Abstract
Among 284 human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected adults starting tenofovir-based antiretroviral therapy (ART) in Zambia, median baseline CD4+ count was 202 cells/mm3 and 41.6% were hepatitis B e-antigen positive. Within 2 years of therapy, 29 (10.2%) participants experienced HBV functional cure (confirmed loss of hepatitis B surface antigen). In multivariable analysis, baseline CD4 count <350 cells/mm3, female sex, and lower baseline HBV deoxyribonucleic acid were associated with increased odds of functional cure. Immune recovery during HIV-HBV treatment with ART may drive higher rates of functional cure than during HBV monoinfection treatment. Understanding the mechanisms underlying this phenomenon could inform immunomodulatory therapies for HBV cure.
Keywords: Africa, antiretroviral therapy outcomes, HBV functional cure, HIV-HBV coinfection
Among human immunodeficiency virus-hepatitis B virus (HIV-HBV) coinfected patients in Zambia, 10.2% experienced hepatitis B surface antigen loss within 2 years of tenofovir-containing antiretroviral therapy. Understanding the mechanisms of HBsAg loss during HIV treatment could inform HBV cure immune therapies.
Chronic infection with hepatitis B virus (HBV), defined by persistent hepatitis B surface antigen (HBsAg), reflects the failure of the host immune system to eliminate the infection. Treatment of chronic hepatitis B with nucleos(t)ide analogs (NA) results in reliable HBV-deoxyribonucleic acid (DNA) suppression, but clearance of HBsAg (also referred to as “functional cure”) occurs at a very slow rate (~1% per year), necessitating lifelong therapy in most patients [1]. Functional cure is desirable because it is associated with reduction in risk of liver disease progression and hepatocellular carcinoma and allows NA therapy to be discontinued [2]. A growing number of novel therapies and therapeutic approaches are being developed to increase the rate of HBV functional cure. Many of these agents are immune modulators, drugs, or vaccines that may restore or enhance HBV-specific host immune responses [3].
Human immunodeficiency virus (HIV)-infected individuals are at increased risk of acquiring HBV infection due to shared routes of transmission and are more likely to develop chronic HBV infection. Furthermore, in HIV-HBV coinfection, HBV DNA and HBsAg levels are increased and hepatitis B e antigen (HBeAg) positivity (an indicator of active viral replication) is more common than in HBV monoinfection. Patients with HIV-HBV coinfection have increased risk of liver disease progression and mortality compared with those with either virus alone. Because of its potency against HBV, tenofovir should be included in the antiretroviral therapy (ART) regimens of coinfected patients. However, mortality in HIV-HBV coinfection remains high relative to those who are HIV positive but HBsAg negative even when optimal ART is started at near-normal CD4+ T-cell counts [4]. Although the mechanisms by which HIV impacts the HBV natural history are not well defined, impaired T-cell responses are thought to play a major contributory role in these observations.
Although HIV impairs the host immune response to chronic HBV infection, during tenofovir-based ART and immune restoration, it has been suggested that achievement of functional cure may be enhanced in HIV-HBV coinfection when compared with HBV monoinfection during NA therapy [5]. To assess this in a larger cohort, we analyzed HBsAg clearance and anti-HBs seroconversion in a prospective cohort of HIV-HBV coinfected patients in Zambia on tenofovir-based ART and assessed baseline and change in CD4 during therapy as possible correlates of functional cure.
MATERIALS AND METHODS
A prospective cohort was established within 2 public sector facilities in Lusaka, Zambia, under the framework of the International Epidemiological Databases to Evaluate AIDS (IeDEA) collaboration in Southern Africa. Human immunodeficiency virus-infected adults (18+ years old) initiating ART per national guidelines were eligible to participate if they were HBsAg positive based on a single positive HBsAg test and were ART-naive [6]. Antiretroviral therapy consisted of fixed-dose combination tenofovir disoproxil fumarate, lamivudine, and efavirenz.
At the time of ART initiation, we measured alanine aminotransferase (ALT), CD4 count, HBV DNA (Roche COBAS Ampliprep/COBAS TaqMan; sensitivity 20 IU/mL), HBeAg and anti-hepatitis delta virus (HDV) (DiaSorin, Brussels, Belgium), and hepatitis C virus (HCV) antibodies (OraQuick; Orasure Technologies) with reflex HCV ribonucleic acid (RNA) testing of positives, and liver stiffness using transient elastography (FibroScan 402; Echosens, Paris, France). Significant fibrosis was defined as liver stiffness measurement ≥7.9 kilopascals (kPa). At follow-up visits, which occurred every 6–12 months during the time of ART clinic visits, we repeated ALT, CD4, HBV DNA, and HBeAg (if the baseline result was positive) and measured HIV viral load. Hepatitis B surface antigen loss was monitored in the clinic using a rapid point of care assay (Determine; Alere). Negative rapid results triggered additional sample collection for confirmatory qualitative HBsAg and anti-HBs testing using a central laboratory assay (Access2Analyzer; Beckman Coulter). Antiretroviral therapy was continued for HIV treatment regardless of change in HBsAg results.
This analysis included cohort participants with at least 1 year of follow-up and at least 1 follow-up HBsAg result. We described the characteristics at ART start. Elevated ALT was defined as >19 U/L for women and >30 U/L for men. Change in CD4 count was calculated from baseline to 1 year on ART. Hepatitis B virus DNA suppression was defined as <20 IU/mL, and HIV RNA suppression was defined as <40 copies/mL by 12 months on ART. Hepatitis C virus positivity was defined as having detectable HCV RNA. We described major HBV outcomes including the proportion who achieved ALT normalization and HBV-DNA suppression and became HBeAg negative by 2 years on therapy. We defined HBV functional cure as confirmed HBsAg clearance with or without anti-HBs positivity. In bivariable logistic regression, we evaluated the following possible correlates of functional cure: sex, age, World Health Organization HIV clinical stage, tuberculosis, elevated baseline ALT, significant fibrosis, baseline CD4 count, HBV-DNA level, CD4 count change (from baseline to 1 year), and HIV viral suppression. Patients with baseline CD4 >350 cells/mm3 were a priori selected as the reference group for analyses because they had the smallest degree of immune suppression in the cohort. Hepatitis B e antigen was excluded from our primary multivariable analyses due to collinearity with HBV DNA and missing data. Factors that were associated with functional cure at P < .2 were included in a multivariable logistic regression model. All data were analyzed using Stata (version 14; StataCorp, College Station, TX). All participants provided written informed consent. Ethical review and approval was provided by the University of Zambia Biomedical Research Ethics Committee and the University of Alabama at Birmingham Institutional Review Board.
RESULTS
From October 2013 to August 2017, 358 HIV-HBV coinfected adults enrolled in the cohort. Of these, we excluded the following from analysis: 21 for death, 12 for transfer, 21 for loss to follow-up before the 1-year repeat HBsAg test, and 20 for having less than 1 year of follow-up at administrative censoring. Among the remaining 284, median age was 34 years (interquartile range [IQR], 28–39 years), 108 (38.2%) were women, 28 had tuberculosis coinfection (9.9%), and none were HCV positive. At ART start, median baseline CD4+ count was 202 cells/mm3 (IQR, 94–338), 89 of the 214 with results (41.6%) were HBeAg positive, and 108 (49.3%) of the 219 with results had HBV DNA >2000 IU/mL. Significant fibrosis, based on elastography (ie, ≥7.9 kPa), was present in 46 (20.0%) of 230 with the test. After ART initiation, 45 of 127 (35.4%) with baseline elevation experienced normalization of ALT. Of the 164 patients with HBV DNA >20 IU/mL at baseline, 68 of 102 (64.2%) with available results achieved full suppression on tenofovir-containing ART. Among the 89 with HBeAg positivity at baseline, 77 had a repeat test on ART and 17 (22.1%) experienced HBeAg loss.
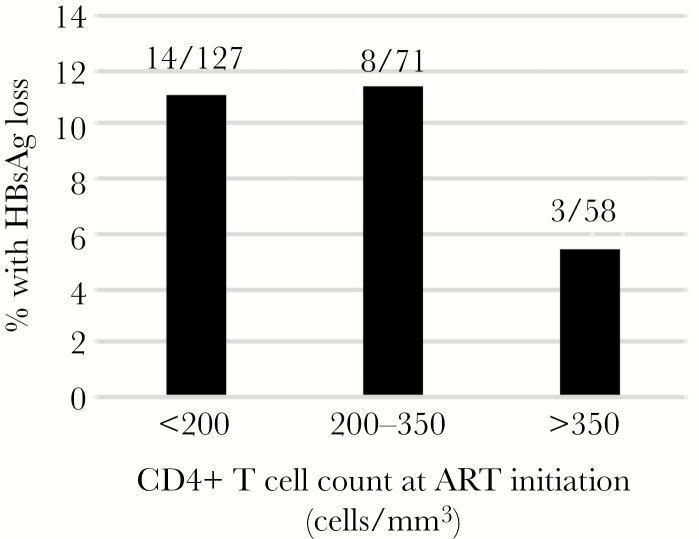
During ART, 58 participants had a negative rapid HBsAg test result in the clinic. Of those, 18 were false negatives (ie, HBsAg was positive on the central laboratory assay), 29 were confirmed to be HBsAg negative (ie, consistent with HBV functional cure), and 11 did not have confirmatory testing. Therefore, 10.2% (29 of 284) met our definition of functional cure. At the time of ascertainment, all 29 had undetectable HBV DNA and normal ALT, and 12 (41.4%) were anti-HBs positive. We plotted the percentage with HBsAg clearance by baseline CD4 (Figure 1) [6]. In multivariable analysis, CD4 <350 cells/mm3 was associated with increased odds of functional cure (adjusted odds ratio [AOR], 4.94; 95% confidence interval [CI], 1.02–23.80), whereas male sex (AOR, 0.38; 95% CI, .14–1.05) and a 1-log increase in HBV DNA (AOR, 0.68; 95% CI, .54–.86) were associated with reduced odds (Table 1). Similar associations were seen when the CD4 was dichotomized at 200 cells/mm3.
Figure 1.
Percentage of human immunodeficiency virus-hepatitis B virus-coinfected patients with hepatitis B surface antigen (HBsAg) loss by 2 years of tenofovir-based therapy, by CD4 count.
Table 1.
Factors Associated With HBV Functional Cure
| Characteristic | n/N (%) With the Outcome or Median (IQR) | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Sex | |||||
| Female | 15/108 (13.9) | Reference | Reference | ||
| Male | 14/175 (8.0) | 0.54 (.25–1.17) | .12 | 0.38 (.14–1.05) | .06 |
| Age, years | 34 (28–39) | 1.00 (.95–1.05) | .92 | ||
| WHO Clinical Stage | |||||
| 1 or 2 | 18/166 (10.8) | Reference | |||
| 3 or 4 | 10/110 (9.1) | 0.82 (.36–1.85) | .64 | ||
| Tuberculosis | |||||
| No | 26/255 (10.2) | Reference | |||
| Yes | 3/28 (10.7) | 1.06 (.30–3.74) | .93 | ||
| Elevated Baseline ALTa | |||||
| No | 16/133 (12.0) | Reference | |||
| Yes | 10/121 (8.3) | 0.66 (.29–1.51) | .32 | ||
| Baseline CD4 Count | |||||
| ≥350 cells/mm3 | 3/58 (5.2) | Reference | Reference | ||
| <350 cells/mm3 | 22/176 (11.1) | 2.29 (.66–7.95) | .19 | 4.94 (1.02–23.80) | .05 |
| Per 1 log HBV DNA increase at baseline | 3.3 (1.0–6.4) | 0.72 (.59–.88) | <.01 | 0.68 (.54–.86) | <.01 |
| Significant Liver Fibrosisb | |||||
| No | 18/184 (9.8) | Reference | |||
| Yes | 3/46 (6.5) | 0.64 (.18–2.29) | .50 | ||
| HIV Viral Suppressionc | |||||
| No | 2/21 (9.5) | Reference | |||
| Yes | 8/79 (10.1) | 1.07 (.21–5.46) | .93 | ||
| Change in CD4 countd | +88 (+8 to +155) | 1.01 (.98–1.03) | .70 |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; DNA, deoxyribonucleic acid; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range; OR, odds ratio; RNA, ribonucleic acid; WHO, World Health Organization.
aDefined as >19 U/L for women and >30 U/L for men.
bBased on liver stiffness measurement on transient elastography of ≥7.9 kPa.
cDefined as HIV RNA level <40 copies/mL within 1 year of starting therapy.
dUnits were cells/mm3, a 1-cell increase was the unit of analysis in models.
DISCUSSION
In a Zambian cohort of HIV-HBV coinfected individuals characterized by relatively low median baseline CD4 count, over 10% attained functional cure within 2 years of initiation of tenofovir-based ART, which is substantially higher than the 1% per year that is reported for tenofovir-treated HBV monoinfection [1]. Possible correlates of an elevated functional cure response included lower pretherapy CD4 count, lower pretherapy HBV DNA, and female sex. Understanding the correlates and mechanisms of HBsAg loss in HIV-infected hosts may help in the development of HBV immunotherapies.
The main finding of our analysis was that HBsAg loss occurred in a high proportion of HIV-HBV patients treated with ART relative to HBV monoinfection. This builds on multiple smaller studies in which 6%–38% of HIV-HBV patients experienced HBsAg loss over 1–2 years on ART [1]. We observed an inverse relationship between baseline HBV DNA and the odds of functional cure, consistent with another report that found higher HBsAg clearance in HIV-HBV coinfected persons with low HBV-DNA levels and low quantitative HBsAg levels [7–10], which we have not yet measured in the cohort. Our finding of a possible association between lower initial CD4 and increased functional cure adds to what is known about HBV control in HIV-HBV coinfection. Substantial declines in quantitative HBsAg (qHBsAg) and loss of HBsAg have been correlated with gains in CD4 after ART initiation [8], and restoration of peripheral HBV-specific T-cell responses was noted with HBV-active ART [10, 11]. It is possible that the immunological boost of ART, which may occur rapidly when HIV is suppressed and immune dysfunction and inflammation are reduced, augments anti-HBV immune responses that may have been less exhausted and more HBV naive in the setting of HIV-induced T-cell depletion. We sought, but did not see, a link between change in CD4 count and HBsAg loss; however, host immune responses in the liver where HBV immunopathogenesis occurs may vary considerably from that in peripheral blood [12].
This study has important clinical and public health implications. A growing number of antiviral and immunomodulatory drugs are under development for HBV, and these data can guide the use of these agents in HIV patients. For example, HIV-infected patients more likely to achieve functional cure under current ART could be preferentially recruited into early stage trials of agents with nontrivial potential risk. Dynamic changes to the immune system that may occur after ART start in HIV-HBV coinfection could guide the development and testing of immune modulators. For example, PD-1 expression in HBV-specific T cells, a potentially important biomarker of HBV control, should be evaluated in patients with HIV who attain HBsAg loss/decline because this could further support the use of PD-1/PD-L1 inhibitors [13]. Finally, high rates of HBsAg loss/decline in HIV-HBV could facilitate the development and validation of surrogate end points for HBV clinical trials testing curative therapies. Although our focus was on HBsAg clearance, we also reported that baseline ALT elevation persisted in approximately one third of HIV-HBV coinfected individuals, despite high levels of HIV-RNA and HBV-DNA suppression. This was likely driven by factors other than HIV and HBV, namely, unhealthy alcohol use [14], rather than hepatitis C and delta, which are rare in Zambia.
Although this is one of the largest prospective HIV-HBV studies in Africa, where coinfection is relatively common, it has several limitations. Because this study was nested within real-world ART clinics, laboratory monitoring during ART was infrequent and 25% were excluded from analysis for insufficient data. These observations may have led us to overestimate HBsAg clearance and HBV-DNA suppression because patients with poorer outcomes may have been less likely to be analyzed. On the contrary, the proportion with HBV functional cure was likely a conservative estimate because an additional 11 patients with possible functional cure did not have confirmatory testing. Inclusion of qHBsAg data would have strengthened our analysis because low HBsAg levels may predict evolution to functional cure. Likewise, lack of viral sequencing data prevented us from speculating on the impact of African genotypes on outcomes. Finally, our definition of functional cure was not fully consistent with HBV monoinfection studies, where sustained HBsAg loss off therapy was a definition recommended by experts. In HIV-HBV coinfection, stopping anti-HBV therapy is generally not possible because patients continuing NA therapy with activity against both viruses is recommended [15]. We are continuing to follow-up these patients to assess the durability of HBsAg clearance. Finally, the overall number of patients experiencing HBsAg loss in the different CD4 strata was low, and this likely reduced the precision of estimates in our multivariable analyses.
CONCLUSIONS
In summary, HIV-HBV coinfected patients taking tenofovir-based ART in Zambia experienced high rates of HBV functional cure early after starting therapy. Further investigation is needed to understand how immune restoration in HIV infection affects control of HBV, which could be relevant to novel therapies for HBV functional cure.
Note
Financial support. This work was funded by the National Institute of Allergy and Infectious Disease (Grant Number U01AI069924) and the Fogarty International Center (Grant Number K01TW009998) at the National Institutes of Health (NIH) and the Swiss National Science Foundation (SNSF; Grant Number PP00P3_176944). R. T. C. (Grants AI082630 and DK078772) and D. B. (Grant HD085862) also received funding from the NIH. M. E. received special project funding (Grant Number 174281) from the SNSF.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 2019 Conference on Retroviruses and Opportunistic Infections, Seattle, WA.
References
- 1. Yeo YH, Ho HJ, Yang HI, et al. . Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: a systematic review and meta-analysis. Gastroenterology 2019; 156:635–646.e9. [DOI] [PubMed] [Google Scholar]
- 2. Kim GA, Lim YS, An J, et al. . HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 2014; 63:1325–32. [DOI] [PubMed] [Google Scholar]
- 3. Gehring AJ. New treatments to reach functional cure: rationale and challenges for emerging immune-based therapies. Best Pract Res Clin Gastroenterol 2017; 31:337–45. [DOI] [PubMed] [Google Scholar]
- 4. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017; 31:2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kouamé G-M, Boyd A, Moh R, et al. . Higher mortality despite early antiretroviral therapy in human immunodeficiency virus and hepatitis B virus (HBV)-coinfected patients with high HBV replication. Clin Infect Dis 2018; 66:112–20. [DOI] [PubMed] [Google Scholar]
- 6. Vinikoor MJ, Sinkala E, Chilengi R, et al. . Impact of antiretroviral therapy on liver fibrosis among human immunodeficiency virus-infected adults with and without HBV coinfection in Zambia. Clin Infect Dis 2017; 64:1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamers RL, Zaaijer HL, Wallis CL, et al. . HIV-HBV coinfection in Southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. J Acquir Immune Defic Syndr 2013; 64:174–82. [DOI] [PubMed] [Google Scholar]
- 8. Matthews GV, Ali RJ, Avihingsanon A, et al. . Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals. PLoS One 2013; 8:e61297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson M, Gaseitsiwe S, Moyo S, et al. . Slow CD4+ T-cell recovery in human immunodeficiency virus/hepatitis B virus-coinfected patients initiating truvada-based combination antiretroviral therapy in Botswana. Open Forum Infect Dis 2016; 3:ofw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyd A, Maylin S, Moh R, et al. . Hepatitis B surface antigen quantification as a predictor of seroclearance during treatment in HIV-hepatitis B virus coinfected patients from Sub-Saharan Africa. J Gastroenterol Hepatol 2016; 31:634–44. [DOI] [PubMed] [Google Scholar]
- 11. Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, et al. . Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J Infect Dis 2012; 206:974–80. [DOI] [PubMed] [Google Scholar]
- 12. Chang JJ, Wightman F, Bartholomeusz A, et al. . Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J Virol 2005; 79:3038–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters MG, Locarnini S. New direct-acting antiviral agents and immunomodulators for hepatitis B virus infection. Gastroenterol Hepatol (N Y) 2017; 13:348–56. [PMC free article] [PubMed] [Google Scholar]
- 14. Vinikoor MJ, Zyambo Z, Muyoyeta M, Chander G, Saag MS, Cropsey K. Point-of-care urine ethyl glucuronide testing to detect alcohol use among HIV-hepatitis B virus coinfected adults in Zambia. AIDS Behav 2018; 22:2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gane E, Gaggar A, Nguyen AH, et al. . A phase1 study evaluating anti-PD-1 treatment with or without GS-4774 in HBeAg negative chronic hepatitis B patients. J Hepatol 2017; 66:S26–7. [Google Scholar]