Abstract
Background
Immunocompromised patients infected with influenza virus require prolonged treatment with neuraminidase inhibitors, because these patients are not able to eradicate the virus from the respiratory tract, leading to the emergence of drug-resistant mutant viruses.
Methods
In this study, we examined the efficacy of baloxavir marboxil in nude mice that were immunologically deficient.
Results
Daily treatment with a suboptimal dose of baloxavir marboxil increased the survival time of the virus-infected nude mice but did not clear the virus from their respiratory organs, resulting in gradual body weight loss after termination of treatment.
Conclusions
Despite the prolonged baloxavir marboxil treatment, few resistant mutants were detected.
Keywords: baloxavir marboxil, drug resistance, immunocompromised, influenza
Prolonged baloxavir marboxil treatment increased the survival time of virus-infected immunocompromised mice but did not clear the virus from their respiratory organs. Despite the prolonged treatment, few resistant mutants were detected.
Treatment of seasonal influenza has relied heavily on neuraminidase (NA) inhibitors, which target the viral NA activity of the NA protein. In 2018, the novel antiviral baloxavir marboxil, which targets the viral endonuclease activity of the PA protein, was approved in Japan and other countries including the United States. Between October 2018 and January 2019 in Japan, baloxavir marboxil was supplied to medical facilities to treat approximately 5.5 million patients [1]. Although treatment with baloxavir marboxil reduced the viral load compared with treatment with an NA inhibitor within 1 day of initiation of treatment, the time to influenza symptom alleviation was similar between baloxavir marboxil and the NA inhibitor [2]. However, detection of viruses that exhibited reduced susceptibility to baloxavir marboxil were frequently reported in immunocompetent patients [3]. This reduced susceptibility was caused by an amino acid substitution of isoleucine to threonine, methionine, or phenylalanine at position 38 of the PA protein [2–4].
METHODS
To treat immunocompromised patients, NA inhibitors have been used, but they fail to achieve virus eradication from the respiratory tract [5–7]. Prolonged treatment with NA inhibitors frequently leads to the selection of viruses that are resistant to these drugs [5–7]. The resistant viruses, in turn, compromise the effectiveness of treatment with NA inhibitors. Because viruses harboring a substitution (isoleucine to threonine, methionine, or phenylalanine) at position 38 in PA could cause influenza even in immunocompetent individuals [1], the emergence of viruses with reduced susceptibility to baloxavir marboxil would be problematic, especially for immunocompromised patients [2–4, 8]. Animal models of immunocompromised hosts have been developed and used in influenza virus research [9–11]. One of these animal models is the nude mouse, which lacks a thymus and cannot generate mature T cells. In nude mice infected with influenza virus, virus clearance is delayed and the survival rate is reduced. Treatment with NA inhibitors and/or the virus polymerase inhibitor favipiravir increases the survival time but fails to prevent virus replication in the respiratory tract of the infected mice, resulting in frequent emergence of viruses resistant to the NA inhibitors but sensitive to favipiravir [12].
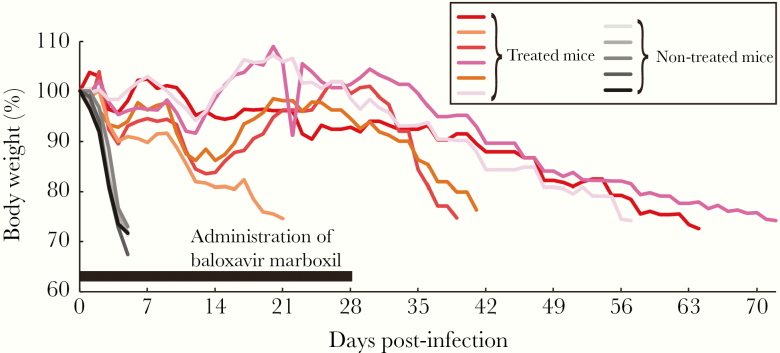
In this study, we used nude mice to assess the therapeutic value of baloxavir marboxil in an immunocompromised host infected with influenza virus. Eleven 6-week-old female nude mice (BALB/c-nu/nu; Japan SLC Inc.) were anesthetized with isoflurane and intranasally infected with 104 plaque-forming units (PFUs) of mouse-adapted A/California/04/2009 (H1N1pdm09; MA-CA04) [13]. Six of the infected mice were treated orally with baloxavir marboxil (10 mg/kg) once a day for 28 days because virus clearance is delayed in nude mice, requiring prolonged treatment with antivirals (cf, the standard treatment with baloxavir marboxil is a single dose for outpatients). The remaining 5 untreated mice served as controls. Body weight changes of these mice were monitored for 72 days, and mice that lost 25% or more of their initial body weight were scored as dead and euthanized according to institutional guidelines. All animal experiments were conducted in accordance with the University of Tokyo’s Regulations for Animal Care and Use, which were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo. Although untreated mice died within 5 days of infection, treatment with baloxavir marboxil significantly increased survival times; the median survival time was 5 days versus 49 days (P = .0016, log-rank test) (Figure 1). During baloxavir marboxil treatment, the body weights of the infected mice ranged from approximately 80% to 110% of the initial weights (Figure 1). One mouse died at 21 days postinfection despite treatment. The other 5 mice survived for the duration of the 28 day-treatment period, but they began to lose body weight once treatment was terminated, and they died within 39–72 days of infection. These results indicate that 28-day treatment with baloxavir marboxil at 10 mg/kg once a day does not eliminate influenza virus from the respiratory tract of infected nude mice.
Figure 1.
Body weight change of nude mice infected with MA-CA04. Eleven mice were infected with 104 plaque-forming units of MA-CA04. Six mice (red-based colored lines) were treated with baloxavir marboxil (10 mg/kg) for 28 days, and the remaining 5 mice were left untreated (black-based colored lines) to serve as controls.
RESULTS
Because all of the mice succumbed to influenza virus infection after the termination of treatment, we next assessed virus titers in the lungs of infected mice at 7 and 28 days postinfection to evaluate the degree of virus clearance. Four nude mice per time point were anesthetized with isoflurane, intranasally infected with 104 PFU of MA-CA04, and treated orally with baloxavir marboxil (10 mg/kg) once a day for 28 days. These mice were then euthanized on day 7 and 28 postinfection, and virus titers in the lungs were determined by using plaque assays in Madin-Darby canine kidney (MDCK) cells. The virus tiers in the lungs of the mice at day 7 postinfection were 7.1–7.5 log10 PFU/g (Table 1). The virus titers in the lungs of the mice at termination of treatment (day 28) decreased to 4.7–5.9 log10 PFU/g, whereas those in the lungs of the mice that died after termination of treatment increased to 6.1–7.0 log10 PFU/g (Table 1). The virus titers in the lungs of the mice at termination of treatment were significantly lower than those in the lungs of the mice at day 7 postinfection and that died after 28 days postinfection (P < .01, one-way analysis of variance followed by Tukey’s multiple comparisons test), indicating that treatment with baloxavir marboxil restricts virus replication to some extent and virus titers rebound after termination of treatment. These results suggest that treatment with baloxavir marboxil for 28 days fails to achieve virus clearance in the nude mouse model.
Table 1.
Titer, Sensitivity, and Mutations in the PA Protein of Viruses in the Lungs of Baloxavir Marboxil-Treated Mice
| Day Postinfection | Outcome | Lung Virus Titer (Log10 PFU/g) | IC50 Valuea (nM) | Amino Acid Mutations in the PA of 9 Plaque-Purified Viruses (%) |
|---|---|---|---|---|
| 7 | Survived | 7.4 | 1.0 | ND |
| 7 | Survived | 7.1 | 1.3 | ND |
| 7 | Survived | 7.5 | 1.4 | ND |
| 7 | Survived | 7.2 | 1.3 | ND |
| 28 | Survived | 5.9 | 8.2 | No mutations |
| 28 | Survived | 4.7 | 4.5 | No mutations |
| 28 | Survived | 5.5 | 5.2 | E199Gb (22.2) |
| 28 | Survived | 5.8 | 3.5 | No mutations |
| 21 | Died | 5.2 | 2.3 | I38M b (11.1) |
| 39 | Died | 6.1 | 2.1 | ND |
| 41 | Died | 6.6 | 4.4 | ND |
| 57 | Died | 7.0 | 4.7 | ND |
| 64 | Died | 6.7 | 3.4 | ND |
| 72 | Died | 6.9 | 1.5 | ND |
Abbreviations: IC50, half-maximal inhibitory concentration; ND, not done; PFU, plaque-forming units.
aThe IC50 values to baloxavir acid of viruses isolated from the lung of mice.
bThe IC50 values of the I38M and E199G mutant viruses, which were plaque-purified from isolated viruses, to baloxavir acid were 122 and 2.3 nM, respectively. The IC50 value of the wild-type virus to baloxavir acid was 4.2 nM.
Discussion
Drug-resistant mutant viruses are known to appear after the long-term treatment of influenza virus-infected immunocompromised patients with NA inhibitors [5–7]. To examine whether drug-resistant mutants emerge in nude mice treated with baloxavir marboxil, we assessed the drug sensitivity of the viruses that were isolated from the lung samples (Table 1). In brief, confluent MDCK cells in 6-well plates were infected with the isolated viruses (approximately 50 PFU). After infection, the viral inoculum was removed and the cells were overlaid with minimal essential medium containing 0.3% bovine serum albumin, 1% agarose, 1 μg/mL tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin, and various concentrations (0.01–1000 nM) of baloxavir acid. The plates were incubated for 2–3 days, and plaques were counted for calculation of half-maximal inhibitory concentration (IC50) values. We found that the IC50 values were not significantly increased (Table 1). To detect minor population of resistant mutants, we performed plaque purification using the lung samples from 4 mice that were euthanized on day 28 and from 1 mouse that died on day 21. Nine plaques were picked from each of the 5 samples, so a total of 45 picked viruses were propagated in MDCK cells. Viral ribonucleic acid (RNA) was extracted from the supernatants of the virus-infected MDCK cells by using the QIAamp Viral RNA Mini Kit (QIAGEN). Reverse-transcription polymerase chain reaction (RT-PCR) was performed by using the Superscript Ⅲ One-Step RT-PCR System with Platinum Taq deoxyribonucleic acid polymerase (Invitrogen) and primers specific for the PA segment of MA-CA04. The PCR products were purified by use of a MinElute Gel Extraction kit (QIAGEN) and sequenced with the BigDye terminator 3.1 kit on an ABI 3130xl (Applied Biosystems). We found that 1 plaque-purified virus possessed the I38M mutation in PA and 2 plaque-purified viruses possessed the E199G mutation in PA (Table 1). Therefore, we examined the sensitivity of the I38M and E199G mutants to baloxavir acid in the plaque reduction assay. The IC50 values of the I38M and E199G mutants to baloxavir acid were 122 and 2.3 nM, respectively, whereas that of the wild-type virus was 4.2 nM, suggesting that the I38M mutation confers resistance to baloxavir marboxil, but the E199G mutation does not. Although previous reports [3, 14] showed that the E199G mutation increased IC50 values by 3–5 times compared with that of the wild-type virus, this difference in the contribution of the E199G mutation to baloxavir acid sensitivity may be strain-dependent. This I38M mutant was 1 of 9 plaque-purified clones of virus isolated from the lungs of a mouse that died at 21 days postinfection; this day 21 virus population as a whole was sensitive to baloxavir acid in vitro, suggesting that the contribution of a minor population of resistant virus to the death of this mouse during treatment may not be high. Our results show that prolonged baloxavir marboxil treatment of immunocompromised mice does not completely suppress viral replication, but baloxavir marboxil-resistant viruses rarely emerge under these conditions.
In this study, we examined the efficacy of baloxavir marboxil treatment in an immunocompromised mouse infection model. Prolonged (ie, 28-day) treatment with baloxavir marboxil improved the survival time of infected nude mice. In a similar study using nude mice, the polymerase inhibitor favipiravir increased the survival time of the infected mice, but NA inhibitors did not [12], suggesting that the efficacy of baloxavir marboxil is similar to that of favipiravir and superior to that of NA inhibitors in this model. However, treatment with baloxavir marboxil for 28 days failed to eradicate the virus from the infected nude mice, resulting in gradual weight loss after termination of treatment. Because monotherapy with baloxavir marboxil is insufficient for virus clearance, combination therapy with baloxavir marboxil and other antivirals, such as favipiravir or an NA inhibitor, may be required.
Prolonged treatment of the immunocompromised host with antivirals always presents the potential for the emergence of resistant mutants. For baloxavir marboxil, resistant viruses frequently emerge in immunocompetent patients [2–4], and the PA-I38T/M mutation is known to increase the IC50 value by 10- to 50-fold [3, 4]. Although nude mice were treated with baloxavir marboxil for 28 days, we detected no resistant isolates out of 10 lung samples and only 1 PA-I38M mutant clone out of 45 plaque-purified clones. In similar studies of prolonged treatment with an NA inhibitor plus favipiravir, viruses frequently became resistant to the NA inhibitor but not to favipiravir [12]. Collectively, the frequency of emergence of virus resistance to baloxavir marboxil is lower than that to the NA inhibitors in the nude mouse model. However, it has been reported that viruses resistant to baloxavir marboxil occasionally emerge within a few days of treatment of human infections [2–4, 8]. In humans, single oral administration of 40 mg of baloxavir marboxil is required to maintain the plasma concentration of baloxavir acid above 6.85 ng/mL for 5 days to exert greater virus reduction compared with oseltamivir [15]. Because the half-life of baloxavir acid in mouse plasma (2.26 hours at 15 mg/kg oral administration) is quite shorter than that in human plasma (85.9 hours at 40-mg single oral administration), oral administration of baloxavir marboxil at 15 mg/kg twice daily for 5 days, which should maintain the plasma concentration of baloxavir acid above 6.85 ng/mL in mice, is considered as a clinically equivalent administration in mice [15]. The plasma concentration of baloxavir acid in our nude mouse study (oral administration of baloxavir marboxil at 10 mg/kg once a day for 28 days) was likely lower than that of clinically equivalent administration (ie, 6.85 ng/mL). We used this dose (ie, 10 gm/kg once a day) to mimic suboptimal dosing conditions. However, resistant viruses rarely emerged under such conditions. This discrepancy in the level of the emergence of resistant viruses between human cases and our nude mouse model is interesting and requires further study.
Conclusions
In summary, treatment with baloxavir marboxil increased the survival time of influenza virus-infected immunocompromised mice but did not result in virus clearance. Novel antiviral approaches, such as combination therapy of baloxavir marboxil and an NA inhibitor [15], are needed to eradicate influenza virus from immunocompromised hosts to help to reduce the possibility of the emergence of resistant viruses in such hosts.
Notes
Acknowledgments. We thank Dr. Kohei Oishi for assistance with experiments and Dr. Susan Watson for editing the manuscript.
Financial support. This work was funded by Leading Advanced Projects for medical innovation (LEAP) (JP19am001007), the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) (JP19fm0108006), a Research Program on Emerging and Re-emerging Infectious Diseases (JP17jm0210042) from the Japan Agency for Medical Research and Development (AMED), Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan (Nos. 16H06429, 16K21723, and 16H06434), and the Center for Research on Influenza Pathogenesis funded by National Institute of Allergy and Infectious Diseases Contract HHSN272201400008C.
Potential conflicts of interest. Y. K. has received speaker’s honoraria from Toyama Chemical and Astellas Inc. and grant support from Chugai Pharmaceuticals, Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Otsuka Pharmaceutical Co., Ltd, Denka Seiken Co., Ltd, and Shionogi & Co., Ltd. Y. K. is also co-founder of FluGen. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Takashita E, Kawakami C, Ogawa R, et al. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Euro Surveill 2019; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 3. Omoto S, Speranzini V, Hashimoto T, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep 2018; 8:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takashita E, Kawakami C, Morita H, et al. Detection of influenza A(H3N2) viruses exhibiting reduced susceptibility to the novel cap-dependent endonuclease inhibitor baloxavir in Japan, December 2018. Euro Surveill 2019; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renaud C, Boudreault AA, Kuypers J, et al. H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerg Infect Dis 2011; 17:653–60; quiz 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis 2009; 199:1435–41. [DOI] [PubMed] [Google Scholar]
- 7. van der Vries E, Stelma FF, Boucher CA. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med 2010; 363:1381–2. [DOI] [PubMed] [Google Scholar]
- 8. Uehara T, Hayden FG, Kawaguchi K, et al. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 2020. 221:63–70. [DOI] [PubMed] [Google Scholar]
- 9. Baz M, Carbonneau J, Rheaume C, Cavanagh MH, Boivin G. Combination therapy with oseltamivir and favipiravir delays mortality but does not prevent oseltamivir resistance in immunodeficient mice infected with pandemic A(H1N1) influenza virus. Viruses 2018; 10:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ison MG, Mishin VP, Braciale TJ, Hayden FG, Gubareva LV. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J Infect Dis 2006; 193:765–72. [DOI] [PubMed] [Google Scholar]
- 11. Fukao K, Ando Y, Noshi T, et al. Baloxavir marboxil, a novel cap-dependent endonuclease inhibitor potently suppresses influenza virus replication and represents therapeutic effects in both immunocompetent and immunocompromised mouse models. PLoS One 2019; 14:e0217307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiso M, Lopes TJS, Yamayoshi S, et al. Combination therapy with neuraminidase and polymerase inhibitors in nude mice infected with influenza virus. J Infect Dis 2018; 217:887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakabe S, Ozawa M, Takano R, Iwastuki-Horimoto K, Kawaoka Y. Mutations in PA, NP, and HA of a pandemic (H1N1) 2009 influenza virus contribute to its adaptation to mice. Virus Res 2011; 158:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gubareva LV, Mishin VP, Patel MC, et al. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Euro Surveill 2019; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukao K, Noshi T, Yamamoto A, et al. Combination treatment with the cap-dependent endonuclease inhibitor baloxavir marboxil and a neuraminidase inhibitor in a mouse model of influenza A virus infection. J Antimicrob Chemother 2019; 74:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]