The experiences of SPRINT research teams indicate that training behavioral scientists to think about their work through a “customer discovery” lens is potentially beneficial, but also demonstrate the challenges interventionists face when bringing their products to market.
Keywords: Scale-up, Implementation, Innovation, Commercialization, Behavioral interventions
Abstract
The National Cancer Institute established a dissemination and implementation accelerator program called Speeding Research-tested INTerventions (SPRINT) in order to expedite the translation of behavioral research into practice. The goal of SPRINT is to introduce researchers to a new method for moving their research into practice in order to increase the real-world impact of their evidence-based interventions. The goal of this article is to present case studies on three teams that have completed the SPRINT program to date. Each case study provides a description of the intervention the team came into the program with, the team’s motivation for participating in the SPRINT program, the team’s experience in the program, lessons learned from “customer discovery” interviews conducted by the team during the course, and the team’s future plans for their intervention. The case studies suggest that by focusing on behavioral researchers, SPRINT addresses an unmet need in the commercialization training space; that the definition of “success” can vary across SPRINT projects; that identifying and engaging “payors” for behavioral interventions is an ongoing challenge; and that there are potential “misalignments” between the research process and market demands. Overall, these examples show that customer discovery is a potentially useful method for making interventions more responsive to the needs of stakeholders, and that researchers can benefit from learning the “language” of business and working with individuals who have business experience before trying to move their research from the lab to the real world.
Implications.
Practice: Practitioners can demand greater use of evidence-based interventions (EBIs) and partner with behavioral scientists to better understand what the marketplace for prevention may demand.
Policy: Promoting and facilitating partnerships between behavioral scientists, industry, and other relevant stakeholders can promote the translation of research into practice and expand the healthcare marketplace.
Research: Providing more opportunities for behavioral scientists to learn about commercialization and the marketplace for their interventions could greatly increase the number of EBIs used in the “real world.”
INTRODUCTION
More than half of the cancers that occur can be prevented by applying existing knowledge and technology [1]. Despite their potential to improve health, the dissemination of evidence-based practices remains inadequate in public health, clinical, and community settings, with the result being that new discoveries are not reaching many of the individuals and communities that could benefit from them. Recognizing that faster translation of scientific knowledge into practical applications is needed in order to reduce the global burden of cancer, the National Cancer Institute (NCI) created the SPeeding Research-tested INTerventions (SPRINT) Training Program—a “boot-camp” for behavioral researchers that offers real-world, hands-on instruction on transforming evidence-based cancer control interventions into market-ready products and services. The ultimate goal of the program is to increase the number of research-tested behavioral interventions that are ready to be put into practice.
SPRINT is largely modeled on the NSF I-Corps program, which utilizes the Lean Launchpad Curriculum created by entrepreneur Steve Blank to help federally funded researchers develop a business model to commercialize their technology [2]. However, SPRINT is unique in that it is targeted specifically to cancer prevention and control interventions that focus on behavior change, maintenance, or adherence. There are several contextual factors that make a program like SPRINT particularly timely and useful for behavioral scientists. First, the size of the healthcare marketplace and the demand for innovation continue to increase as the burden of chronic disease continues to grow; second, broad penetration of technology has the potential to expand the reach of behavioral innovations; and third, burgeoning academic and industry partnerships could play an important role in helping move evidence-based science into real-world applications.
Although described elsewhere in detail [3] briefly, the SPRINT program provides 8 weeks of training around business concepts (delivered through a combination of online and in-person lectures), networking opportunities, and personalized guidance from instructors with extensive start-up experience. By the end of the training, researchers are expected to (a) identify a suitable market for their intervention, (b) become familiar with the steps involved in the process of commercializing interventions and overcoming the various barriers to intervention adoption, and (c) learn how to make their research more “stakeholder focused” and commercially viable from the outset (although researchers generally come into the program with a fully developed intervention, they are encouraged to apply the principles learned in the course to their future work and consider customer needs in the initial stages of intervention development going forward).
To date, three cohorts have completed the SPRINT program: 10 teams participated in the program in 2016, 10 teams participated in 2017, and 9 teams participated in 2018. Few SPRINT program participants have prior experience with starting a company or business, developing a business model, or explaining their intervention to investors. The aim of the SPRINT program is to teach behavioral scientists how to refine the different components that are required for a successful business (pricing models, sales channels, etc.) and to prepare them to effectively pitch their business idea to funders, especially those in the private sector [2]. The program achieves this by encouraging Principle Investigators to obtain a mentor with business experience to guide them through the program, and by leading research teams through the process of constructing a Business Model Canvas, which is a template that organizes the nine fundamental components of a business model1 in a clear and systematic way [4]. The canvas is a tool that helps teams brainstorm, articulate, and frame “hypotheses” regarding their business venture. The canvas also guides participants through the “Customer Discovery” process, where teams work to test their hypotheses through customer interviews in order to identify a viable business model (i.e. the logic behind the way an organization intends to create, deliver, and capture value [4]). At the end of the course, teams finalize their business model and create a “minimum viable product” (MVP), or a plan for commercializing their intervention that is more aligned with customer needs and market realities, as revealed by the customer discovery interviews.
At the SPRINT closeout session, teams give their final presentations and receive feedback on their final business model canvas, MVP, and other program components. Teams also create and present a 2-min video that describes their journey through the SPRINT program. During the closeout session, program participants also learn about the process for preparing an NIH Small Business Innovative Research (SBIR) or Small Business Technology Transfer (STTR) application as one of several possible “next steps” they can consider if they decide to continue their work in this space. Additional information about the course, including the full SPRINT curriculum, is available on the SPRINT website (https://www.nci-sprint.com).
METHODS
Customer discovery
Stakeholder interviews are an essential component of the SPRINT course, as they are at the heart of the lean startup “Customer Discovery” process—a method for turning a potential business venture into a series of business model hypotheses and testing customer reactions to those hypotheses [5]. In the SPRINT course, teams develop hypotheses about different aspects of their potential business (e.g., the pricing model they will use) and obtain data to either validate or disprove each hypothesis by interviewing various stakeholders (“customers”) that have insight into the market or the product being sold (such as potential end users/beneficiaries, payors, partners, experts, competitors, etc.) [2]. If a hypothesis is validated, teams can move on to the next hypothesis, but if data suggest that the assumption being made is incorrect or the chosen approach will not work in the real world, the team needs to make a substantive change to the business model (i.e., “pivot”), which may involve changing the product itself or changing the dissemination approach. The Customer Discovery process also reflects the “experiential learning” paradigm that is at the core of the program, which posits that the best way for researchers to discover a viable business model for their intervention is to “get out of the building” and learn by doing [2].
Case studies
In this article, we present three examples of teams that completed the SPRINT program. Because the marketplace for behavioral interventions can vary greatly based on intervention characteristics, these three cases were selected to showcase the variability and scope in the types of interventions that come through the SPRINT program (e.g., a device vs. a training curriculum), the diverse settings these interventions operate in (e.g., clinics vs. churches), the different experiences investigators have in the program, and the diverse outcomes they experience after the training program concludes. The purpose of these case studies is to demonstrate how the SPRINT program, through the customer discovery process, enables researchers to think about implementation of evidence-based programs in the marketplace. Given the importance of context [6] and stakeholder engagement [7] in the field of Dissemination & Implementation, customer discovery offers a practical way to approach the scale-up of evidence-based interventions (EBIs) with the realities of the healthcare marketplace in mind.
The case studies below describe the experiences of three SPRINT teams in the course and the insights they gained from the customer discovery process. Each case study includes (a) a summary of the team’s behavioral intervention; (b) an explanation of the team’s motivation for participating in SPRINT; (c) a summary of their SPRINT program experience; and (d) next steps. The three SPRINT teams are referred to herein as “Project HEAL” led by Cheryl Knott, “REWARD” led by Nora L. Nock, and “Witness CARES” led by Deborah Erwin. Details about each case study are summarized in Table 1. The table illustrates the purpose of the intervention and the stakeholders that were interviewed during the SPRINT program as part of the “customer discovery” process.
Table 1.
| Summary of SPRINT program case study examples
| Team | University/Institution | Team composition | Intervention description | Setting | No. of interviews | Who was interviewed |
|---|---|---|---|---|---|---|
| Project HEAL | University of Maryland, College Park | •PI: Cheryl Knott •EL: Sherie Lou Santos •Mentor: Jimmie L. Slade •Project HEAL team members: Laundette Jones & Felicia Davenport |
Evidence-based online system to train lay persons as community health advisors and deliver a 3-workshop series on early cancer detection (breast, prostate, colorectal) | Faith-based organizations | 41 | •2 payors •3 pastors •2 church leaders •16 recommenders/influencers •12 key opinion leaders •2 community health advisors •4 church members |
| REWARD | Case Western Reserve University | •PI: Nora L. Nock •EL/Co-I: Jay Alberts •Mentor: Mark Milligan |
Lifestyle behavioral intervention evaluating ‘assisted’ exercise (along with group-based nutritional counseling) in obese endometrial cancer survivors [Efforts in the SPRINT program focused on the ‘assisted’ exercise technology] | 3 clinical / community sites | 41 | •26 physical therapists (Neuro, Ortho) and managers of rehabilitation programs •7 regulatory related (FDA, specialized exercise equipment owners/CEOs) •6 stationary bike dealers/sales managers •2 health and fitness industry managers/directors |
| Witness CARES | Roswell Park Comprehensive Cancer Center | •PI: Deborah Erwin •EL: Detric (“Dee”) Johnson •Mentor: Patrick Emmerling |
Group intervention to address cognitive and affective determinants of screening in order to positively influence behavior change and increase CRC screening | Community and faith-based organizations | 51 | •7 end-user/healthy community members •5 Medicaid patients •17 health insurance staff/executives •9 health care clinic/hospital executives •5 primary care physicians •3 gastroenterologists •2 surgeons •2 nurses •1 Medicare/Medicaid executive |
PI Principle Investigator; EL Entrepreneurial Lead.
RESULTS
Case Example 1: Project HEAL (Knott)
Intervention summary
Project HEAL (Health through Early Awareness and Learning) is an evidence based intervention that aims to increase cancer awareness and screening behaviors by working through African American faith-based organizations [8]. The intervention was developed using a community-engaged approach and had demonstrated efficacy in previous randomized trials [9–11]. Using a community health advisor approach, lay church members were trained and certified to teach their peers about early detection of breast, prostate, and colorectal cancer (CRC), and about living a healthy lifestyle [8,12]. An implementation trial demonstrated that Project HEAL community health advisors can receive their training and intervention materials using a web-based system [12,13] and that community health advisors trained online are as effective in delivering the intervention and improving outcomes (e.g., cancer awareness and screening behaviors) as those trained in the classroom [14]. Given the great potential reach of the internet, the team began to think about approaches for scaling-up Project HEAL to achieve population-wide health improvements using computer/internet access.
Motivation for participating in SPRINT
The Project HEAL team was attracted to the SPRINT opportunity because it appeared to be the next logical step in the translational continuum, from intervention development to the establishment of efficacy to considering wider intervention reach at a population level. At the time the team was applying to SPRINT, they had an intervention for which efficacy had been established through several previous randomized trials and felt that by participating in SPRINT they would learn more about if and how Project HEAL could be scaled up, what modifications would need to be made to the intervention in order to bring it to scale, and whether a sustainable financial model could be developed. The team therefore reached out to Jimmie Slade, the Executive Director of a community-based organization that worked with Project HEAL, to be their mentor in the program due to his familiarity with both the intervention and with faith-based organizations in general.
Summary of SPRINT program experience
The project team received training on how to think about Project HEAL using a business model framework. The team constructed a Value Proposition, which is a statement that describes the benefits a customer can expect to obtain from the product or service [15]. The following Value Proposition was crafted for Project Heal: “Our intervention helps African American churches that want to provide evidence-based cancer education, by capacity building to enhance the sustainability of health programming and providing reliable information, unlike sporadic, non-evidence-based health promotion activities with no continuity.” When developing the value proposition the team noted that there are few “competitors” in church-based health promotion and the main “competing” factors are the limited time and resources available in these settings.
The team conducted discovery interviews with key stakeholders in the Project HEAL “ecosystem.” Key findings are summarized here, as previously discussed in Jones et al. [16]. The team interviewed a total of 41 stakeholders within the church ecosystem including church members, pastors, community health advisors, and other key opinion leaders. The discovery interviews provided several key insights, which led the team to think in different ways about intervention scale-up and commercialization (i.e., to “pivot”).
First, though the internet provides the ability to reach people almost anywhere, offering a potentially effective channel for intervention scale-up, the team learned that the challenges to intervention scale-up in community settings like churches are unique and likely greater in comparison to healthcare contexts where health promotion is a primary focus. Churches are largely de-centralized organizations and make decisions independently, sometimes based on the pastor’s authority and other times in consultation with a church leadership team. Therefore, the team realized that scale-up would need to take a more incremental approach. The team also learned that although people can technically be reached through the internet, this does not mean that they can actually be engaged through internet or email outreach in the absence of a personal connection or motivation. This implies that engaging churches for health promotion will continue to require intensive relationship building, a considerable challenge to broader scale-up.
Second, the team realized that Project HEAL will need additional development in order to bring it to greater scale. In particular, the community health advisor training curriculum and the intervention materials will need to cover additional health topics beyond cancer. Though cancer is a significant health concern, it is not the only concern among community members, who also need information about managing other chronic diseases (e.g., hypertension, diabetes), as well as mental health and opioid addiction. Inclusion of a broader range of health topics in the intervention poses a considerable challenge in the face of a traditionally siloed funding environment.
Third, in consideration of a sustainable fiscal model to support Project HEAL, the team encountered challenges in thinking about who would pay for the intervention in the churches. There may be some churches, though not many, that are willing to pay for the intervention. However, churches often are challenged with their own financial needs and may not have the funds available to pay for training if offered. Most church leaders indicated that they would not be willing to adopt Project HEAL if there was a cost involved. This creates another significant challenge to intervention scale-up.
Next steps
By the end of the SPRINT training, the team arrived at a number of potential next steps. First, the team will continue to think about channels and approaches through which Project HEAL can be scaled to reach more churches. This will include broadening the intervention content to include health topics other than cancer and considering how to remotely offer technical assistance to end users. The team plans to develop and pilot test strategies for scale-up, based on established models and frameworks [17–19], and may seek subsequent research support to test such strategies. Since completing the SPRINT training, the research team attended two faith-based conferences in an attempt to disseminate the intervention and network to reach more churches. At this time the team does not plan to pursue commercialization, but has been in contact with the University’s Office of Technology and Commercialization and applied for internal pilot funds to broaden the intervention content and explore scale-up strategies. The team also applied for licensing of the intervention through the Creative Commons, which allows users to access, adapt, and implement the intervention materials but not to benefit from them commercially.
Case Example 2: REWARD (Nock)
Intervention summary
The REWARD (evving-up Exercise for Sustained Weight Loss by Altering Neurological Reward and Drive) trial, is evaluating the potential impact of ‘assisted’ exercise, an innovative technology that provides mechanical assistance to enable patients to pedal faster than they voluntarily pedal on their own. This ‘assisted’ exercise technology uses a “smart” motor and “smart” algorithm system that senses and utilizes cadence, power, and torque exhibited by the patient to control and adjust, in real time, the assistance from the motor system, which ensures active patient engagement. This active patient engagement is what differentiates the ‘assisted’ exercise technology from currently available passive motorized systems. The REWARD trial is evaluating the effects of this ‘assisted’ exercise technology on physiological (weight, body fat, fitness) and behavioral (eating behavior, exercise motivation) changes in obese endometrial cancer survivors [20]. In addition, the trial is evaluating potential changes in the neural response to high-calorie visual food cues and stop/go signals in brain regions associated with food reward, motivation, and inhibition using functional magnetic resonance imaging (MRI) [20].
The team’s research has previously shown that ‘assisted’ exercise on stationary cycles provides therapeutic benefit to Parkinson’s disease (PD) and stroke patients. More specifically, in PD patients, the team found global improvements in motor function and increased activity in cortical and subcortical brain regions consistent with neural activation patterns after applying a dopamine agonist, suggesting that ‘assisted’ exercise may be modulating dopamine levels in the brain [21]. In addition, ‘assisted’ cycling in stroke patients, when used as a supplement to standard repetitive motor task practice (RTP) therapy, has been shown to improve motor function (Fugl–Meyer Assessment [FMA] Upper Extremity [UE] motor scores), and these improvements in motor function were found to be superior to cycling on a standard stationary bike and persisted 4 weeks after the exercise intervention was completed [22]. Because the team’s prior studies suggest that ‘assisted’ exercise may be modulating dopamine levels in the brain, the team hypothesizes that ‘assisted’ exercise may help to reduce food and substance use cravings in individuals with obesity and substance use disorders, respectively [23]. In summary, the ‘assisted’ exercise technology may provide therapeutic benefits beyond those achievable from standard exercise equipment in several different patient populations.
Motivation for participation in SPRINT
The team’s overall motivation for participating in the SPRINT program was to evaluate ways to potentially scale-up the ‘assisted’ exercise technology for wider-dissemination and to modify the technology to become more cost-effective. The team also felt that the SPRINT program could help the team’s short-term efforts to build additional ‘assisted’ exercise cycles more efficiently so that the REWARD program could be offered at additional sites to enhance recruitment efforts. Moreover, the team thought the training and experience acquired from the SPRINT program would provide additional skills that would enable the team members to better disseminate future interventions and overcome barriers to their adoption, which would enable subsequent research endeavors to be more ‘stakeholder focused and commercially viable’ from the initial project planning phases.
Summary of SPRINT experience
When the team entered the SPRINT program, the intervention was in its third year of NIH R01 funding. The PI of the REWARD Trial (Nock) served as the PI for the SPRINT program. A co-investigator on the parent trial and a patent holder of the “assisted” exercise technology (Alberts) served as the Entrepreneurial Lead. The team’s Mentor was Mark Milligan, a Medical Product Manager for Woodway, USA (a company that manufactures specialized exercise equipment), who had prior experience bringing a technology developed in a university setting to the commercial market.
The team’s first major task was to develop a “Value Proposition” and corresponding initial “Business Model Canvas.” This initially sounded like a relatively easy assignment (since the team had been working with the technology for a few years); however, it required quite a bit of thought, discussion and additional reading about customer segments, channels, cost structures, and revenue streams. The next major task was to “get out of the building and talk to customers.” The SPRINT customer discovery interviews are deemed exempt from IRB oversight under clause 45 CFR 46.101(b)(2) of the Department of Health and Human Services regulations (Chesapeake IRB; Protocol Number 00021164); thus, the team was able to quickly devise a semi-structured interview guide and begin setting up meetings. The team completed over 40 interviews (in-person, by telephone, and via Skype) over an 8-week period. The interviews, which ranged in length from approximately 30 min to 3 hr, included “customers” from neuro- and ortho-physical therapy (PT) clinics (including managers of rehabilitation centers), health and fitness club representatives (marketing managers, regional representatives) and dealers, as well as key informants on regulatory matters (University Technology Centers and Industrial Relations, Food and Drug Administration [FDA] representatives, bike manufacturer owners/CEOs).
These interviews confirmed interest in the ‘assisted’ exercise technology and helped the team develop and refine the Business Model Canvas, Minimum Viable Product (MVP), Customer Ecosystems, and Customer workflows. The feedback from the interviews also led the team to a few key insights, including that PT clinics, particularly those treating patients with neurological disorders and diseases, wanted the ability to couple the technology with functional electrical stimulation (FES). They also wanted a reliable product with a good service network that would be able to fix any issues with the equipment in 24–48 hr or less. The team also gained insight on price points of passive motorized stationary bikes, and although these manufacturers do not provide the same features and benefits that the ‘assisted’ exercise technology can achieve, they were identified as the largest potential “competitor”.
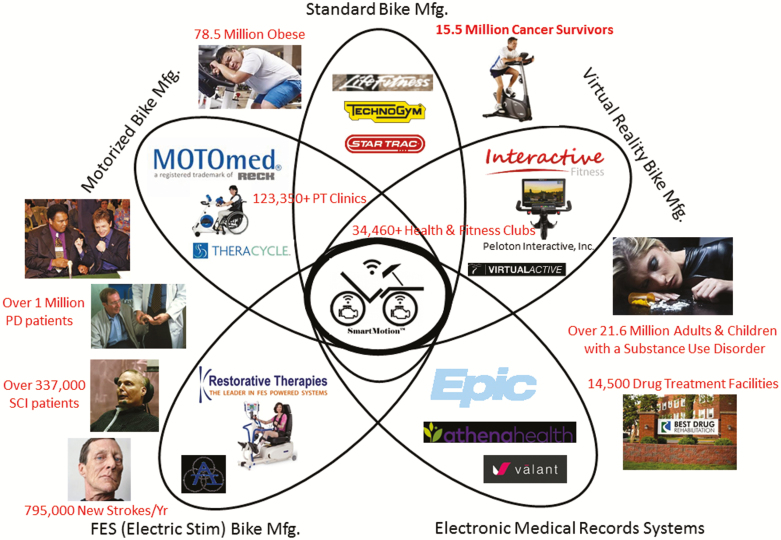
To better understand where ‘assisted’ exercise technology might fit in the overall market, the team created a Petal Diagram (Fig. 1), which highlights how the ‘assisted’ exercise technology could potentially help millions of patients with neurological diseases, cancer, and obesity. Through the customer discovery interviews, the team learned that the ‘assisted’ exercise bikes could potentially be purchased using Medicaid waivers and/or the services could potentially be reimbursed through various CPT PT codes. Further, the team realized that the FDA approval process is complex and may require hiring a product liability attorney. In addition, the team learned that FDA Premarket Notification 510(K) approval could potentially be achieved more readily using a “predicate” (e.g., Class I: Motorized Exercise Equipment [BXB]; Class II: FES Motorized Devices [GZI]) rather than a “de novo” approval. However, the team learned that even though the “de novo” approval would take longer and be much more involved, the “de novo” process would enable them to clearly differentiate the therapeutic benefits of the ‘assisted’ exercise technology and demonstrate why this technology is superior to existing products on the market.
Fig. 1.
| Petal diagram of potential market fit for the ‘assisted’ exercise technology.
Next steps
After the SPRINT training ended, the team executed license agreements with the Cleveland Clinic and with Rockwell Automation, Inc. to continue to collaboratively modify the ‘assisted’ exercise technology. The team continues to discuss ways of further improving the efficiency and effectiveness of the technology for different patient populations (including adding FES ports) and exploring design options to further drive down the price point. The team also continues to pursue NIH grants as well as other funding mechanisms to support these enhancements to the technology and additional studies in other patient populations where the ‘assisted’ exercise technology may provide benefit.
Case Example 3: Witness CARES (Erwin)
Intervention summary
Witness Cares is a behavioral intervention study that aims to increase colonoscopy screening among African American adults in New York City and Buffalo, NY. The research conducted as part of this study showed the important mediating impact of affective associations on colorectal cancer (CRC) screening and the fact that educational interventions can positively change negative feelings about colonoscopy [24–26]. In addition, 25 years of community-based interventions with African American women through the National Witness Project have demonstrated the positive impact of culturally appropriate methods to engage lower income and minority women in screening [27–29]. The R01 study for Witness CARES was entering its fourth year when the team decided to apply to the SPRINT program.
Motivation for participating in SPRINT
The Witness CARES team was informed about the launch of a new training program at the NCI and was intrigued with the idea of learning more about dissemination and especially commercialization opportunities. The PI (Erwin) and Entrepreneurial Lead (Johnson) had investigated the SBIR/STTR application program in the past but were stymied by the challenge of commercializing a program and related services for which, seemingly, no one would pay. Academic and public health specialists undoubtedly recognized the value and significance of the culturally tailored intervention(s) but finding funding and support for this labor-intensive specialized service outside of research grants, the Centers for Disease Control and Prevention, and possibly hospitals and cancer centers, had been challenging. Certainly, the patients that most needed the services were not in a position to pay for this assistance.
Summary of SPRINT experience
Before starting the program, the PI (Erwin) and Entrepreneurial Lead (Johnson, who had served as the Project Coordinator of the R01) had to recruit a mentor for their SPRINT team and turned to the technology transfer office at their institution, which provided an exceptionally talented Mentor (Emmerling) who is still working with the team 2 years later. Finding the business mentor was essential to future developments and supported the team as it navigated the world of “start-ups” and “tech transfer” opportunities.
Participation in SPRINT was an intensive process over 8 weeks where the Witness CARES team was forced to confront what had been (for years) an academic research study and begin to conceptualize the potential for it to be a commercially successful business. This work required intensive “get out of the building” experiences to determine who the real customer is (and is not)—that is, who would be willing to pay for Medicaid patients to get CRC screening. In consultation with the IRB chair at the PI’s institution, it was determined that stakeholder interviews could be considered a “quality improvement” process and therefore the team did not need to obtain IRB approval or signed informed consent to conduct the planned interviews.
Through 51 in-person conversations and calls conducted by telephone/Skype, various stakeholders (e.g., insurance executives, primary care physicians, Medicaid patients, gastroenterologists, and hospital executives) were interviewed to obtain data on potential customer “Pains and Gains.” With regard to CRC screening, “pains” for insurers and healthcare providers were quickly determined to be all of the patients 50–75 years of age who had not completed either a stool test or colonoscopy and were therefore considered “gaps in care” patients. Notably, the highest proportion of these insured patients were those covered by Medicaid. Therefore, the “gains” for insurers and providers would be to have more Medicaid patients complete CRC screening and reduce these “gaps in care.” We discovered that insurers and providers were penalized for having a large proportion of patients classified as “gaps in care,” and therefore had a lot to “gain” from addressing this problem. “Pains” for patients included not understanding how to prepare for the test(s), and not knowing how to obtain a colonoscopy appointment, where to go, how to get there, or how to get home after undergoing a colonoscopy. The “gains” for patients were described as preventing cancer (through colonoscopy) or knowing they did not have signs of cancer (through stool testing).
Customer Discovery data were collected through methods similar to qualitative interviews, but rather than using a structured or semi-structured interview, the questions and format varied by stakeholder, by where the team was in the process, by data that had been obtained previously, and other factors. This type of intensive market research is an unfamiliar approach for NIH investigators. Key insights from this customer discovery process included the following: (a) how CRC screening can be improved for Medicaid patients is a mystery to most primary care physicians (PCPs) and insurers; (b) PCPs and insurance companies are well incentivized to increase screening in these underscreened patient groups (referred to as “gaps in care”); and (c) health insurance companies will pay for someone else to help improve these screening rates.
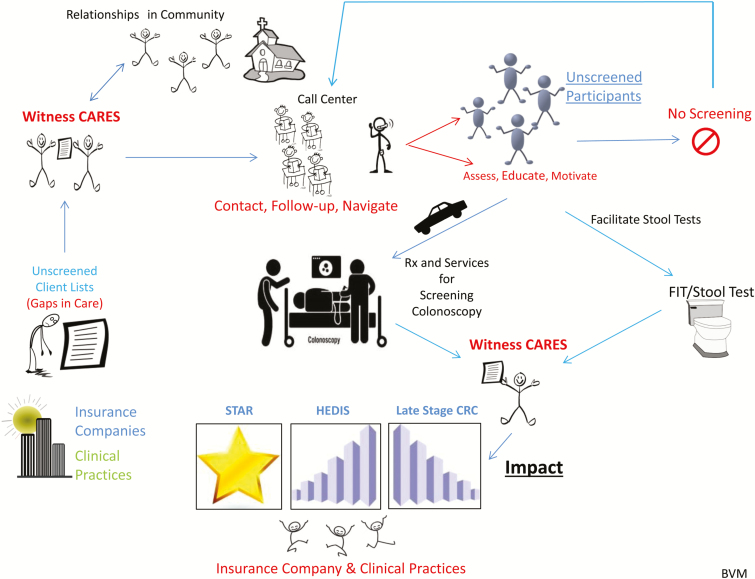
The customer discovery process resulted in changes to business development strategies as well. The Witness CARES team recognized that patient acquisition would now need to focus on addressing the insurers’ “gaps in care” patient list, rather than relying on the community-based approaches used in the R01 study. Additionally, scalability of the business would require development of thoughtful, tested process measures and directives which were not part of the R01 study. The SPRINT process was very productive—resulting in a composition of facts defining and/or informing the team about customers, services (i.e., the Value Proposition), technology needs, delivery of services, revenue streams, and cost structure, as well as generating an outline for a Minimal Viable Product (Fig. 2) and a business model canvas draft.
Fig. 2.
| Minimal Viable Product (MVP) for Witness CARES, LLC.
Next steps
The SPRINT experience inspired the Witness CARES team to incorporate in New York State in October 2016 as a Limited Liability Corporation (LLC) with Johnson (51%) and Erwin (49%) as partners.
Witness CARES, LLC is a business that helps people by facilitating access to healthcare—initially CRC screening—particularly for those least likely to obtain screening on their own (i.e., Medicaid patients). Financed by contracts from health insurance companies and primary care practices, Witness CARES focuses on building social health capital among health insurers, clinical practices, and their African American and lower income/Medicaid patients to optimize access to and use of quality clinical services. Witness CARES achieves its mission through providing personalized, culturally/racially-customized services (e.g., education, navigation, transportation) for end users (i.e., patients) while improving metrics and cost-effective access for its customers (i.e., health insurance companies and primary care physicians). The company has pending certification from New York State for Minority Women Business Enterprise, African American Business Enterprise, Small Disadvantaged Business, Women Business Enterprise, and Women-Owned Small Business. Johnson and Erwin attended the SBIR/STTR Annual Conferences in 2016 and 2017, developed innovative technology plans for the business, and successfully obtained a Small Business Technology Transfer (STTR) Phase 1 grant.
DISCUSSION
The SPRINT training program teaches behavioral scientists about commercialization, as well as considerations for offering their intervention in the marketplace (whether through a for-profit or mission-based model). The goal of the program is to introduce these researchers to a new method for moving their research into practice and increasing the impact of their EBIs. Scale-up and sustainability are emerging as increasingly important outcomes in the field of implementation science [30,31]. A 2015 review by Milat et al. [31] identified key components of frameworks that specifically focus on health intervention scale-up, such as monitoring and evaluation systems, and strong leadership. SPRINT directly addresses several of the components highlighted in the review, by (a) encouraging researchers to engage with stakeholders (through the customer discovery process), (b) supporting research teams with gathering information that enables their intervention to be tailored to a target market, (c) emphasizing the systematic use of evidence to inform the scale-up approach, (d) providing a structure for creating a well-defined scale-up strategy through the use of the Business Model Canvas framework, (e) underscoring the importance of considering cost, revenue, and other financial aspects of intervention scale-up, and (f) providing infrastructure to support scale-up efforts through the training program itself as well as by connecting program alumni to additional resources.
The three case examples discussed in this article are very different in terms of the product or program the teams were attempting to commercialize, how far along they were in testing the efficacy of their intervention, and what they decided to do with their intervention after the program. However, all three teams felt that they benefited from participating in the program and gained invaluable insights about their intervention and about the healthcare marketplace. There were four major findings from these case studies: (a) what “success” looks like for teams in the SPRINT program varies by type of intervention, maturity of the intervention, and other factors; (b) by focusing on behavioral researchers, SPRINT addresses an unmet need in the commercialization training space; (c) identifying and engaging “payors” for behavioral interventions is an ongoing challenge; and (d) there are potential “misalignments” between the research process and market demand. We expand on each of these findings below.
There is no single model for what it means to be a “successful” SPRINT team. While the curriculum and customer discovery methodology are standardized, the determination of marketplace and “value” varies by the type of the intervention (e.g. exercise equipment vs. a training program for peer educators), as well as stakeholder interests. Even if at the end of the SPRINT program teams decide that their current intervention is not ready or suitable for commercialization, they have gained a new set of tools and a new way of thinking about designing behavioral interventions that will enable them to make their future work more responsive to the needs of various stakeholders (including potential end users and payors). Creating this “paradigm shift” in the way behavioral scientists think about their interventions and encouraging them to think about what they will do with their intervention “beyond the grant cycle” should increase the rate at which federally funded research is translated into practice and increase the number of interventions that have a real-world impact on population health.
The featured case examples also highlight the unique language and orientation needed to discuss the commercialization of behavioral interventions. For example, “competitors” in the context of behavioral interventions may be different in comparison to other scientific innovations, where—for example—behavioral interventions might be competing against similar (but scientifically less rigorous) resources provided by for-profit or nonprofit organizations. SPRINT is unique in that it is geared towards the kinds of “products” that usually emerge from behavior change research—in some instances, like in the case of the ‘assisted’ exercise technology, these products are actual devices, gadgets, or inventions and are easily conceptualized in the standard language of business. However, more often, the outputs of behavioral research are programs, curricula, trainings, educational materials, message or video libraries, and decision aids, that can be more difficult to market and sell. Generally, the teams found that while the potential public health significance of their intervention may be high, the marketplace response to an intervention is not based solely on potential health impact, but also on other considerations that are important to potential stakeholders (such as the intervention’s price, its potential to save costs, etc.).
In a marketplace historically driven by a focus on acute care rather than prevention, identifying a payor or buyer for prevention services can be challenging. However, the healthcare marketplace is progressively placing a larger emphasis on chronic disease, and consequently, demand for chronic disease prevention programs and products will likely increase [32,33]. In the meantime, partnerships and innovative pivots, such as broadening the scope of an intervention to serve different patient populations or to serve multiple needs of a single patient population, may help increase value for payors and bring interventions into practice more quickly.
As the three case examples highlight, finding and creating value for “payors” is especially difficult in the context of behaviorally focused interventions. The target population of the intervention is often not the group that would be expected to pay for the intervention, and so behavioral scientists need to think about ways they can change their product (or change how it is framed and marketed) to appeal to payors such as medical practices or hospital systems, insurers, corporate wellness programs, etc. In some cases, researchers will realize that the changes they would need to make to ensure their intervention is attractive to potential payors would threaten its efficacy or pose other substantial problems that make it impossible to move forward. For example, during the customer discovery process, a researcher may find that insurers would only be interested in an intervention if it were turned into an “app,” but this may make the intervention inaccessible to the low-income, underserved populations for whom the researcher originally developed that intervention. Balancing the demands of the market with ethical concerns, intervention fidelity, and researchers’ desire to contribute to public health (rather than make a profit) is an ongoing challenge.
Models for behavioral research focus on investigating and understanding mechanisms of behavior change in interventions. This work often requires narrowing scope and population. Yet profitability (or even just sustainability) may require pursuing the largest possible market share, which can create a misalignment between scientific goals and what the marketplace may demand. For example, the market may prioritize end-user relevance, but may not place a high value on community engagement. Interventions that were initially developed with community involvement raise important ethical considerations around translating interventions developed through community-based participatory research (CBPR) into the marketplace. As the design of the intervention changes to be more responsive to broader marketplace needs, how can the team continue to engage the community as partners, if at all? Taking a CBPR model and approaches from engineering (such as people-centered design) may be a way to reconcile the goals of community engagement in intervention development with the need to eventually implement the intervention in the real world. Lean start-up models often emphasize “fail fast and often” but in engaged approaches, models that encourage researchers to “fail, get feedback, iterate, repeat” may be more appropriate.
CONCLUSION
While not all behavioral researchers who go through the SPRINT program will want to start their own business, these case examples highlight other positive outcomes that can accrue from efforts to promote knowledge around the commercialization of behavioral interventions, such as opportunities to build partnerships with industry and the healthcare system, making interventions more responsive to the needs of various stakeholders, and encouraging an orientation towards designing interventions with dissemination and implementation in mind. The case studies also show that researchers find it beneficial to learn the “language” of business and to work with people who have business experience and are knowledgeable about the healthcare marketplace. Case examples presented in this paper also uncovered contrasts between a worldview shaped by the standards of research and existing models of commercialization, indicating that a further introduction of the marketplace to science and vice versa could facilitate efforts to more successfully translate behavioral interventions into the marketplace.
Despite these tensions, the case examples (and the consistently high demand for participation in the SPRINT program) demonstrate that there is a high level of interest from behavioral researchers in learning about ways to bring their interventions to scale, including through commercialization, and that researchers perceive a substantial value in having this type of training. SPRINT is currently the only program we are aware of that provides commercialization training specifically for federally funded researchers leading behavioral interventions. Providing more opportunities for behavioral scientists to learn about commercialization and the marketplace for their interventions could greatly increase the number of EBIs that are actually used, which is vital for ensuring that research efforts achieve their ultimate goal of reducing the burden of disease in the population.
Acknowledgements
Project HEAL research is funded by the National Cancer Institute: R01 CA147313. The parent study was approved by the University of Maryland Institutional Review Board (IRB; #10–0691), and the SPRINT initiative was determined by the Chesapeake IRB as exempt (#00021164). We would like to acknowledge Project HEAL team members who participated in SPRINT, including Laundette Jones, Jimmie Slade, Sherie Lou Santos, and Felicia Davenport.
•The REWARD trial is being funded by NIH R01 CA175100 (PI: Nock) and the SPRINT training/ participation was funded through a supplement to the parent trial (R01 CA175100-S1; PI: Nock). We would like to thank all of the “customers” for taking time out of their busy schedules to talk with us.
•Witness CARES research and the SPRINT Supplement were funded by the National Cancer Institute: R01 CA171935. The parent study was approved by Roswell Park Comprehensive Cancer Center Institutional Review Board (IRB # I 159309). We would like to acknowledge the support of Patrick Emmerling, PhD, Roswell Park Technology Transfer and Commercial Development, and the entrepreneurial spirit of Dee Johnson.
Funding: SPRINT is funded by the National Cancer Institute. Activities reported in the article were funded by administrative supplements from the National Cancer Institute as well as a contract with ICF Inc. (HHSN261201400002B; HHSN26100011).
Conflicts of Interest: Authors declare that they have no conflicts of interest.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Activities reported in this manuscript were deemed exempt from IRB oversight under clause 45 CFR 46.101(b)(2) of the Department of Health and Human Services regulation (Chesapeake IRB; Protocol Number 00021164).
Informed Consent: Exempt protocol.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Footnotes
These nine components are: key partnerships, key activities, key resources, value proposition, customer relationships, channels, customer segments, cost structure, and revenue streams.
References
- 1. Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med. 2012;4(127):127rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blank S. Innovation Corps: A Review of a New National Science Foundation Program to Leverage Research Investments. Subcommittee on Research and Science Education Committee on Science, Space, and Technology, Washington, DC: U.S. House of Representatives. [Google Scholar]
- 3.Gaysynsky A, Oh A, Vinson CA. Development and evaluation of the SPeeding Research-tested INTerventions (SPRINT) training program. Transl Behav Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osterwalder A, Pigneur Y.. Business Model Generation: A Handbook for Visionaries, Game Changers, and Challengers. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 5. Blank S, Dorf B.. The Startup Owner’s Manual: The Step-by-step Guide for Building a Great Company. Pescadero, CA: BookBaby; 2012. [Google Scholar]
- 6. Pfadenhauer LM, Gerhardus A, Mozygemba K, et al. Making sense of complexity in context and implementation: The Context and Implementation of Complex Interventions (CICI) framework. Implement Sci. 2017;12(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lobb R, Colditz GA. Implementation science and its application to population health. Annu Rev Public Health. 2013;34:235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holt CL, Tagai EK, Scheirer MA, et al. Translating evidence-based interventions for implementation: Experiences from Project HEAL in African American churches. Implement Sci. 2014;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holt CL, Klem PR. As you go, spread the word: Spiritually based breast cancer education for African American women. Gynecol Oncol. 2005;99(3 Suppl 1):S141–S142. [DOI] [PubMed] [Google Scholar]
- 10. Holt CL, Litaker MS, Scarinci IC, et al. Spiritually based intervention to increase colorectal cancer screening among African Americans: Screening and theory-based outcomes from a randomized trial. Health Educ Behav. 2013;40(4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holt CL, Wynn TA, Litaker MS, Southward P, Jeames S, Schulz E. A comparison of a spiritually based and non-spiritually based educational intervention for informed decision making for prostate cancer screening among church-attending African-American men. Urol Nurs. 2009;29(4):249–258. [PMC free article] [PubMed] [Google Scholar]
- 12. Santos SL, Tagai EK, Scheirer MA, et al. Adoption, reach, and implementation of a cancer education intervention in African American churches. Implement Sci. 2017;12(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santos SL, Tagai EK, Wang MQ, Scheirer MA, Slade JL, Holt CL. Feasibility of a web-based training system for peer community health advisors in cancer early detection among African Americans. Am J Public Health. 2014;104(12):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holt CL, Tagai EK, Santos SLZ, et al. Web-based versus in-person methods for training lay community health advisors to implement health promotion workshops: Participant outcomes from a cluster-randomized trial. Transl Behav Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osterwalder A, Pigneur Y, Bernarda G, Smith A.. Value Proposition Design: How to Create Products and Services Customers Want. Hoboken, NJ: John Wiley & Sons; 2014. [Google Scholar]
- 16. Jones LP, Slade JL, Davenport F, Santos SLZ, Holt CL. Planning for scale-up of an evidence-based intervention in community settings: Project HEAL insights from the SPRINT initiative. Health Promot Pract.; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Browne D. Scaling Up, Staying True: A Wallace Conference Report on Spreading Innovations in Expanded Learning. New York, NY: Wallace Foundation; 2014 [Google Scholar]
- 18. Barker PM, Reid A, Schall MW. A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci. 2016;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milat AJ, King L, Newson R, et al. Increasing the scale and adoption of population health interventions: Experiences and perspectives of policy makers, practitioners, and researchers. Health Res Policy Syst. 2014;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nock NL, Dimitropoulos A, Rao SM, et al. Rationale and design of REWARD (revving-up exercise for sustained weight loss by altering neurological reward and drive): A randomized trial in obese endometrial cancer survivors. Contemp Clin Trials. 2014;39(2):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alberts JL, Phillips M, Lowe MJ, et al. Cortical and motor responses to acute forced exercise in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linder SM, Rosenfeldt AB, Dey T, Alberts JL. Forced aerobic exercise preceding task practice improves motor recovery poststroke. Am J Occup Ther. 2017;71(2):7102290020p1–7102290020p9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nock NL, Minnes S, Alberts JL. Neurobiology of substance use in adolescents and potential therapeutic effects of exercise for prevention and treatment of substance use disorders. Birth Defects Res. 2017;109(20):1711–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellis EM, Erwin DO, Jandorf L, et al. Designing a randomized controlled trial to evaluate a community-based narrative intervention for improving colorectal cancer screening for African Americans. Contemp Clin Trials. 2018;65:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiviniemi MT, Klasko-Foster LB, Erwin DO, Jandorf L. Decision-making and socioeconomic disparities in colonoscopy screening in African Americans. Health Psychol. 2018;37(5):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiviniemi MT, Jandorf L, Erwin DO. Disgusted, embarrassed, annoyed: Affective associations relate to uptake of colonoscopy screening. Ann Behav Med. 2014;48(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erwin DO. The Witness Project: Narratives that shape the cancer experience for African American women. Paper presented at: The In Confronting Cancer: Metaphors, Advocacy, and Anthropology; 2009; Sante Fe, CA: School for Advanced Research Seminar Series. [Google Scholar]
- 28. Erwin DO, Ivory J, Stayton C, et al. Replication and dissemination of a cancer education model for African American women. Cancer Control. 2003;10(5 Suppl):13–21. [DOI] [PubMed] [Google Scholar]
- 29. Erwin DO, Spatz TS, Stotts RC, Hollenberg JA. Increasing mammography practice by African American women. Cancer Pract. 1999;7(2):78–85. [DOI] [PubMed] [Google Scholar]
- 30. Proctor E, Luke D, Calhoun A, et al. Sustainability of evidence-based healthcare: Research agenda, methodological advances, and infrastructure support. Implement Sci. 2015;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milat AJ, Bauman A, Redman S. Narrative review of models and success factors for scaling up public health interventions. Implement Sci. 2015;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaw FE, Asomugha CN, Conway PH, Rein AS. The patient protection and affordable care act: Opportunities for prevention and public health. Lancet. 2014;384(9937):75–82. [DOI] [PubMed] [Google Scholar]
- 33. Health Research & Educational Trust. Managing population health: The role of the hospital 2012. Available at http://www.hpoe.org/Reports-HPOE/managing_population_health.pdf. Accessibility verified October 10, 2018.