI=1.8–8.1) and low vitality (OR=2.2, 95%CI=1.1–4.4), while a history of spinal disorders was associated with reporting low physical function (OR=3.0, 95%CI=1.2–7.7). Short stature was associated with an increased likelihood of reporting poor quality of life related to physical functioning (OR=3.1, 95% CI=1.4–6.9) and role limitations due to physical health (OR=3.1, 95% CI=1.3–7.4; Table 3). Survivors with one or more grade 3–4 condition were 3- to 4-times more likely to report bodily pain (OR=4.2 95%CI=1.0–17.0), low vitality (OR=3.3, 95%CI=1.0–10.2) and to not be currently working (OR=3.2, 95%CI=1.2–8.5), and 6 times more likely to report poorer general health outcomes (OR=6.6, 95%CI=1.2–8.5) compared to survivors with no severe/disabling or life-threatening conditions.
Table 3:
Selected chronic conditions and associations with HRQOL, emotional distress, and social attainment among neuroblastoma survivors
| HEARING LOSS1 N=33 |
OBESITY2 N=43 |
SPINAL DISORDERS1,3 N=21 |
SHORT STATURE4 N=30 |
≥1 GRADE 3–4 CHRONIC CONDITON N=95 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR5 (95%CI) | p-value | PR5 (95%CI) | p-value | PR5(95%CI) | p-value | PR5 (95%CI) | p-value | PR5 (95%CI) | p-value | |
| HRQOL | ||||||||||
| Physical function | 1.1 (0.4–3.5) | 0.83 | 0.6 (0.3–1.5) | 0.32 | 3.0 (1.2–7.7) | 0.02 | 3.1 (1.4–6.9) | 0.01 | 2.3 (0.7–7.2) | 0.17 |
| Role physical | 1.8 (0.7–5.0) | 0.24 | 0.5 (0.2–1.1) | 0.10 | 1.4 (0.4–4.8) | 0.63 | 3.1 (1.3–7.4) | 0.01 | 1.8 (0.5–6.0) | 0.35 |
| General health | 2.2 (1.2–4.1) | 0.01 | 1.4 (0.7–2.7) | 0.30 | 1.3 (0.7–2.7) | 0.43 | 1.8 (0.9–3.6) | 0.10 | 6.6 (1.6–26.9) | 0.01 |
| Bodily pain | 2.6 (1.2–5.9) | 0.02 | 3.0 (1.1–8.1) | 0.03 | 1.2 (0.4–3.8) | 0.77 | 2.1 (1.0–4.4) | 0.06 | 4.2 (1.0–17.0) | 0.04 |
| Social function | 2.6 (1.1–6.6) | 0.04 | 0.8 (0.3–2.0) | 0.61 | 1.5 (0.5–4.3) | 0.44 | 2.2 (0.9–5.1) | 0.07 | 1.3 (0.4–4.0) | 0.60 |
| Role emotional | 1.6 (0.6–4.0) | 0.33 | 0.9 (0.4–2.1) | 0.87 | 0.9 (0.3–3.2) | 0.90 | 1.7 (0.7–3.8) | 0.22 | 1.2 (0.4–3.5) | 0.76 |
| Mental health | 1.5 (0.8–2.9) | 0.18 | 1.7 (0.8–3.6) | 0.17 | 0.9 (0.4–2.2) | 0.87 | 0.9 (0.5–1.9) | 0.89 | 1.9 (0.8–4.2) | 0.13 |
| Vitality | 1.8 (0.9–3.7) | 0.10 | 2.2 (1.1–4.4) | 0.04 | 1.6 (0.7–3.8) | 0.24 | 1.1 (0.5–2.2) | 0.81 | 3.3 (1.0–10.2) | 0.04 |
| EMOTIONAL DISTRESS | ||||||||||
| Anxiety | 1.8 (0.8–4.2) | 0.17 | 2.7 (1.0–7.2) | 0.04 | 2.4 (1.0–6.2) | 0.06 | 1.4 (0.6–3.2) | 0.42 | 8.1 (1.1–58.0) | 0.04 |
| Depression | 1.7 (0.7–4.5) | 0.25 | 2.0 (0.7–5.8) | 0.20 | 1.0 (0.3–3.8) | 0.98 | 1.9 (0.8–4.6) | 0.15 | 2.0 (0.5–7.0) | 0.30 |
| Somatization | 1.7 (0.8–3.9) | 0.18 | 2.1 (0.9–4.6) | 0.08 | 0.6 (0.2–1.7) | 0.31 | 1.5 (0.7–3.1) | 0.24 | 2.0 (0.7–5.6) | 0.17 |
| SOCIAL ATTAINMENT | ||||||||||
| Non-independent living | 1.2 (0.8–2.0) | 0.39 | 1.1 (0.5–2.6) | 0.76 | 1.0 (0.5–1.9) | 0.99 | 1.4 (0.9–2.3) | 0.14 | 1.2 (0.8–2.0) | 0.40 |
| Not married | 1.1 (0.9–1.4) | 0.36 | 1.0 (0.7–1.3) | 0.85 | 0.9 (0.6–1.4) | 0.77 | 1.2 (0.9–1.7) | 0.20 | 1.2 (0.9–1.7) | 0.24 |
| Not currently working | 1.6 (0.9–3.0) | 0.15 | 1.3 (0.7–2.5) | 0.41 | 0.9 (0.4–2.2) | 0.90 | 1.4 (0.7–2.6) | 0.34 | 3.2 (1.2–8.5) | 0.02 |
| Less than college degree6 | 1.1 (0.6–1.9) | 0.85 | 1.7 (0.9–2.9) | 0.08 | 0.6 (0.3–1.5) | 0.29 | 1.3 (0.7–2.1) | 0.40 | 1.6 (0.9–3.0) | 0.12 |
CTCAE Grade 2 or higher.
CTCAE Grade 3 or higher.
Includes skeletal spine disorder, scoliosis, intervertebral disc disorder, and kyphosis.
Defined as adult height Z-score more than 1.3 SD below expected values.
Models adjusted for sex, age at follow-up, and race/ethnicity. Model for obesity also adjusted for education level, employment status and household income.
Educational attainment dichotomized as being ≤high school graduate vs. having some training after high school, some college, or being ≥college graduate.
HRQOL – health-related quality of life; PR – prevalence ratio; CI – confidence interval
DISCUSSION
This study, through comprehensive health evaluations, characterized health outcomes, HRQOL and social attainment of adults treated for neuroblastoma, the most common solid malignancy of infancy. In contrast to previous studies where health outcomes were ascertained through self-report, our clinical assessments allowed characterization of multimorbidity, with two-thirds of survivors affected by a severe or life-threatening health condition by 35 years of age. Moreover, we found that many of the most frequently identified conditions among survivors, such as hearing loss, obesity, spinal disorders, and short stature, were associated with reduced HRQOL across multiple domains. Survivors with one or more grade 3–4 condition were between 2- and 6-times more like to report increased bodily pain, poorer general health, and reduced vitality relative to survivors without a grade 3–4 condition. This is important given that the cumulative burden of chronic disease was consistently higher among survivors than controls (2.9 vs. 1.3 grade 3–4 conditions by age 40 years) and continued to rise with increasing age. The anticipated increase in morbidity with aging underscores the importance of identifying interventions to optimize health and functional status, symptom control, and overall quality of life among adult survivors of neuroblastoma.
In these analyses, 95% of neuroblastoma survivors, including those with lower stage disease, experienced at least one chronic illness. This is in contrast to previous work where studies among children diagnosed with advanced disease and/or who had received autologous transplantation, report late effects in the majority (>90%) of survivors,2,3,6,7 but not among survivors with lower stage disease. For example, a summary of outcomes among neuroblastoma survivor participants (all stages of disease) in the CCSS reports a 20-year cumulative incidence of a chronic condition at 41%9; and a smaller study of 43 Japanese survivors identified health conditions in only 28% of participants.4 The higher prevalence of chronic conditions observed in our study is likely because outcomes were ascertained uniformly and clinically, and because SJLIFE participants were older (median 31.9 years) when compared to survivors in previous studies. Interestingly, we did not find the cumulative burden of chronic conditions to vary significantly by disease stage at diagnosis. As this study only included neuroblastoma survivors who were adults and more than 10 years from diagnosis at follow-up, those individuals who developed very serious and life-threatening illnesses because of aggressive treatments for advanced disease may not have survived to participate in the SJLIFE study. Nevertheless, the high degree of multimorbidity identified among neuroblastoma survivors highlights the medically complex needs of this population.
Late effects in the cohort were consistent with the clinical presentation of neuroblastoma and therapeutic approaches used over the years.2–9 Radiation therapy provided for local control of paraspinal tumors contributed to the increased risk of endocrine and musculoskeletal late effects. Systemic therapies that are still currently used for neuroblastoma have well-established organ toxicities prevalent among survivors. For example, hearing loss was common and almost exclusively occurred among individuals treated with cisplatin or carboplatin. Cardiometabolic conditions (hypertriglyceridemia, hypercholesterolemia, abnormal glucose homeostasis, or obesity), prevalent among 25% and 58% of survivors in this cohort, have been infrequently noted in previous studies of neuroblastoma survivors. Indeed, in a report from the CCSS, male survivors of neuroblastoma were more likely to be underweight than sibling controls.22 As the prevalence of these outcomes did not differ among survivors and controls, and because we observed few significant differences in treatment exposures between survivors with and without cardiometabolic impairments, it is likely that these outcomes are a consequence of lifestyle rather than treatment exposure. Like the general population, health conditions develop in survivors of neuroblastoma as they age, increasing their risk for serious illnesses including cardiovascular diseases and diabetes.
In our sample, neuroblastoma survivors reported worse physical HRQOL compared to controls. This is not particularly surprising given the heightened prevalence of medical late effects observed among survivors; however, this finding contrasts with a past CCSS report that observed no decrement in physical HRQOL among survivors of neuroblastoma compared to sibling controls.23 This difference may be due to the nature of the comparative groups employed across studies. However, consistent with past studies, we observed that survivors were 2 times more likely to report symptoms of anxiety and somatization compared to community controls. We also found that spinal disorders, short stature, obesity, and hearing loss were associated with increased odds of reduced HRQOL. Though not all associations achieved statistical significance, likely due to our small sample size, the effect estimates were quite large and underscore the potential consequences of health status on survivors’ quality of life. In addition, we observed that survivors were significantly less likely to report being married and living independently. These results highlight the potential emotional and social consequences decades following treatment for neuroblastoma and identify subgroups who may benefit from psychosocial interventions. Moreover, as some survivors may not be aware of the presence of organ dysfunction contributing to HRQOL deficits, clinical assessments that provide opportunities for early diagnosis and intervention are important for minimizing adverse outcomes in this high-risk population.
The strengths of this study included the comprehensive and systematic clinical and laboratory assessments that survivors underwent to identify chronic conditions, and that the median age of assessment (32 years) of participants was older than that reported for most prior studies of neuroblastoma survivors. Accordingly, these analyses provide information on the occurrence of chronic conditions many years after therapy. Additionally, this study had a clinically-assessed comparison group against which to compare health conditions, HRQOL, and social attainment in survivors. Our study was limited by our sample size, which prevented robust examination of associations between treatment exposures with late chronic events. Finally, as most participants were treated prior to 1990s, our ability to generalize findings to patients treated more recently, or with new therapeutics, may be limited. As survival rates for more recently treated patients with advanced disease have improved significantly over the past several decades, our findings may underestimate the true burden of morbidity among neuroblastoma survivors.
Out findings suggest that, in addition to many endocrine, auditory, renal and neurological late effects, the prevalence of cardiometabolic conditions is also high among aging survivors of childhood neuroblastoma. Moreover, multimorbidity is frequent among survivors regardless of stage at diagnosis. These findings highlight the wide range and high number of chronic conditions that can occur among neuroblastoma survivors, which in turn, may be informative for clinicians who care for survivors. Considering the high burden of chronic diseases among neuroblastoma survivors and associations between chronic diseases, such as hearing loss, obesity, spinal disorders, and short stature, with reduced HRQOL and social attainment, further development of screening and interventions, which provide opportunities to optimize health, are important for this population.
Supplementary Material
Funding:
This project was funded by Cancer Center Support Grant number CA021765 (PI, C Roberts), CA195547 (MPI, M Hudson, L Robison) and the American Lebanese Syrian Associated Charities.
Financial disclosures: The authors have no financial disclosures.
References
- 1.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 2.Perwein T, Lackner H, Sovinz P, et al. Survival and late effects in children with stage 4 neuroblastoma. Pediatr Blood Cancer. 2011;57:629–635. [DOI] [PubMed] [Google Scholar]
- 3.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. [DOI] [PubMed] [Google Scholar]
- 4.Kubota M, Okuyama N, Hirayama Y, Asami K, Ogawa A, Watanabe A. Mortality and morbidity of patients with neuroblastoma who survived for more than 10 years after treatment--Niigata Tumor Board Study. J Pediatr Surg. 2010;45:673–677. [DOI] [PubMed] [Google Scholar]
- 5.French AE, Irwin MS, Navarro OM, Greenberg M, Nathan PC. Long-term hepatic outcomes in survivors of stage 4S and 4 neuroblastoma in infancy. Pediatr Blood Cancer. 2012;58:283–288. [DOI] [PubMed] [Google Scholar]
- 6.Hobbie WL, Moshang T, Carlson CA, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40:741–746. [DOI] [PubMed] [Google Scholar]
- 8.Paulino AC, Mayr NA, Simon JH, Buatti JM. Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys. 2002;52:1025–1031. [DOI] [PubMed] [Google Scholar]
- 9.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota M, Yagi M, Kanada S, et al. Long-term follow-up status of patients with neuroblastoma after undergoing either aggressive surgery or chemotherapy--a single institutional study. J Pediatr Surg. 2004;39:1328–1332. [DOI] [PubMed] [Google Scholar]
- 11.Simon T, Berthold F, Borkhardt A, Kremens B, De Carolis B, Hero B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: results of German trials. Pediatr Blood Cancer. 2011;56:578–583. [DOI] [PubMed] [Google Scholar]
- 12.Zheng DJ, Krull KR, Chen Y, et al. Long-term psychological and educational outcomes for survivors of neuroblastoma: A report from the Childhood Cancer Survivor Study. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics. 2007;120:e1229–1236. [DOI] [PubMed] [Google Scholar]
- 14.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 5.0. Children’s Oncology Group; 2018. [Google Scholar]
- 18.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 20.Derogatis L Brief Symptom Inventory (BSI) 18: Administration, scoring, and procedures manual Minneapolis, MN: NCS Pearson;2000. [Google Scholar]
- 21.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390:2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–1739. [DOI] [PubMed] [Google Scholar]
- 23.Nathan PC, Ness KK, Greenberg ML, et al. Health-related quality of life in adult survivors of childhood Wilms tumor or neuroblastoma: A report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49:704–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


