Abstract
Background
Entamoeba histolytica kills human cells by ingesting fragments of live cells until the cell eventually dies, a process termed amebic trogocytosis. In a previous study, we showed that acidified amebic lysosomes are required for both amebic trogocytosis and phagocytosis, as well as cell killing.
Methods
Amebic cysteine proteases (CPs) were inhibited using an irreversible inhibitor, E-64d.
Results
Interfering with amebic CPs decreased amebic trogocytosis and amebic cytotoxicity but did not impair phagocytosis.
Conclusions
We show that amebic CPs are required for amebic trogocytosis and cell killing but not phagocytosis. These data suggest that amebic CPs play a distinct role in amebic trogocytosis and cell killing.
Keywords: amebiasis, amebic trogocytosis, Entamoeba histolytica
Entamoeba histolytica kills by amebic trogocytosis. We show that cysteine proteases (CPs) are required for trogocytosis and cell killing but not phagocytosis. CPs inhibition decreased trogocytosis, but not phagocytosis, suggesting that CPs play a distinct role in amebic trogocytosis.
Human amebiasis, caused by the highly cytotoxic protozoan parasite Entamoeba histolytica, is characterized by massive intestinal ulceration or abscesses in multiple sites [1]. Entamoeba histolytica is able to kill human cells by ingesting fragments of the live human cell, resulting in cell death, a process termed amebic trogocytosis [2]. Trogocytosis involves the rapid transfer (within minutes) of cell fragments but not whole cells [3]. It is interesting to note that E histolytica has been shown to preferentially trogocytose live human cells and phagocytose dead human cells [2].
Amebic trogocytosis is initiated by the interaction of the parasite surface protein Gal/GalNAc lectin with the target cell. Ingestion of cell fragments containing cell membrane, cytoplasm, and mitochondria continues until the target cell dies [2]. Amebic trogocytosis is an active process, requiring a functional parasite actin cytoskeleton, signaling via phosphatidylinositide 3-kinase (PI3K) and C2-domain containing protein kinase (C2PK) and the AGC family kinase EhAGCK1 [1, 4]. The precise mechanism of amebic trogocytosis has not been determined.
We have previously shown that the inhibition of lysosomal acidification significantly impairs both amebic trogocytosis and phagocytosis, as well as cell killing [5], potentially by impairing the function of pH-dependent lysosomal proteases [6, 7]. To test the hypothesis that functional amebic lysosomes are essential for the efficient degradation of cell material ingested via trogocytosis, we investigated the impact of cysteine protease (CP) inhibitors on trogocytosis and cell killing. Amebic lysosomal CPs are involved in the digestion of material acquired by phagocytosis, including bacteria and human cells [8]. Using imaging flow cytometry to quantitatively assess the rates of trogocytosis and host cell killing, we found that inhibition of amebic CPs significantly decreased amebic trogocytosis and cell killing. We were surprised to find that inhibition of amebic CPs had no impact on phagocytosis, suggesting a unique role for CPs in trogocytosis.
METHODS
Cell Culture
Amebic trophozoites (HM1:IMSS) and human Jurkat T cells (Clone E6-1; ATCC) were cultured and harvested as previously described [5].
Amebic Trogocytosis and Cell Killing Assay
Amebic trogocytosis was measured as described previously with some modifications [2, 5]. In brief, amebae were labeled with 200 nM CellTracker Green 5-chloromethylfluorescein diacetate ([CMFDA] Invitrogen) then treated with 100 µM E-64d (EST; EMD Millipore) or an equal volume of dimethyl sulfoxide (DMSO) for 1 hour at 37°C. Jurkat cells were labeled with 5 µM DiIC18(5)-DS (DiD; Molecular Probes). The CMFDA-labeled amebae pretreated with E-64d or DMSO were washed twice with M199s and then coincubated with DiD-labeled Jurkat cells in biological duplicate at 37°C for 5 minutes, 20 minutes, or 40 minutes in M199s. At the end of each time point, samples labeled with live/dead fixable violet (Invitrogen) then fixed with 4% paraformaldehyde as previously described [5]. Flow cytometry was performed using ImageStreamX Mark II (EMD Millipore) as previously described [5]. Example images in Figures 1 and 2 have been cropped to minimize blank space.
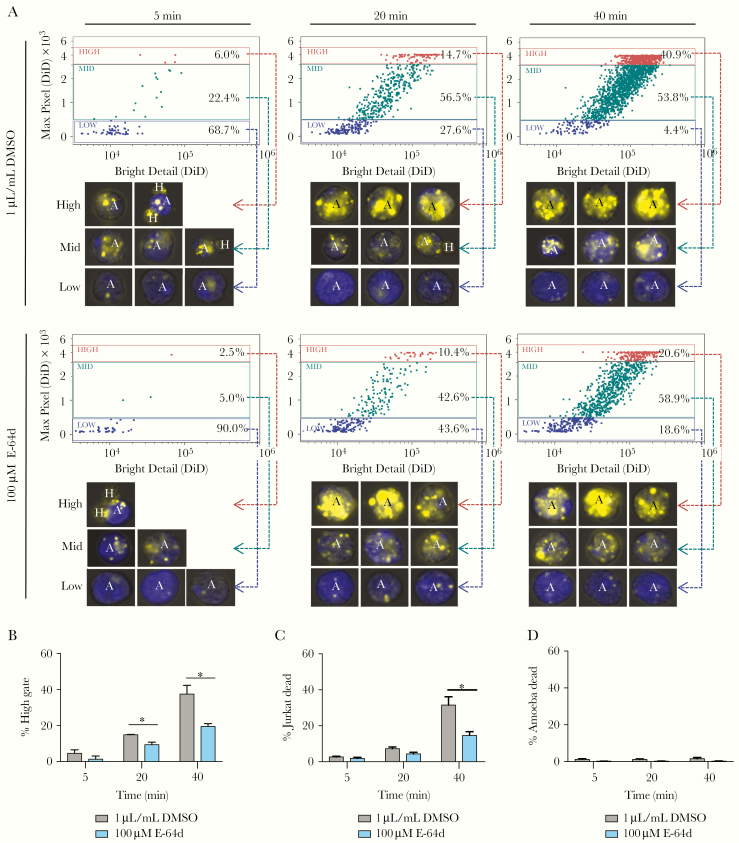
Figure 1.
E-64d treatment decreases amebic trogocytosis and cell killing. Amebae labeled with CMFDA (blue, amines) were pretreated with 100 µM E-64d or 1 µL/mL dimethyl sulfoxide ([DMSO] vehicle) for 1 hour, washed, and incubated with DiD (yellow, membrane) Jurkat-T cells for 5, 20, or 40 minutes at 37°C in media without inhibitor or vehicle. Afterwards, all cells were stained with Live/Dead Violet on ice for 30 minutes and then fixed. Amebic trogocytosis and cell killing were analyzed using imaging flow cytometry. (A) Measurement of fragmentation of ingested material over time. Representative images are shown for each gate. (B) Percent of high gate measures the percentage of events in the high gate, reflecting the quantity of fragments that have been ingested by the parasites. (C) Percent of Jurkat dead measures the percentage of host cells staining with Live/Dead Violet. Means and standard deviations are for biological duplicates. (D) % ameba dead measures the percentage of amebae staining with Live/Dead Violet. Data shown are representative of 5 independent experiments. Data were analyzed by Student’s t test using Prism 6. *, P ≤ .05.
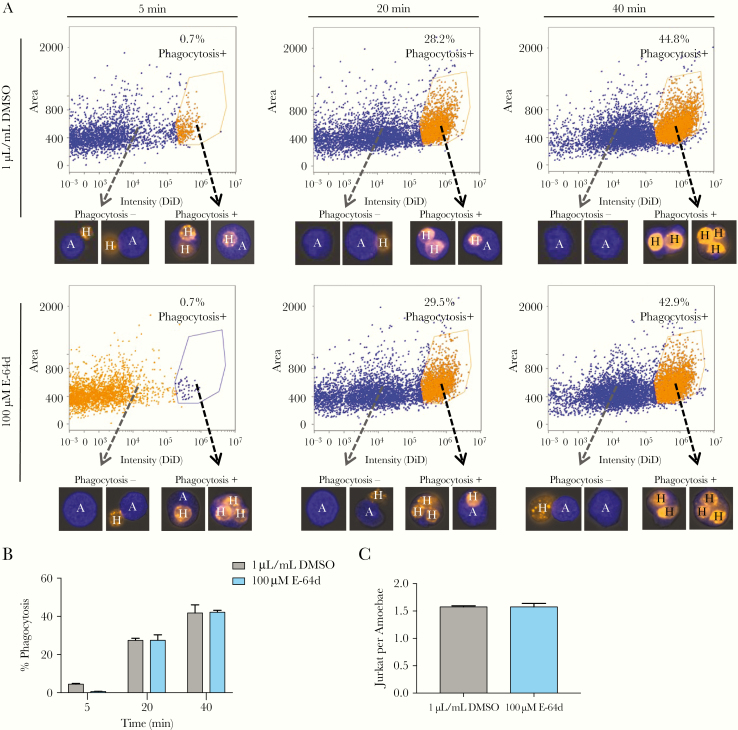
Figure 2.
E-64d treatment does not affect phagocytosis. CMFDA-labeled (blue, amines) amebae pretreated with 100 µM E-64d or 1 µL/mL dimethyl sulfoxide ([DMSO] vehicle) for 1 hour were washed and incubated with heat-killed DiD-labeled (orange, membrane) Jurkat-T cells for 5, 20, or 40 minutes at 37°C in media without inhibitor or vehicle. After coincubation, all cells were fixed. Phagocytosis was analyzed using imaging flow cytometry. (A) Measurement of phagocytosis over time. Representative images are shown for each population. (B) Percent phagocytosis measures the percentage of single amebae that ingested 1 or more Jurkat-T cells. (C) The number of ingested Jurkats per ameba was counted manually (100 images/sample). Means and standard deviations are for biological duplicates. Data shown are representative of 3 independent experiments. Data were analyzed by Student’s t test using Prism 6.
Phagocytosis Assay
Jurkat cells were labeled with 5 µM DiD and then incubated at 55°C for 15 minutes to induce necrotic cell death as previously described [2]. Amebae were labeled with 200 nM CMFDA and then treated with 100 µM E-64d or an equal volume of DMSO as a vehicle control for 1 hour at 37°C. Amebae were washed with M199s and coincubated with DiD-labeled, heat-killed Jurkat cells in M199s at 37°C. Cells were fixed with 4% paraformaldehyde, and flow cytometry was performed using ImageStreamX Mark II as previously described [5]. Focused events were selected and enriched for single events, then single amebae and clusters of amebae single events were plotted as previously described [2]. “Single amebae” were plotted based on area and intensity of DiD staining to distinguish between “phagocytosis+” amebae (containing ≥1 Jurkat cells) and “phagocytosis−” (containing no Jurkat cells). “% Phagocytosis+ amebae” was calculated by dividing the number of “phagocytosis+ amebae” by the number of “single amebae” and multiplying by 100. The number of ingested Jurkats per ameba in the first 100 phagocytosis+ images was counted manually. Example images in Figure 2 were cropped to minimize blank space.
Cathepsin B Activity Assay
Cathepsin B activity was assessed using a fluorometric Cathepsin B Activity Assay Kit (abcam) according to the manufacturer’s directions with some modifications. IN brief, amebae were treated with E-64d or an equal volume of DMSO for 1 hour at 37°C and then resuspended at 2 × 105 cells/mL in M199S. A total of 500 µL aliquots was immediately flash frozen in liquid nitrogen. Additional aliquots were washed, incubated in M199s for 40 minutes at 37°C, then flash frozen in liquid nitrogen, and stored at −80°C. For cell lysis, samples were thawed on ice, washed with ice-cold phosphate-buffered saline, resuspended in 50 µL chilled CB Cell lysis buffer (abcam), and then incubated at 4°C for 15 minutes. Samples were centrifuged for 5 minutes at 4°C at maximum speed, and the supernatant was transferred to a clean tube and then stored at −80°C. For the Cathepsin B activity assay, sample lysates were thawed on ice, and an aliquot was diluted 1:100. Reactions were set up in a black, clear-bottom, 96-well plate (Corning) according to the manufacturer’s instructions. The plate was incubated at 37°C for 15 minutes and protected from light. Fluorescence output was measured at excitation/emission = 400/505 nm on a fluorescence plate reader (BioTek).
Statistical Analysis
Data were analyzed using Prism 6 (GraphPad Software); *, P ≤ .05; **, P ≤ .01; ***, P ≤ .001.
RESULTS
E-64d Decreases Amebic Trogocytosis and Cell Killing
We previously demonstrated that impairing amebic vesicle acidification drastically decreased amebic trogocytosis and cell killing [5], potentially by impairing amebic CPs that function at low pH. Under culture conditions, the majority (>95%) of amebic CP activity is attributed to 4 CPs (EhCP1, EhCP2, EhCP5, and EhCP7) that are structurally similar to human Cathepsin-L and appear to have human Cathepsin-B like activity [9]. Secreted CPs have been shown to degrade colonic mucus and the extracellular matrix [9]. Intracellular cathepsin-like amebic CPs are localized to lysosomes and phagosomes where they aid in the digestion of phagocytosed material [8].
Previous work has shown that treatment of E histolytica with E-64 or its derivatives, E-64c and E-64d, decreased amebic CP activity and impaired the destruction of a host cell monolayer in vitro, a proxy for tissue disruption [9]. To determine whether amebic CPs are required for continued amebic trogocytosis and cell killing, we inhibited amebic CPs using E-64d. We found that treatment with 100 µM E-64d for 1 hour was sufficient to inhibit 97.1% of amebic Cathepsin B-like CP activity, and this effect is maintained for at least 40 minutes after the removal of the inhibitor (Supplemental Figure S1). The CMFDA-labeled parasites were treated with 100 µM E-64d for 1 hour. The parasites were then washed extensively and coincubated with DiD-labeled human Jurkat T-cells or media alone. Thus, only the amebae, not the Jurkat T-cells, were exposed to E-64d. After coincubation of the parasites with the human cells, all cells were stained with Live/Dead Violet, fixed, and analyzed by imaging flow cytometry (Figure 1). To quantify trogocytosis, we measured fragmentation of the human material within the amebae (Figure 1A) [2].
As expected, 100 µM E-64d treatment dramatically reduced amebic CP activity. In control amebae, the percentage of amebae that ingested a high number of human cell fragments increased over time from 6.0% at 5 minutes to 40.9% at 40 minutes. However, the inhibitor-treated parasites showed significantly less ingestion, with only 20.6% of parasites treated with 100 µM E-64d ingesting a high number of human cell fragments after 40 minutes (Figure 1A and 1B). Treatment with E-64d also decreased cell killing (Figure 1C). This finding is consistent with an earlier report that pretreatment of amebae with 50 µM E-64d for 4 hours significantly reduced cell killing [10]. Treatment with E-64d alone did not directly cause amebic death (Figure 1D). Together, these data suggest an important role for amebic CPs in amebic trogocytosis and cell killing.
E64-d Does Not Impair Phagocytosis
Amebic trogocytosis shares many similarities phagocytosis: they are receptor-dependent, requiring attachment to a human cell via the amebic Gal/GalNAc lectin, and they require amebic actin rearrangement and PI3K signaling [1]. However, the mechanism of trogocytosis remains poorly understood, and to date only 1 protein with a unique role in trogocytosis has been discovered in any organism [4]. Therefore, we assessed the impact of CP inhibition on phagocytosis. Entamoeba histolytica has been shown to preferentially phagocytose dead human cells and trogocytose live human cells [2]. To determine whether CP inhibition impairs phagocytosis, CMFDA-labeled parasites were treated with 100 µM E-64d for 1 hour at 37°C, washed extensively, and coincubated with DiD-labeled heat-killed human Jurkat T-cells. All cells were then fixed, and phagocytosis was assessed using imaging flow cytometry (Figure 2A). We were surprised to find that treatment with 100 µM E-64d did not impair phagocytosis when measured as the percentage of amebae that had phagocytosed ≥1 Jurkat T-cell (Figure 2B) or when measured as the number of ingested Jurkat T-cells per ameba (Figure 2C). Therefore, we conclude that amebic Cathepsin B-like CPs are required for efficient amebic trogocytosis and cell killing but not phagocytosis.
DISCUSSION
Trogocytosis has been observed in a wide range of eukaryotes, yet its basic mechanism remains poorly understood. Entamoeba histolytica is able to kill human cells by ingesting fragments of the live cells via amebic trogocytosis [2]. We have recently discovered that amebic lysosomes play a critical role in both trogocytosis and cell killing [5], although their function remains unclear. In this study, we present evidence that amebic lysosomes may play a crucial role in the efficient degradation of ingested material.
Amebic lysosomal and phagosomal CPs function in the digestion of phagocytosed material [8]. In this work, we have shown that the irreversible CP inhibitor E-64d impairs amebic trogocytosis and cell killing (Figure 1). This is consistent with previous work showing that prolonged treatment of parasites with moderate amounts of E-64d significantly reduced cell killing [10]. Entamoeba histolytica has been shown to acidify phagosomes within 2 minutes, suggesting that lysosomes are rapidly recruited and fuse with the phagosome [7]. Our data are consistent with the hypothesis that the inhibitor-treated parasites fail to efficiently degrade material in the phagolysosome, resulting in reduced ingestion. Continued ingestion of multiple human fragments seems to be required to kill human cells [2], thus treated parasites are also impaired in their ability to kill human cells (Figure 1B).
Treatment with E-64d resulted in approximately a 50% decrease in amebic trogocytosis. In contrast, our previous work using 2 different acidification inhibitors resulted in >90% decrease in amebic trogocytosis [5]. The difference in the degree of inhibition of trogocytosis in these 2 studies may be attributable to the efficiency of the inhibition of lysosomal function by these 2 distinct approaches. Multiple amebic lysosomal functions are expected to be impaired by increased lysosomal pH, because most lysosomal functions are likely dependent on a low pH environment. In contrast, E-64d is a specific inhibitor of a single class of proteases, the CPs [11]. More important, however, these 2 distinct approaches both reduce amebic trogocytosis and cell killing. Taken together, these findings suggest that lysosomal functions, in addition to protease activity, may be required for efficient trogocytosis. The rapid recycling of amebic surface receptors may be one such activity.
CONCLUSIONS
Amebic trogocytosis likely involves an endocytic pathway distinct from that of phagocytosis, requiring trogocytosis-specific signaling and effectors. In previous work, we demonstrated that interfering with vesicle acidification blocked both trogocytosis and phagocytosis. In contrast, we found that CP inhibition significantly reduced trogocytosis (Figure 1) but did not impair phagocytosis (Figure 2). There are several possible distinct roles that CPs might play in trogocytosis: amebic CPs may be required to activate trogocytosis-specific receptors or to expose ligands for trogocytosis-specific signaling. It is also possible that trogosomes (vesicles containing ingested fragments) mature more quickly than phagosomes containing dead human cells; therefore, trogocytosis is more sensitive to protease inhibition than phagocytosis. These possibilities offer interesting avenues for further investigation of the pathways and effectors unique to amebic trogocytosis and the potential for the development of novel therapeutics.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplemental Figure 1. E64-d inhibits amebic cysteine proteases in a dose-dependent manner. Amebae pretreated with media (untreated), vehicle (1 µl/mL DMSO), or E-64d (1–100 µM) for 1 hour were either (A) flash frozen and lysed or (B) washed, incubated in media for 40 minutes, then flash frozen, and lysed. Z-Phe-Phe-FMK (irreversible Cathepsin B and L inhibitor) was added to an aliquot of vehicle-treated cell lysate as a negative control. All samples were assayed for their ability to degrade AC-RR-AFC (mammalian Cathepsin B substrate) using a fluorescence plate reader. Means and standard deviations represent biological duplicates. Curve fitting was done using nonlinear regression in Prism 6.
Notes
Financial support. This work was funded by National Institutes of Health (NIH) Grant R01 AI026649. A. A. G. was funded by NIH Biodefense Training Grant T32 AI055432 and Ruth L. Kirschstein Individual Predoctoral MD/PhD Fellowship F30 AI114136-02.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Ralston KS. Chew on this: amoebic trogocytosis and host cell killing by Entamoeba histolytica. Trends Parasitol 2015; 31:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA Jr. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 2014; 508:526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dopfer EP, Minguet S, Schamel WW. A new vampire saga: the molecular mechanism of T cell trogocytosis. Immunity 2011; 35:151–3. [DOI] [PubMed] [Google Scholar]
- 4. Somlata, Nakada-Tsukui K, Nozaki T. AGC family kinase 1 participates in trogocytosis but not in phagocytosis in Entamoeba histolytica. Nat Commun 2017; 8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilmartin AA, Ralston KS, Petri WA. Inhibition of amebic lysosomal acidification blocks amebic trogocytosis and cell killing. mBio 2017; 8:e01187-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravdin JI, Schlesinger PH, Murphy CF, Gluzman IY, Krogstad DJ. Acid intracellular vesicles and the cytolysis of mammalian target cells by Entamoeba histolytica trophozoites. J Protozool 1986; 33:478–86. [DOI] [PubMed] [Google Scholar]
- 7. Mitra BN, Yasuda T, Kobayashi S, Saito-Nakano Y, Nozaki T. Differences in morphology of phagosomes and kinetics of acidification and degradation in phagosomes between the pathogenic Entamoeba histolytica and the non-pathogenic Entamoeba dispar. Cell Motil Cytoskeleton 2005; 62:84–99. [DOI] [PubMed] [Google Scholar]
- 8. Nakada-Tsukui K, Tsuboi K, Furukawa A, Yamada Y, Nozaki T. A novel class of cysteine protease receptors that mediate lysosomal transport. Cell Microbiol 2012; 14:1299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serrano-Luna J, Piña-Vázquez C, Reyes-López M, Ortiz-Estrada G, de la Garza M. Proteases from Entamoeba spp. and pathogenic free-living amoebae as virulence factors. J Trop Med 2013; 2013:890603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faust DM, Marquay Markiewicz J, Danckaert A, Soubigou G, Guillen N. Human liver sinusoidal endothelial cells respond to interaction with Entamoeba histolytica by changes in morphology, integrin signalling and cell death. Cell Microbiol 2011; 13:1091–106. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto K, Mizoue K, Kitamura K, Tse WC, Huber CP, Ishida T. Structural basis of inhibition of cysteine proteases by E-64 and its derivatives. Biopolymers 1999; 51:99–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.