Abstract
Background
Clostridium difficile infection (CDI) causes diarrhea and colitis. We aimed to find a common pathogenic pathway in CDI among humans and mice by comparing toxin-mediated effects in human and mouse colonic tissues.
Method
Using multiplex enzyme-linked immunosorbent assay, we determined the cytokine secretion of toxin A– and B–treated human and mouse colonic explants.
Results
Toxin A and toxin B exposure to fresh human and mouse colonic explants caused different patterns of cytokine secretion. Toxin A induced macrophage inflammatory protein (MIP) 1α secretion in both human and mouse explants. Toxin A reduced the expression of chloride anion exchanger SLC26A3 expression in mouse colonic explants and human colonic epithelial cells. Patients with CDI had increased colonic MIP-1 α expression and reduced colonic SLC26A3 (solute carrier family 26, member 3) compared with controls. Anti–MIP-1 α neutralizing antibody prevented death, ameliorated colonic injury, reduced colonic interleukin 1β (IL-1β) messenger RNA expression, and restored colonic SLC26a3 expression in C. difficile–infected mice. The anti–MIP-1 α neutralizing antibody prevented CDI recurrence. SLC26a3 inhibition augmented colonic IL-1 β messenger RNA expression and abolished the protective effect of anti–MIP-1 α neutralizing antibody in mice with CDI.
Conclusion
MIP-1 α is a common toxin A–dependent chemokine in human and mouse colon. MIP-1 α mediates detrimental effects by reducing SLC26a3 and enhancing IL-1 β expression in the colon.
Keywords: Infection, biologic, therapy
Macrophage inflammatory protein 1 α is a toxin A–mediated chemokine in human and mouse colons. Its neutralization ameliorates Clostridium difficile colitis in mice, and it mediates down-regulation of SLC26A3 and up-regulation of interleukin 1 β, mediating effects of late-stage C. difficile infection.
Clostridium difficile infection (CDI) is a common nosocomial infection after antibiotic exposure. Toxigenic C. difficile bacteria produce toxins A and B [1] , which mediate intestinal inflammation, tissue damage, and clinical symptoms in CDI in animals and humans. C. difficile–infected patients experience watery diarrhea, bloody stool, abdominal pain, fever, and weight loss. Antibiotics such as vancomycin, metronidazole, and fidaxomicin are effective against many primary CDI cases. However, some CDI cases are refractory to antibiotic treatment, leading to multiple relapses. These patients may eventually require surgical resection of the involved tissue, which adversely affects their quality of life [2]. Moreover, the high cost of fidaxomicin has limited its clinical use, despite its effectiveness against relapsing CDI [3]. Anti–toxin B monoclonal antibodies, together with standard antibiotic care, are also associated with fewer recurrent CDI episodes [4]. Despite this progress, it is still necessary to develop new medications against both acute and, particularly, relapsing CDI-associated colitis.
Over the last several years, our group and others have generated preclinical data associated with the use of several therapeutic approaches against CDI, including antibiotics, probiotics, and monoclonal antibodies [5–9]. The use of preclinical CDI research approaches includes the use of fresh human colonic explants, cultured cells, and animal models (mice and hamsters). However, each model has its advantages and disadvantages. Moreover, humans and mice have different responses to C. difficile toxins. To circumvent these difficulties, comparisons of gene expression in human and mouse colon using a systems biology approach may help identify common targets of toxin A– and B–mediated responses in animals and humans.
We hypothesize that cytokines commonly regulated by toxins A and B in human and mouse colon may be important targets for developing CDI therapy. This study used a systems biology approach, including multiplex enzyme-linked immunosorbent assay (ELISA) and whole-transcriptome next-generation sequencing (NGS), to compare gene expression responses of human and mouse colonic tissues exposed to toxins A and B. Our results elucidate the mechanistic roles of a chemokine (macrophage inflammatory protein [MIP] 1 α [or CCL3]), a chloride anion exchanger (solute carrier family 26, member 3 [SLC26a3]), and a cytokine (interleukin 1 β [IL-1 β ]) in mediating toxin-associated downstream immune responses and colonic injury in CDI. The results of the current study illustrate the therapeutic potential of anti–MIP-1 α neutralizing antibodies against CDI.
MATERIALS AND METHODS
C. difficile Culture and Toxin Purification
C. difficile strain A+B+ VPI 10463 (American Type Culture Collection stock 43255) and A−B+ Ribotype 017 (American Type Culture Collection stock 43598) were cultured in Difco cooked meat medium (no. 226730 BD; Fisher Scientific) at 37oC in anaerobic conditions [10]. Wild-type toxins A and B and a mutant noncleavable toxin B (TcdB-L543A) were purified and validated as described elsewhere [10, 11]. The cytotoxicity of the toxins was determined by cell rounding in 3T3 fibroblasts [5].
Human and Mouse Colonic Explants
Formalin-fixed human colonic samples embedded in paraffin blocks, with or without CDI, were obtained from Ciaran P. Kelly at the Beth Israel Deaconess Medical Center of Harvard Medical School. Fresh human colonic explants were obtained from the UCLA Surgical Pathology Department. Fixed non-CDI colonic tissues and fresh human colonic explants were collected from noncancerous regions of patients with colon cancer, as described elsewhere [6].
Inclusion criteria were detection of C. difficile by polymerase chain reaction tests, with confirmation of CDI diagnosis by board-certified gastroenterologists. Pregnant women, prisoners, or minors (age <18 years) were excluded. Detailed patient information is provided elsewhere [12].
Mice were euthanized with carbon dioxide gas. Fresh mouse colonic explants were obtained from male and female normal C57BL/6J mice. Fresh human and mouse colonic explants were cut into 3 × 3-mm pieces. The explants were placed in cell culture medium and treated with phosphate-buffered saline (PBS), toxin A, or toxin B for 5 hours. The experiments were divided into 2 cohorts.
The human colonic explants in the exploratory cohort were placed in serum-free Roswell Park Memorial Institute 1640 medium and treated with PBS (1 μ L/mL), toxin A (0.1 μ g/mL), and toxin B (0.1 μ g/mL). The mouse colonic explants in the exploratory cohort were placed in serum-free Dulbecco modified Eagle medium and treated with PBS, toxin A (10 μ g/mL), and toxin B (10 μ g/mL). In the validation cohort, the human and mouse colonic explants were placed in serum-free Roswell Park Memorial Institute 1640 medium (human) or Dulbecco modified Eagle medium (mouse) and treated with PBS (1 μ L/mL), toxin A (0.01–10 μ g/mL), or toxin B (0.01–10 μ g/mL).
RESULTS
MIP-1 α and IL-1 β as Common Cytokines Expressed in Human and Mouse Colonic Explants Exposed to Toxin A
We compared the responses of human and mouse colonic explants to C. difficile toxins. In the exploratory cohort, human colonic explants from 4 control subjects and mouse colonic explants from 4 mice were exposed to toxins A and B (0.1 μ g/mL). Low concentrations of toxins were sufficient to mediate histological damage (Figure 1A) and increase histology scores (Figure 1C) and cytokine expression (Figure 1E) in human colonic explants [8]. Mouse colonic explants required a high toxin A concentration (10 μ g/mL) to mediate histological damages (Figure 1B) and increase histology score (Figure 1D). The same high toxin A concentration was also needed to produce enteritis in mice [5, 12]. Toxin B (10 μ g/mL) produced only a mild increase in histology score in mouse colonic explants (Figure 1D).
Figure 1.
Macrophage inflammatory protein (MIP) 1 α and interleukin (IL) 1β are common toxin A–mediated cytokines in fresh human and mouse colonic explants. A, Fresh human colonic explants were treated with phosphate-buffered saline (PBS), toxin A, or toxin B for 5 hours (hematoxylin-eosin [HE] staining of fresh human colonic explants in the exploratory cohort, ×100 magnification). B, Fresh mouse colonic explants were treated with PBS, toxin A, or toxin B for 5 hours (HE staining of fresh mouse colonic explants in the exploratory cohort, ×100 magnification). C, Histology score of fresh human colonic explants in the exploratory cohort. Both toxins increased histology scores significantly. D, Histology scores of fresh mouse colonic explants in the exploratory cohort. Only toxin A increased histology scores significantly. E, Cytokine levels in the human and mouse colonic explant-conditioned media in the exploratory cohort. The cytokine levels were measured with multiplex enzyme-linked immunosorbent assay. The exploratory cohort included 4 human tissue donors and 4 mice. F, Consistently altered messenger RNA (mRNA) expression in toxin A– or toxin B–treated fresh mouse colonic explants, determined with next-generation sequencing (NGS). Toxin A consistently decreased expression of SLC26a3 (solute carrier family 26, member 3) mRNA in fresh mouse colonic explants. No consistently altered genes were found in the toxin B–treated fresh mouse colonic explants. Abbreviations: FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IP-10, interferon gamma inducible protein 10kDa; KC, chemokine (C-X-C motif) ligand 1; MCP, monocyte chemoattractant protein; NS, not significant; PDGF-BB, platelet-derived growth factor BB; RANTES, regulated on activation, normal T-expressed, and presumably secreted; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The multiplex ELISA results showed that toxins A and B induced different patterns of cytokine secretion in human and mouse colonic explants (Figure 1E). Notably, toxin A induced MIP-1 α and IL-1 β secretion in both human and mouse colonic explants (Figure 1E). This finding suggests that MIP-1 α and IL-1 β are common toxin A–mediated cytokines in human and mouse colons.
Whole-Transcriptome NGS Identification of Toxin A–Dependent Genes in Fresh Mouse Colonic Explants
We used NGS to search for downstream targets of toxins A and B in fresh mouse colonic explants. Toxins A and B caused different patterns of gene expression in fresh colonic explants, as shown by the principal component analysis and heat map (Supplementary Figure 1A and 1B). Sample identifier interpretation is shown in Supplementary Figure 1C. Full lists of toxin A– and toxin B–mediated differentially expressed genes in each set of mouse colonic explants are shown in Supplementary Figure 1D and 1E. NGS identified SLC26a3 as a toxin A–mediated differentially expressed gene in the exploratory cohort (Figure 1F). No toxin B–mediated differentially expressed genes were found.
Increased Colonic MIP-1 α and Reduced Colonic SLC26A3 Expression in Patients With CDI
Our study also included a validation cohort of human and mouse colonic explants for assessing toxin dose-effect relationship. Toxin A induced MIP-1 α secretion at 0.1 μ g/mL in human and at 10 μ g/mL in mouse colonic explants (Figure 2A and 2B). At all tested concentrations, toxin B failed to increase MIP-1 α secretion in the mouse validation cohort (Figure 2A and 2B). In the same cohort, toxin B inhibited SLC26A3 messenger RNA (mRNA) expression in fresh human colonic explants (Figure 2C), whereas toxin A, but not toxin B, significantly reduced SLC26a3 mRNA expression (Figure 2D) and significantly increased IL-1 β secretion (Supplementary Figure 2A) in fresh mouse colonic explants. The toxin B–mediated induction of IL-1 β secretion in fresh human colonic explants had been shown in our group’s previous study [8], and another group reported increased serum IL-1 β levels in C. difficile–infected patients [13].
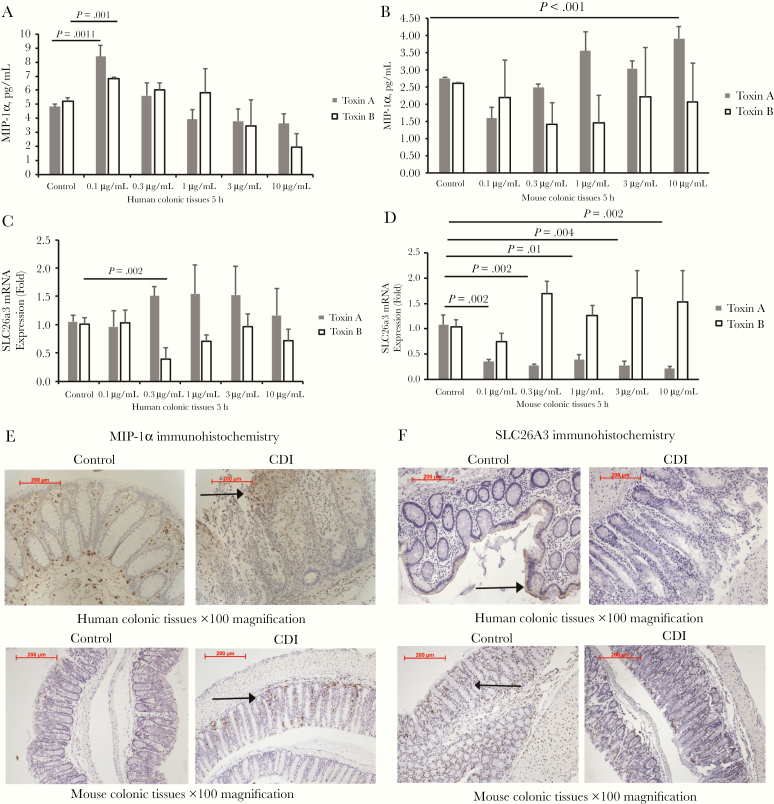
Figure 2.
Clostridium difficile infection (CDI) increased macrophage inflammatory protein (MIP) 1 α and decreased SLC26A3 (solute carrier family 26, member 3) protein expression in the colonic mucosa. Fresh human and mouse colonic explants were treated with toxin A or B for 5 hours. Conditioned media were collected for enzyme-linked immunosorbent assay, and tissues were collected for real-time reverse-transcription polymerase chain reaction testing. The validation cohort included 5 human tissue donors and 5 mice. A, MIP-1 α secretion in fresh human colonic explants in the validation cohort. B, MIP-1 α secretion in fresh mouse colonic explants in the validation cohort. C, SLC26A3 messenger RNA (mRNA) expression in fresh human colonic explants in the validation cohort. D, SLC26a3 mRNA expression in fresh mouse colonic explants in the validation cohort. E, Upper panel, Immunohistochemistry of MIP-1 α in the colonic tissues of patients with or without CDI. Lower panel, Immunohistochemistry of MIP-1 α in the mouse colonic tissues with or without CDI. MIP-1 α -positive signals were identified as brown spots in the colonic mucosa (arrows). F, Upper panel, Immunohistochemistry of SLC26A3 in the colonic tissues of patients without or with CDI. Lower panel, Immunohistochemistry of SLC26a3 in the mouse colonic tissues with or without CDI. SLC26A3-positive signals were identified as brown spots in the colonic mucosal epithelial layer (arrows).
In the colonic tissues of patients without CDI, baseline levels of MIP-1 α and SLC26A3 immunoreactivity were found throughout the colonic mucosa (Figure 2E and 2F). Colonic tissues of patients with CDI, however, showed increased MIP-1 α and decreased SLC26A3 immunoreactivity (Figure 2E and 2F). Mice with CDI also showed increased MIP-1 α and decreased SLC26A3 immunoreactivity in the colon (Figure 2E and 2F). Consistent with our findings, another group demonstrated that colons of patients with CDI had reduced SLC26A3 protein expression [14].
Alteration of Colonic MIP-1 α (CCL3), IL-1 β, and SLC26a3 mRNA Expression in Later-Stage CDI
To understand the correlation of colonic gene expression and disease development in CDI, the time-dependent change of colonic gene expression was determined in C. difficile–infected mice. CDI caused progressive deterioration of histological injury in the colon, with increasing histology score from day 0 to day 3 after infection (Figure 3A and 3B). CDI moderately increased colonic CCL3, tumor necrosis factor (TNF), and CXCl1 mRNA expression (Figure 3C). Notably, colonic IL-1 β mRNA expression was sharply increased, whereas colonic SLC26a3 mRNA expression began to decrease on day 3 after infection (Figure 3C). Therefore, the most severe phenotypes of CDI occurred on day 3 after infection.
Figure 3.
Colonic expression of SLC26a3 (solute carrier family 26, member 3) is reduced at the late stage of Clostridium difficile infection (CDI). A, Hematoxylin-eosin staining of mouse colonic tissues on days 0, 1, 2, and 3 of CDI. The colonic mucosal integrity deteriorated progressively (×100 magnification). B, Histology score. C, Colonic tumor necrosis factor (TNF), interleukin 1 β (IL-1 β ), CXCl1, CCL3, and SLC26a3 expression from day 0 to day 3. SLC26a3 messenger RNA (mRNA) expression was significantly reduced on day 3. IL-1 β mRNA expression had the most dramatic increase on day 3 after infection, compared with TNF, CXCl1, and CCL3 (5 mice per group). D, E, Serum-starved NCM460 cells were treated with toxin A, toxin B, or macrophage inflammatory protein (MIP) 1 α for 5 hours. Relative MIP-1 α and SLC26A3 mRNA expression was determined by means of real-time reverse-transcription polymerase chain reaction. Toxin A or B, but not MIP-1 α, significantly reduced SLC26A3, but not MIP-1 α mRNA expression in human colonocytes. F, Serum-starved mouse macrophages were treated with toxin A, toxin B, or MIP-1 α for 2 hours. Inflammasome activity was measured by means of luminescence assay. Toxins A and B, but not MIP-1 α, significantly increased inflammasome activity in macrophages. Results are pooled from 3 independent experiments.
Exposure to either toxins or MIP-1 α did not affect MIP-1 α mRNA expression in NCM460 colonocytes, suggesting that colonic epithelial cells may not be the source of MIP-1 α (Figure 3D). Consistent with findings of a previous study [14], toxins A and B, but not MIP-1 α, inhibited SLC26A3 mRNA expression in human colonic epithelial NCM460 cells (Figure 3E). On the other hand, toxins A and B, but not MIP-1 α, increased inflammasome activity in mouse macrophages (Figure 3F). Because inflammasome mediates pro–IL-1 β cleavage [15], this finding suggests that C. difficile toxins, but not MIP-1 α, can induce IL-1 β production.
Effectiveness of Systemic Neutralization of MIP-1 α Against Primary CDI in Mice
We next determined the functional importance of MIP-1 α in mice with CDI (Figure 4A). After C. difficile inoculation, diarrhea developed as early as 6 hours and showed a 20% mortality rate in mice on day 3 (Figure 4B). Intraperitoneal injection of anti–MIP-1 α neutralizing antibody prevented death of the infected mice (Figure 4B) but did not affect colonic histological structure or body weight in normal uninfected mice (Figure 4C and 4D). Although anti–MIP-1 α neutralizing antibody treatment did not affect body weight loss in the infected mice (Figure 4C), it still improved survival through day 10 after infection (Supplementary Figure 2B and 2C). CDI caused moderate disruption of colonic epithelial layer, accumulation of immune cells in colonic mucosa, and increase in histology score (Figure 4D and 4E). These effects were partially reversed by an anti–MIP-1 α neutralizing antibody injection provided 6 hours after C. difficile inoculation (Figure 4D and 4E), suggesting its therapeutic potential.
Figure 4.
Neutralization of macrophage inflammatory protein (MIP) 1 α ameliorated acute colitis associated with Clostridium difficile infection (CDI) in mice. A, Experimental plan of the CDI primary infection model. B, Survival rate at day 3. C, Body weight change. D, Hematoxylin-eosin staining of mouse colons and histology score. Colonic mucosal injury was characterized by damaged epithelial layer and neutrophil infiltration (black bars represent 200 μm). E, Histology score. F, Colonic MIP-1 α, SLC26a3 (solute carrier family 26, member 3), and interleukin 1 β (IL-1 β ) messenger RNA (mRNA) expression on day 3 after infection. CDI increased colonic MIP-1 α and IL-1 β, and reduced colonic SLC26a3 mRNA expression in mice. These effects were reversed by anti–MIP-1 α neutralizing antibody (NAb) treatment. Each group included 8 mice. Abbreviation: IgG, immunoglobulin G.
In mice with CDI, colonic IL-1 β and MIP-1 α mRNA expression was increased, whereas colonic SLC26a3 mRNA expression was reduced (Figure 4F). These changes were reversed by the injection of the anti–MIP-1 α antibody (Figure 4F). CDI also increased colonic proinflammatory cytokine (TNF-α and CXCl1) mRNA expression in mice, but their expression was not affected by the anti–MIP-1 α antibody treatment (Figure 5A).
Figure 5.
Neutralization of macrophage inflammatory protein (MIP) 1 α restored colonic expression of SLC26A3 (solute carrier family 26, member 3) in mice with Clostridium difficile (CDI) infection. A, CDI increased colonic tumor necrosis factor (TNF) and CXCl1 messenger RNA (mRNA) expression, which were not affected by anti–MIP-1 α neutralizing antibody (NaB) treatment. B, Serum cytokine levels were measured by multiplex enzyme-linked immunosorbent assay. CDI or anti–MIP-1 α antibody treatment did not affect serum MIP-1 α or interleukin 1β (IL-1 β ) levels in mice. C, CDI increased serum interleukin 6 (IL-6) and granulocyte colony-stimulating factor (G-CSF) levels that were reversed by anti–MIP-1 α neutralizing antibody treatment. D, CDI or anti–MIP-1 α antibody treatment did not affect MIP-1 α or IL-1 β mRNA expression in the circulating blood cells of mice. E, Immunohistochemistry of SLC26A3 in the mouse colonic tissues. SLC26A3-positive signals were identified as brown spots. CDI reduced colonic SLC26A3 protein expression that was restored by anti–MIP-1 α neutralizing antibody treatment. Each group included 8 mice. F, Serum-starved CCD-18Co fibroblasts were treated with either control construct or SLC26A3-overexpressing construct overnight, followed by exposure to phosphate-buffered saline (PBS), toxin A, or toxin B for 1 hour. Toxins caused cell rounding with loss of spindle cell shape. SLC26A3 overexpression did not alter toxin-mediated cell rounding. Results are representative of 3 independent experiments. Abbreviations: IFN, interferon; IgG, immunoglobulin G; KC, chemokine (C-X-C motif) ligand 1.
Multiplex ELISA indicated that circulating MIP-1 α and IL-1 β protein levels in mice were low in all groups (Figure 5B). Circulating MIP-1 α, TNF-α, IL-1 β, and chemokine (C-X-C motif) ligand 1 (KC) levels did not differ between infected mice with or without anti–MIP-1 α neutralizing antibody treatment (Figure 5B). The anti–MIP-1 α antibody treatment significantly reduced elevated circulating interleukin 6 (IL-6) and granulocyte colony-stimulating factor (G-CSF) levels in C. difficile–infected mice (Figure 5C). Neither CDI nor anti–MIP-1 α neutralizing antibody treatment affected IL-1 β and MIP-1 α mRNA expression in circulating blood cells of mice, suggesting that the intestine, but not circulating blood cells, may be the source of these 2 cytokines (Figure 5D). SLC26a3 mRNA signal was undetectable in the circulating blood cells in all groups (data not shown).
Anti–MIP-1 α Antibody Reversal of Colonic SLC26a3 Down-Regulation in Mice With CDI
Immunohistochemical findings indicate that SLC26A3 protein expression was primarily localized in the colonic mucosa of control and C. difficile–infected mice (Figure 5E). Consistent with colonic SLC26a3 mRNA expression (Figure 4F), CDI significantly reduced colonic SLC26a3 protein expression in mice, which was restored by anti–MIP-1 α antibody treatment (Figure 5E). Toxins A and toxin B caused cell rounding in fibroblasts; however, toxin-mediated cell rounding was not affected by SLC26A3 overexpression (Figure 5F). Because cell rounding often occurs before cell death [16], this finding suggests that SLC26A3 is not directly involved in toxin-mediated cytoskeletal disruption and cell death.
Role of SLC26a3 Restoration in Inhibiting IL-1 β Expression and Mediating the Therapeutic Effects of Anti–MIP-1 α Antibody in Mice With CDI
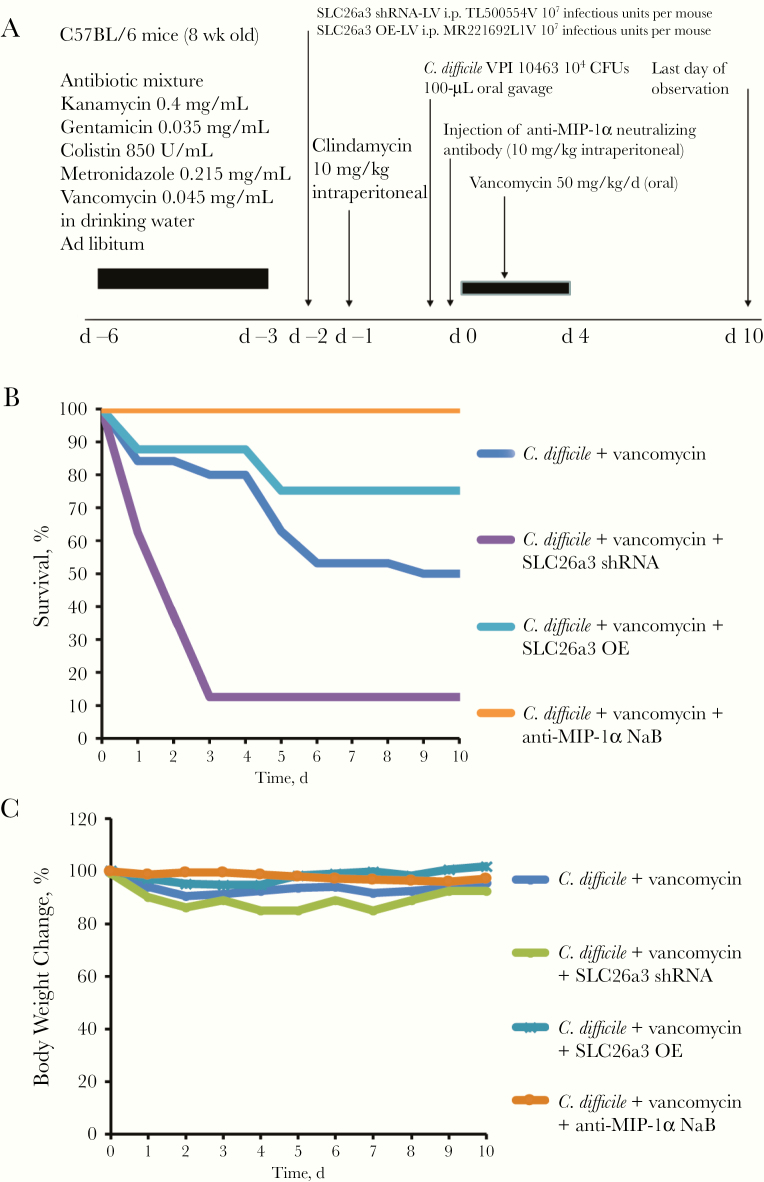
To determine the functional role of SLC26a3 in mice with CDI, we inhibited its expression using SLC26a3 short hairpin RNA (shRNA) lentivirus and increased its expression using SLC26a3-overexpressing lentivirus (Figure 6A). Inhibition of SLC26a3 did not alter the survival or change in body weight up to day 10 after infection (Figure 6B and 6C and Supplementary Figure 2B and 2C), but it did worsen the colonic injury with increased histology score in these mice (Figure 6D). Importantly, SLC26a3 shRNA lentivirus reversed the protective effects of the anti–MIP-1 α antibody treatment against survival (Figure 6B), body weight loss (Figure 6C), and colonic injury (Figure 6D) in the infected mice, while lentiviral SLC26a3 overexpression ameliorated CDI by minimizing body weight loss (Figure 6C) and reducing colonic injury and histology scores in the infected mice (Figure 6D). SLC26a3 overexpression prevented further deaths beyond day 3 after infection (Supplementary Figure 2B), suggesting that SLC26A3 is necessary for mediating detrimental effects during the late stage of CDI.
Figure 6.
SLC26A3 (solute carrier family 26, member 3) mediates the protective effects of anti–macrophage inflammatory protein (MIP) 1 α neutralizing antibody (NAb) in Clostridium difficile infection (CDI). A, Experimental plan of the CDI primary infection model. Lentiviral vectors were injected into mice before CDI infection. Anti–MIP-1 α neutralizing antibody was injected into mice 6 hours after infection. B, Survival rate at day 3. C, Body weight change at day 3. D, Hematoxylin-eosin (HE) staining of mouse colons and histology scores on day 3 after infection (×100 magnification). SLC26A3 inhibition abolished the protective effects of anti–MIP-1 α antibody against colonic injury in CDI. SLC26a3 overexpression ameliorated colonic injury in mice with CDI. E, Colonic SLC26a3 and interleukin 1 β (IL-1 β) messenger RNA (mRNA) expression on day 3 after infection. F, Serum IL-1 β levels on day 3 after infection. SLC26a3 inhibition increased colonic IL-1 β mRNA expression and serum IL-1 β levels in infected mice with or without anti–MIP-1 α antibody treatment. SLC26a3 overexpression inhibited colonic IL-1 β mRNA expression and serum IL-1β levels in infected mice. Each group included 8 mice. Abbreviations: IgG, immunoglobulin G; shRNA, short hairpin RNA.
SLC26a3 shRNA lentivirus further reduced colonic SLC26a3 mRNA expression in mice with CDI, whereas SLC26a3-overexpressing lentivirus significantly increased colonic SLC26a3 mRNA expression in these mice (Figure 6E). The lentiviral SLC26a3 inhibition augmented the increased colonic IL-1 β mRNA expression and serum IL-1 β levels in C. difficile–infected mice with or without anti–MIP-1 α neutralizing antibody treatment (Figure 6E and 6F). SLC26a3 overexpression significantly reduced IL-1 β mRNA expression and serum IL-1 β levels in the infected mice (Figure 6E and 6F).
Administration of Anti–MIP-1 α Neutralizing Antibody and Restoration of SLC26A3 to Prevent Vancomycin-Associated CDI Recurrence in Mice
Vancomycin is associated with CDI recurrence [17]. We treated the infected mice with vancomycin and an anti–MIP-1 α antibody at the early stage (day 0) of CDI (Figure 7A). CDI caused a high mortality rate rapidly, with a final 50% survival rate by day 10 after infection (Figure 7B). Twenty percent of mice died during vancomycin treatment, and an another 30% had CDI relapse and died after vancomycin withdrawal (Figure 7B). Administration of anti–MIP-1 α antibody after C. difficile inoculation prevented vancomycin-dependent relapse and death (Figure 7B). These results suggest that the anti–MIP-1 α antibody prevents CDI recurrence in mice.
Figure 7.
Neutralization of macrophage inflammatory protein (MIP) 1 α reduced vancomycin-associated Clostridium difficile infection (CDI) recurrence in mice. A, Experimental plan of the CDI recurrence model. Lentiviral vectors were injected into mice before CDI infection. Anti–MIP-1 α neutralizing antibody (NAb) was injected into mice at 6 hours after infection. Vancomycin was injected daily from day 0 to day 4. B, Survival rate. C, Body weight change. Anti–MIP-1 α neutralizing antibody or SLC26a3 (solute carrier family 26, member 3) overexpression prevented CDI recurrence. SLC26a3 inhibition caused rapid death even with vancomycin treatment. Each group included 8 mice. Abbreviations: CFUs, colony-forming units; shRNA, short hairpin RNA.
Interestingly, lentiviral inhibition of SLC26a3 markedly increased the mortality rate in vancomycin-treated infected mice (Figure 7B). Most of the mice died within the first 3 days. SLC26a3 overexpression did not affect survival in the first 4 days, but it improved survival after vancomycin withdrawal, which suggests that SLC26a3 restoration reduced CDI relapse (Figure 7B). After vancomycin withdrawal, body weight changes of individual relapsing mice did not significantly affect the average values of the groups’ body weight, because relapse occurred randomly (Figure 7C).
DISCUSSION
We report that MIP-1 α and IL-1 β are common toxin A–targeted cytokines in both human and mouse colonic tissues (Figure 1). Our study is the first to explore their mechanistic relationship to the MIP-1 α –SLC26A3–IL-1 β pathway. The therapeutic effects of anti–MIP-1 α antibody in protecting survival rates, ameliorating colonic tissue damage, and minimizing body weight loss in mice with primary CDI (Figure 4) were consistent with findings from Jose and colleagues [18]. Importantly, our results also demonstrate that anti–MIP-1 α antibody successfully prevented relapse and death in a mouse model of recurrent CDI. Taken together, these results suggest that MIP-1 α may represent a potential therapeutic target in both first episodes and recurrent CDI in humans.
MIP-1 α mediates immune cell chemotaxis, which promotes inflammatory responses. In the current study, we observed reduced immune cell infiltration in the colons of anti–MIP-1 α antibody–treated mice with CDI (Figure 4D), in line with previous results using MIP-1 α deficient mice in the toxin A–treated ileal loop model [19]. Because MIP-1 α binds to the CCR1 receptor [20], genetic deficiency of CCR1 protects mice against toxin A–mediated enteritis [19]. CCR1 seems to have multifaceted roles in injury, including significant input in reepithelialization during the wound healing process [21].
We found that neutralization of MIP-1 α reduced circulating levels of IL-6 and G-CSF during CDI in mice (Figure 5C). The anti–MIP-1 α antibody–mediated modulation of circulating IL-6 levels during CDI in mice may reflect CDI disease activity in humans and animals [22]. Although the role of G-CSF in CDI is unknown, it may be associated with activation of neutrophils [23] that play a crucial role in the pathogenesis of CDI in both animals and humans [24, 25]. Along these lines, Wang et al [9] have observed activation of circulating neutrophils in C. difficile–infected mice.
SLC26A3 is involved in chloride absorption in intestinal epithelial cells. Thus, SLC26a3-deficient patients experience congenital chloride diarrhea [26, 27]. The reduced colonic SLC26A3 mRNA and protein expression in mice with CDI (Figures 4F and 5E) is a typical phenotype in colitis and diarrhea, as observed in the colons of patients with CDI and ulcerative colitis, as well as in human colonic epithelial cells treated with toxin A, toxin B, and TNF- α [14, 28]. Deficiency of SLC26a3 causes reduction of fluid absorption and loss of intestinal barrier, which mimics colitis [29]. Overexpression of SLC26a3 also prevents dextran sulfate–mediated colitis in mice [30]. To our knowledge, our group is the first to demonstrate that SLC26A3 regulates IL-1 β expression as SLC26a3 overexpression reduced colonic IL-1 β mRNA expression and circulating IL-1 β levels, whereas SLC26a3 inhibition reversed anti–MIP-1 α antibody–mediated suppression of IL-1 β expression in CDI (Figure 6E and 6F). Therefore, SLC26A3 mediates the pathogenic effects of MIP-1 α via IL-1 β expression in CDI.
We believe that SLC26A3 does not mediate early-stage CDI because colonic SLC26a3 mRNA expression was reduced only in late-stage CDI (Figure 3C). At the early stage, C. difficile toxins lead to progressive colonic injury and trigger MIP-1 α production (Figures 1E and 3A). Increased MIP-1 α expression promotes immune cell infiltration that drives various inflammatory responses and tissue injury indirectly. In late-stage CDI (day 3), increasing levels of C. difficile toxins are associated with the down-regulation of colonic epithelial SLC26A3 expression [14, 28, 30]. SLC26a3 seems to be crucial for survival at the late stage of infection because SLC26a3 overexpression prevented additional deaths only beyond day 3 after infection, whereas SLC26a3 inhibition did not affect the mortality rate before day 3 (Supplementary Figure 2B). SLC26A3 down-regulation is associated with damage to the intestinal barrier, which may allow more toxin molecules to enter the colonic mucosa.
Although MIP-1 α does not directly activate inflammasome activity in macrophages (Figure 3F), the increased levels of toxins activate macrophage production of inflammasome and IL-1 β [15]. This response may be associated with the dramatically high colonic IL-1 β mRNA expression in late-stage CDI (Figure 3C). Exposure to toxins A and B did not induce IL-1 β secretion in human colonic epithelial NCM460 cells (data not shown), suggesting that both toxins do not activate inflammasome activity in human colonic epithelial cells. Toxin A– and toxin B–mediated intestinal epithelial cell death is independent of inflammasome [31]. Therefore, the anti–MIP-1 α neutralizing antibody treatment diminishes the positive feedback loop of IL-1 β production.
IL-1 β is a crucial pathogenic mediator in CDI in humans, because they have significantly increased circulating levels of IL-1 β [13]. Cowardin et al [13] demonstrated interleukin 1 receptor–dependent interleukin 23 (IL-23) expression. The same research group showed that inhibition of IL-23 by genetic deficiency or neutralizing antibody prevented death in mice with CDI [32]. Inhibition of the IL-1 β pathway shows therapeutic effects against CDI, as treatment with the recombinant interleukin 1 receptor antagonist anakinra protects mice from CDI-associated colitis [15], whereas inhibition of toxin B–mediated IL-1 β secretion is associated with the anti-inflammatory effect of fidaxomicin in fresh human colonic explants [8]. Thus, anti–MIP-1 α antibody treatment may prevent initiation of the IL-1 β –dependent IL-23 signaling cascade.
One goal of the current study was to compare the responses of human and mouse colonic explants to C. difficile toxins. Key findings from human studies are not fully reflected in mouse models. For example, exposure to toxin B failed to cause significant histological injury or MIP-1 α secretion in mouse colonic tissues (Figures 1 and 2B). CDI with a toxin A−B+ strain did not cause death in mice (Supplementary Figure 3A). The difference in CDI responses among humans and mice could be explained by several factors, including host responses and possibly intestinal microbiota. A 2017 study demonstrated that a recombinant toxin B–based nanoparticle vaccine protects mice from CDI, suggesting that toxin B mediates immune responses in mice [33].
Interestingly, exposure of mouse colonic explants to a mutant toxin B caused severe histological damage, increased MIP-1 α and IL-1 β secretion, and reduced SLC26a3 mRNA expression in mouse colonic explants (Supplementary Figure 3B and 3E). This mutant toxin B (TcdB-L543A) is noncleavable by cysteine protease [11]. This result was consistent with the recent finding that autoprocessing of the toxins regulates their inflammatory activities [34]. Although these ex vivo experiments could only be performed within 24 hours and immune cell infiltration could not be demonstrated using fresh colonic explants, this model mimicked the toxin B–mediated increased MIP-1 α and IL-1 β secretion and decreased SLC26A3 mRNA expression in human colonic explants (Figures 1E, 2A, and 2C). We speculate that the MIP-1 α –SLC26A3–IL-1 β pathway can mediate the pathogenic effects of toxin B in human colon.
In summary, MIP-1 α and IL-1 β are common toxin A–mediated cytokines in human and mouse colonic tissues. Neutralization of MIP-1 α is effective in inhibiting primary and recurrent CDI in mice. SLC26A3 regulates IL-1 β expression and detrimental effects in late-stage CDI. Inhibition of MIP-1 α restored colonic SLC26a3 expression and reduced colonic IL-1 β expression in C. difficile–infected mice, which is relevant to the reversal of CDI colitis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Data acquisition: J. W., C. O., L. F., R. M., and Y. X. Production of toxin A and wild-type toxin B: X. C. Production of mutant toxin B: H. F. Study design: C. P. and H. W. K. Study supervision and critical revision of the manuscript: C. P. Data analysis, and writing of the manuscript: H. W. K.
Financial support. This work was supported by Merck, the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant UO-1 AI124290 to C. P.), the Blinder Research Foundation for Crohn’s Disease, the Eli and Edythe Broad Foundation, and the National Institutes of Health (grants R03 DK103964 and R21 AI137663 to H. W. K. and Center for Ulcer Research and Education (CURE) grant P30 DK041301 to the UCLA Center for Systems Biomedicine).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. El Feghaly RE, Bangar H, Haslam DB. The molecular basis of Clostridium difficile disease and host response. Curr Opin Gastroenterol 2015; 31:24–9. [DOI] [PubMed] [Google Scholar]
- 2. Kaiser AM, Hogen R, Bordeianou L, Alavi K, Wise PE, Sudan R; CME Committee of the SSAT Clostridium difficile infection from a surgical perspective. J Gastrointest Surg 2015; 19:1363–77. [DOI] [PubMed] [Google Scholar]
- 3. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 99. [DOI] [PubMed] [Google Scholar]
- 4. Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 5. Koon HW, Ho S, Hing TC, et al. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother 2014; 58:4642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koon HW, Shih DQ, Hing TC, et al. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother 2013; 57:3214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koon HW, Su B, Xu C, et al. Probiotic Saccharomyces boulardii CNCM I-745 prevents outbreak-associated Clostridium difficile-associated cecal inflammation in hamsters. Am J Physiol Gastrointest Liver Physiol 2016; 311:G610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koon HW, Wang J, Mussatto CC, et al. Fidaxomicin and OP-1118 inhibit C. difficile toxin A- and B-mediated inflammatory responses via inhibition of NF-кB activity. Antimicrob Agents Chemother 2017; 62:e01513–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Ghali S, Xu C, et al. Ceragenin CSA13 reduces Clostridium difficile infection in mice by modulating the intestinal microbiome and metabolites. Gastroenterology 2018; 154:1737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pothoulakis C, LaMont JT, Eglow R, et al. Characterization of rabbit ileal receptors for Clostridium difficile toxin A: evidence for a receptor-coupled G protein. J Clin Invest 1991; 88:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li S, Shi L, Yang Z, et al. Critical roles of Clostridium difficile toxin B enzymatic activities in pathogenesis. Infect Immun 2015; 83:502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hing TC, Ho S, Shih DQ, et al. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 2013; 62:1295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowardin CA, Kuehne SA, Buonomo EL, Marie CS, Minton NP, Petri WA Jr. Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. MBio 2015; 6:e02386–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coffing H, Priyamvada S, Anbazhagan AN, et al. Clostridium difficile toxins A and B decrease intestinal SLC26A3 protein expression. Am J Physiol Gastrointest Liver Physiol 2018; 315:G43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng J, Hirota SA, Gross O, et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 2010; 139:542–552, 52 e1-3. [DOI] [PubMed] [Google Scholar]
- 16. Rello S, Stockert JC, Moreno V, et al. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005; 10:201–8. [DOI] [PubMed] [Google Scholar]
- 17. Warren CA, van Opstal EJ, Riggins MS, et al. Vancomycin treatment’s association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob Agents Chemother 2013; 57:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jose S, Mukherjee A, Abhyankar MM, et al. Neutralization of macrophage migration inhibitory factor improves host survival after Clostridium difficile infection. Anaerobe 2018; 53:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morteau O, Castagliuolo I, Mykoniatis A, et al. Genetic deficiency in the chemokine receptor CCR1 protects against acute Clostridium difficile toxin A enteritis in mice. Gastroenterology 2002; 122:725–33. [DOI] [PubMed] [Google Scholar]
- 20. Chou CC, Fine JS, Pugliese-Sivo C, et al. Pharmacological characterization of the chemokine receptor, hCCR1 in a stable transfectant and differentiated HL-60 cells: antagonism of hCCR1 activation by MIP-1 β. Br J Pharmacol 2002; 137:663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, Waaijman T, Scheper RJ, Gibbs S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol 2012; 132:216–25. [DOI] [PubMed] [Google Scholar]
- 22. Yu H, Chen K, Sun Y, et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol 2017; 24:e00037–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors 2005; 23:33–41. [DOI] [PubMed] [Google Scholar]
- 24. Jose S, Madan R. Neutrophil-mediated inflammation in the pathogenesis of Clostridium difficile infections. Anaerobe 2016; 41:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelly CP, Becker S, Linevsky JK, et al. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest 1994; 93:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Höglund P, Haila S, Socha J, et al. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 1996; 14:316–9. [DOI] [PubMed] [Google Scholar]
- 27. Byeon MK, Westerman MA, Maroulakou IG, et al. The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene 1996; 12:387–96. [PubMed] [Google Scholar]
- 28. Ding X, Li D, Li M, Tian D, Yu H, Yu Q. Tumor necrosis factor-α acts reciprocally with solute carrier family 26, member 3, (downregulated-in-adenoma) and reduces its expression, leading to intestinal inflammation. Int J Mol Med 2018; 41:1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao F, Yu Q, Li J, et al. Slc26a3 deficiency is associated with loss of colonic HCO3- secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol 2014; 211:161–75. [DOI] [PubMed] [Google Scholar]
- 30. Ding X, Li D, Li M, et al. SLC26A3 (DRA) prevents TNF-alpha-induced barrier dysfunction and dextran sulfate sodium-induced acute colitis. Lab Invest 2018; 98:462–76. [DOI] [PubMed] [Google Scholar]
- 31. Saavedra PHV, Huang L, Ghazavi F, et al. Apoptosis of intestinal epithelial cells restricts Clostridium difficile infection in a model of pseudomembranous colitis. Nat Commun 2018; 9:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buonomo EL, Madan R, Pramoonjago P, Li L, Okusa MD, Petri WA Jr. Role of interleukin 23 signaling in Clostridium difficile colitis. J Infect Dis 2013; 208:917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu YW, Chen YH, Chen JW, Tsai PJ, Huang IH. Immunization with recombinant TcdB-encapsulated nanocomplex induces protection against Clostridium difficile challenge in a mouse model. Front Microbiol 2017; 8:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Li S, Yang Z, et al. Cysteine protease-mediated autocleavage of Clostridium difficile toxins regulates their proinflammatory activity. Cell Mol Gastroenterol Hepatol 2018; 5:611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.