Abstract
Radial glia-like neural stem cells (RGLs) in the dentate gyrus subregion of the hippocampus give rise to dentate granule cells (DGCs) and astrocytes throughout life, a process referred to as adult hippocampal neurogenesis. Adult hippocampal neurogenesis is sensitive to experiences suggesting that it may represent an adaptive mechanism by which hippocampal circuitry is modified in response to environmental demands. Experiential information is conveyed to RGLs, progenitors and adult-born DGCs via the neurogenic niche that is comprised of diverse cell-types, extracellular matrix, and afferents. Understanding how the niche performs its functions may guide strategies to maintain its healthspan and provide a permissive milieu for neurogenesis. Here, we first discuss representative contributions of niche-cell types to regulation of NSC homeostasis and maturation of adult-born DGCs. We then consider mechanisms by which the activity of multiple niche cell-types maybe coordinated to communicate signals to NSCs. Finally, we speculate how NSCs integrate niche-derived signals to govern their regulation.
In Brief
Vicidomini, Guo et al offer a framework for thinking about how the niche sustains adult hippocampal neurogenesis by supporting communication, cross talk and signal integration.
Introduction
Radial glia-like neural stem cells (RGLs) in the dentate gyrus subregion of the hippocampus give rise to dentate granule cells (DGCs) and astrocytes throughout life, a process referred to as adult hippocampal neurogenesis(Bonaguidi et al., 2012; Garcia et al., 2004; Goncalves et al., 2016b; Pilz et al., 2018; Seri et al., 2001). While much less is known about adult-born astrocytes, adult-born DGCs integrate into hippocampal circuitry by remodeling the network and ultimately, contribute to hippocampal dependent learning and memory and regulation of emotion (Anacker and Hen, 2017; Goncalves et al., 2016b; Miller and Sahay, 2019; Toni and Schinder, 2015; Tuncdemir et al., 2019). Levels of adult hippocampal neurogenesis are highly sensitive to experience (Cope and Gould, 2019; Goncalves et al., 2016b; Kempermann et al., 1998; Mirescu and Gould, 2006; van Praag et al., 2000; Yun et al., 2016) suggesting that neurogenesis may represent an adaptive mechanism by which hippocampal circuit performance is optimized in response to demands of the environment. Experience is conveyed to RGLs, neuroblasts and immature adult-born DGCs via signals sensed by the hippocampal neurogenic niche that is comprised of diverse local cell-types including astrocytes, DGCs, inhibitory interneurons, endothelial cells, extracellular matrix (ECM), and subcortical neurons that project to the DG. Thus, the local and extended niche enables NSCs to listen and respond to changes in neural activity and systemic factors (Guo and Sahay, 2017). Understanding how the niche performs its functions may guide strategies to maintain its health throughout the lifespan and provide a permissive milieu for adult hippocampal neurogenesis.
A swath of evidence generated over several decades describes how different kinds of experiences affect neural stem cell and progenitor proliferation, and differentiation and survival of adult-born DGCs(Cope and Gould, 2019; Dranovsky et al., 2011; Encinas et al., 2008; Goncalves et al., 2016b; Song et al., 2016). However, much less is understood about how different cell-types within the local and extended niche communicate to NSCs and adult-born DGCs to mediate the effects of experience on adult hippocampal neurogenesis. Experience modulates NSCs by governing quiescence (state of reversible growth arrest) or activation decisions and symmetric/asymmetric self- renewal. These fundamental decisions made by the NSC are essential for homeostasis: maintenance of reservoir of NSCs ready for mobilization in response to experiential demands. Not surprisingly, NSCs do not act autonomously, but instead, sense and integrate a plethora of niche-derived signals communicated by local, distal and systemic actors. Transplantation studies exemplify the role of niche in instructing and respecifying fate of biased progenitors (Gage et al., 1995; Seidenfaden et al., 2006). Additionally, many of these local niche cell-types also govern the maturation and synaptic integration of adult-born DGCs. Here, we first discuss representative contributions of distinct niche-cell types to regulation of NSC homeostasis and maturation of adult-born DGCs with each section conveying outstanding questions. We then consider mechanisms by which the activity of multiple niche cell-types maybe coordinated to communicate signals to NSCs. Finally, we speculate how NSCs integrate these multiple niche-derived signals to make decisions.
Anatomical constraints of the neurogenic niche
Ultrastructural analysis and high resolution imaging provides a ground truth for understanding how NSCs and immature adult-born DGCs may respond to local niche signals. The subgranular zone of the DG, where neural stem cells differentiate into DGCs, is highly vascularized(Palmer et al., 2000). EM analysis has revealed that RGL cell bodies have concave edges presumably reflecting the convex curvature of adjacent DGC bodies. The primary (apical) processes of RGLs navigate the granule cell layer to branch extensively in the inner molecular layer (Moss et al., 2016). Secondary and tertiary processes contact DGC dendritic spines and apposing axon terminals of entorhinal cortical, subcortical projections and mossy cells. RGL processes do not establish synaptic contacts, but much like astrocytes, wrap around or form tight appositions with axon terminals and spines(Moss et al., 2016). Larger diameter processes, like astrocytic endfeet, wrap local blood vessels creating a blanket of coverage along with astrocytic processes. Basal processes project along the subgranular zone axis and into the hilus where they are positioned to sense signals from hilar neurons(Moss et al., 2016).
Electron microscopy analysis of retrovirally labeled adult-born DGCs has revealed that dendritic spines of adult-born DGCs establish contacts with perforant path boutons that have already formed synapses onto mature DGCs(Toni et al., 2007)(Figure 1). Local inhibitory interneurons form synaptic contacts with early neuroblasts, maturing abDGCs and DGCs(Catavero et al., 2018; Dieni et al., 2016; Esposito et al., 2005; Freund and Buzsaki, 1996; Ge et al., 2006; Heigele et al., 2016; Marin-Burgin et al., 2012; Miller and Sahay, 2019; Overstreet-Wadiche et al., 2006; Pelkey et al., 2017; Song et al., 2016; Song et al., 2013). Serial section immune electron microscopy and electrophysiological recordings have demonstrated existence of astrocytic perisynaptic ensheathments of dendritic spines and mossy fiber terminals of adult-born DGCs(Krzisch et al., 2015; Sultan et al., 2015). 2 photon imaging has shown that dendrites of immature adult-born DGCs undergo pruning both at steady state and following enriched experience(Goncalves et al., 2016a). Although the maturation of dendritic spines and mossy fiber terminals of adult-born DGCs continues for several months(Faulkner et al., 2008; Goncalves et al., 2016a; Lemaire et al., 2012; Sun et al., 2013; Toni et al., 2008; Toni et al., 2007), the tempo of maturation is modifiable by experience(Alvarez et al., 2016; Piatti et al., 2011; Snyder et al., 2009; Vivar et al., 2013; Zhao et al., 2006) suggesting that different niche cell-types relay experiential information to regulate the rate of neurogenesis. Imaging studies also identify microglia, oligodendrocytes as other local niche residents(Braun et al., 2015; Ribak et al., 2009; Shapiro et al., 2009; Sierra et al., 2010). The structural organization of DG neurogenic niche suggests extensive cross talk between niche cell-types and positions NSCs and adult-born DGCs as integrators of diverse signals from the niche. We discuss examples of these dialogues in the next sections in the hope of shedding light on the logic underlying anatomical constraints of the niche. In each section, we begin with the relationship between niche cell-type and NSCs prior to transitioning to dialogue between niche-cell type and adult-born DGCs.
Figure 1. Local DG circuits regulate NSC homeostasis and integration of adult-born neurons.
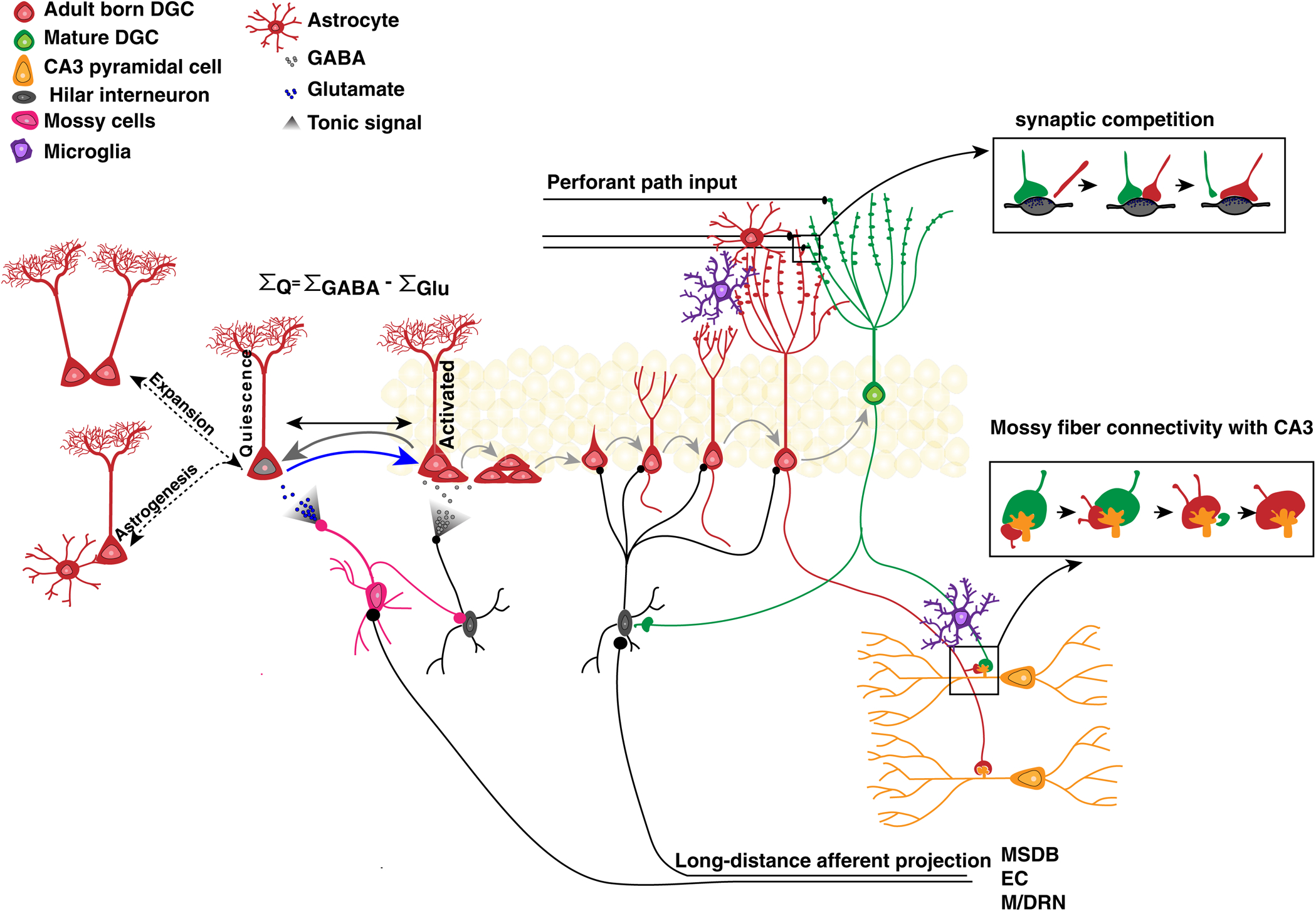
Local hilar inhibitory interneurons, mossy cells and potentially, astrocytes relay local and distal inputs onto NSCs and differentiating adult-born DGCs. NSCs summate GABAergic and Glutamatergic inputs to mediate quiescence-activation and self-renewal decisions. Tonic GABA release onto NSCs promotes quiescence (grey arrow) whereas mossy cell dependent glutamate release promotes activation of NSCs (blue arrow). Afferent and efferent synaptogenesis of adult-born DGCs occurs at pre-existing synapses and astrocytes and microglia may modulate synaptic competition through vesicular release of D-serine and secreted synaptogenic factors and trogocytosis. MSDB: medial septum/diagonal band, M/DRN: median/dorsal raphe, EC: entorhinal cortex. See text for details.
Local neuronal cell-types coordinate neurogenesis and network activity
Dentate granule cells are abundant niche cells and ensconce the cell bodies of NSCs located in the subgranular zone (Figure 1). DGCs receive excitatory inputs from diverse cortical and subcortical circuits and other hippocampal subregions and as such, are well positioned to relay signals to NSCs and immature adult-born DGCs (Miller and Sahay, 2019). Growing evidence suggests that neuronal activity may recruit DGCs to modulate NSC homeostasis via secretion of factors. Several studies have identified activity dependent production of pro-neurogenic secreted factors. A pioneering study found that electroconvulsive shock treatment induced a transient increase in GADD45b expression in DGCs (Ma et al., 2009). Gadd45b functions as a DNA demethylase and promotes expression of secreted factors such as BDNF and FGF to stimulate neural progenitor proliferation and dendritic maturation of adult-born DGCs. Neural activity and running were shown to decrease levels of sFRP3, secreted frizzled-related protein 3, an extra-cellular Wnt inhibitor, in DGCs (Jang et al., 2013). Reduction of sFRP3 levels promoted NSC activation without affecting lineage choice. Additionally, loss of sFRP3 also accelerated dendritic growth and spine formation suggesting that it normally functions as a brake that calibrates the tempo of neuronal differentiation. DGCs also express other neurogenic ligands such as vascular endothelial growth factor C (VEGF-C) that signals through VEGFR3 in NSCs to promote neurogenesis(Han et al., 2015). Exercise, enriched environment and administration of selective serotonin reuptake inhibitors, have been shown to decrease BMP signaling by either reducing BMP levels or increasing expression of noggin, an extra-cellular BMP inhibitor, in DGCs (Brooker et al., 2017; Gobeske et al., 2009). Viral overexpression of noggin in DGCs (Gobeske et al., 2009) or genetic ablation of BMP signaling in neural stem and progenitor cells (NSPCs, when neither population is selectively targeted) promoted proliferation (Mira et al., 2010). Interestingly, inducible elimination of dendritic spines of mature DGCs is accompanied by robust activation of NSCs (McAvoy et al., 2016) and elevation in noggin expression in DGCs (McAvoy and Sahay, unpublished results). These observations suggest that DGCs modulate NSC quiescence by regulation of BMP signaling in NSCs. Interestingly, the RNA-binding protein FXR2, which controls the stability of noggin mRNA, is expressed in mature DGCs. Loss of function experiments have shown that FXR2 deficiency results in increased expression of Noggin and proliferation of NSCs(Guo et al., 2011). In addition to these ligands, many other ligand-receptor pairs that are expressed in complementary manner in NSCs and DGCs (Dong et al., 2019; Engler et al., 2018; Lie et al., 2005; Semerci et al., 2017) are also likely to mediate different kinds of experiential signals. Together, these examples illustrate how extrinsic signals induce expression of DGC derived secreted factors to regulate NSCs and immature adult-born DGCs. However, many questions remain to be addressed regarding the mechanisms by which neural activity governs release of secreted factors from DGCs to modulate NSCs. Since most DGCs do not project into the inner molecular layer [except for semilunar granule cells(Williams et al., 2007) and a few adult-born DGCs (Luna et al., 2019)] where RGL’s primary apical branches are located, it is unclear whether DGC derived ligands signal to their receptors on NSCs via contact between cell bodies or mossy fiber dependent release into the hilus where RGLs have basal branches (Figure 1, 2a). Do specific patterns of neuronal activity in DGCs release different neurogenic factors? How are transcription factors that regulate NSC homeostasis and maturation of adult-born DGCs (Andersen et al., 2014; Beckervordersandforth et al., 2015; Hsieh, 2012; Hsieh and Zhao, 2016) recruited to govern expression of secreted factors?
Figure 2. NSCs integrate secreted and juxtacrine signals from diverse niche cell-types to maintain homeostasis.
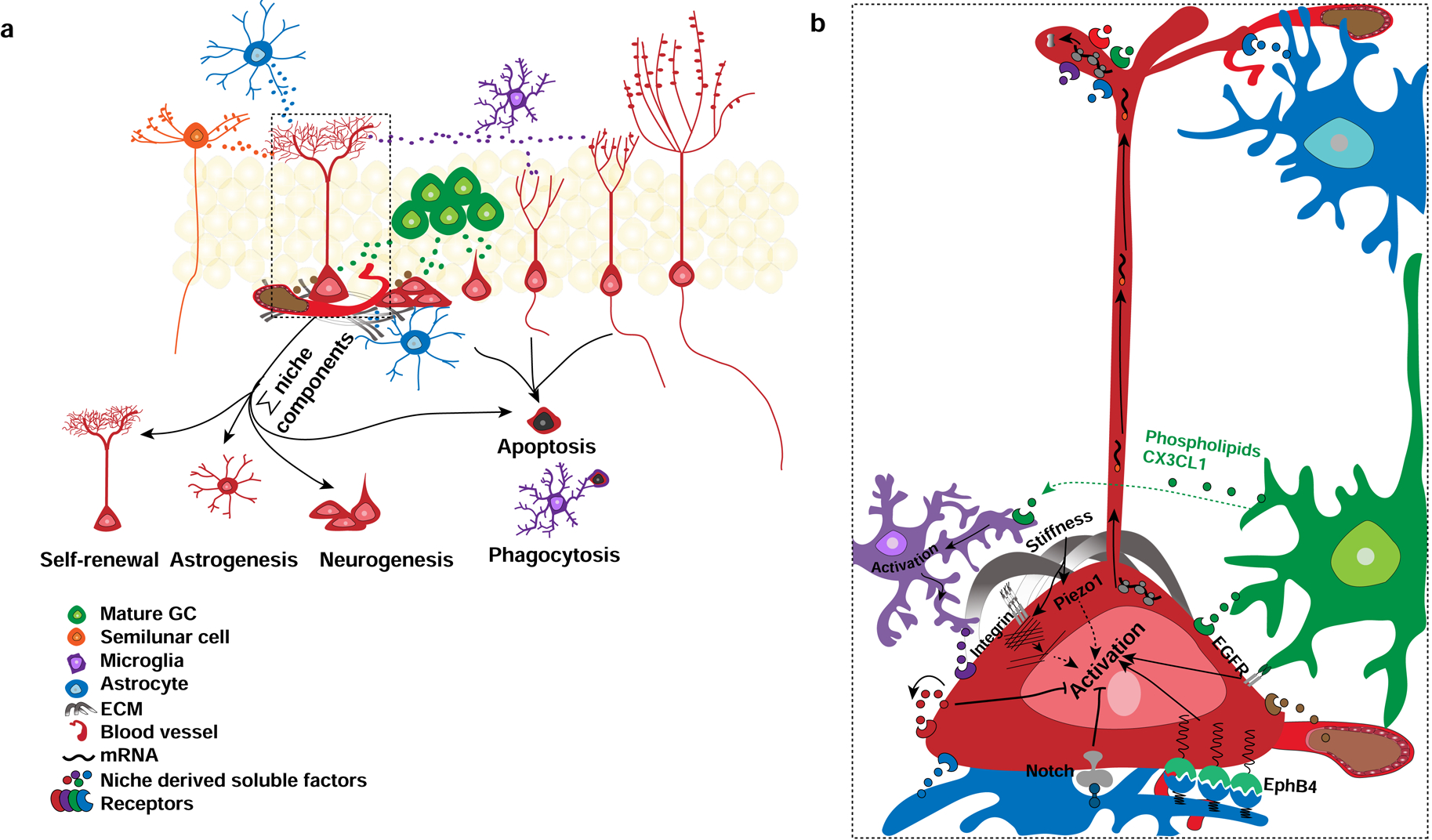
a. NSCs integrate signals from astrocytes, DGCs, semilunar granule cells, inhibitory interneurons, blood vessels, extracellular matrix (ECM) and inhibitory interneurons to mediate quiescence-activation and self-renewal decisions. Different niche actors may release ligands onto discrete domains of NSCs. Receptors in the apical process of RGLs may sense ligands released by axon terminals of DG afferents or of semilunar granule cells in the inner molecular layer. Competition for niche-derived factors may trigger microglial dependent pruning of NSC numbers to maintain homeostasis. b. Magnification of RGL in dashed line box in a. conveying niche derived secreted ligands [astrocyte derived factors (blue): IL1b, Wnt3a; vasculature derived factors (brown): IGF; mature DGC derived factors (Green): sFRP3, VEGF-C, Noggin, BMP, Fracktalkine, microglia derived factors (purple)] signal in paracrine or juxtacrine modes. DGCs may regulate NSC behavior through recruitment of microglia via release of phospholipids and fracktalkine. NSCs and progenitors may regulate their fate choices by autocrine signaling [(red): VEGF, Mfge8]. Extracellular matrix (ECM) regulates NSC behavior through ligands such as laminin, reelin and stiffness dependent modulation of Piezo signaling in NSCs. Within NSCs, mRNAs encoding ligands maybe transported along the radial apical process for local translation in response to physiological signals. See text for details.
DGCs also recruit circuit mechanisms mediated by local inhibitory interneurons (INs) and mossy cells to modulate NSC homeostasis and maturation of abDGCs (Figure 1). The DG is populated by distinct INs such as parvalbumin (PV) basket cells and axo-axonic cells, somatostatin hilar perforant path, hilar commissural associational pathway, neurogliaform/Ivy cells, molecular perforant path (Deshpande et al., 2013; Li et al., 2013; McAvoy et al., 2016; Vivar et al., 2012), CCK, NPY, Calretinin, and VIP interneurons (Freund and Buzsaki, 1996; Pelkey et al., 2017). NSCs express GABAA receptors in both their cell bodies and processes, whereas glutamate transporters and AMPA receptors are found in the cell bodies and apical processes, respectively (Renzel et al., 2013). Activation of PV INs, but not somatostatin (SST) and vasoactive intestinal polypeptide (VIP) INs, promoted quiescence of NSCs and reversed social isolation induced increase in symmetric divisions (Song et al., 2012). Because NSCs appear to lack functional GABAergic synapses, PV INs are thought to regulate NSCs by tonic inhibition mediated by GABA spillover from PV-DGC synapses (Song et al., 2012; Tozuka et al., 2005; Wang et al., 2005). In contrast to NSCs, neural progenitors have functional GABAergic synapses (Tozuka et al., 2005) and PV INs (but not SST INs) inhibit their proliferation and survival (Song et al., 2013). DG interneurons receive inputs from different extra-hippocampal and subcortical circuits(Freund and Buzsaki, 1996; Pelkey et al., 2017). The medial septum/diagonal band (MSDB) GABAergic neurons constitute a major projection to DG INs and PV INs in particular (Freund and Antal, 1988; Salib et al., 2019). Consistent with this innervation pattern, optogenetic stimulation of MS GABAergic projections depolarized PV INs and promoted NSC quiescence (Bao et al., 2017).
Mossy cells project both ipsilaterally and contralaterally to form monosynaptic excitatory synapses onto DGCs and disynaptically inhibit DGCs via hilar INs(Scharfman and Myers, 2012). Ex vivo chemogenetic or optogenetic activation of mossy cells depolarized NSCs and hilar INs including PV INs(Yeh et al., 2018) (Figure 1). In vivo chemogenetic activation or inhibition of mossy cells promoted NSC quiescence and activation through recruitment of interneurons, respectively. The same study suggested that mossy cells may recruit hilar INs to exert their effects on quiescence whereas, direct release of glutamate from mossy cells depolarizes and activates NSCs.
Electrophysiological and viral synaptic tracing studies have demonstrated that adult-born DGCs progressively recruit different INs during their differentiation to regulate synapse and dendritic maturation (Bergami et al., 2015; Dieni et al., 2016; Esposito et al., 2005; Ge et al., 2006; Marin-Burgin et al., 2012; McAvoy et al., 2016; Miller and Sahay, 2019; Overstreet-Wadiche et al., 2006; Vivar et al., 2012). Like that seen during embryonic and early brain development (Ben-Ari et al., 1997), dendritic depolarizing GABAergic synapses coupled with calcium signaling and CREB dependent transcription promotes the establishment of first glutamatergic synapses onto adult-born DGCs(Esposito et al., 2005; Ge et al., 2006; Jagasia et al., 2009; Markwardt et al., 2009; Overstreet-Wadiche et al., 2006). Ivy/neurogliaform (NGF) cells are amongst the earliest GABAergic presynaptic partners and are thought to couple newborn DGC depolarization with disinhibition of mature DGCs (Markwardt et al., 2011). During this window, hilar mossy cells are the first to establish glutamatergic synapses onto DGCs thereby potentially coordinating NMDA receptor activation dependent AMPA receptor insertion into silent synapses and disynaptic GABAergic depolarization (Chancey et al., 2013; Kumamoto et al., 2012). Dendritic elaboration and dendritic spine formation are modified by experience during the first few weeks of adult-born DGC maturation (Bergami et al., 2015; Goncalves et al., 2016a; Sun et al., 2013). Mature DGCs recruit PV INs during this stage to depolarize immature DGCs and regulate these experience sensitive developmental processes (Alvarez et al., 2016). Interestingly, pairing of glutamatergic subthreshold potentials with strong stimulation of GABAergic inputs suppressed firing of immature adult-born DGCs by shunting inhibition(Heigele et al., 2016). Thus, GABAergic INs can regulate immature DGC maturation by both excitation and shunting inhibition in response to network activity. Establishment of perisomatic inhibition and perforant path-DGC synapses is followed by an extended period of maturation, over several months, during which dendritic spine and dendritic complexity continue to be refined by experience (Figure 1).
Together, these studies suggest that DGCs, local INs and mossy cells regulate NSC homeostasis and maturation of adult-born DGCs (Figure 1). Growing evidence supports a role for extended inhibitory neuron networks that traverse brain regions and project across hippocampal subregions (Caputi et al., 2013; Freund, 2003; Klausberger and Somogyi, 2008; Salib et al., 2019; Szabo et al., 2017)to regulate and coordinate principal cell activity. However, more studies are needed to delineate the precise contributions of different local-DG INs and DG projecting-INs to NSC homeostasis and neurogenesis. It is likely that just as in the case of PV INs, distinct DG INs are also regulated by different extra-hippocampal inputs to modulate NSCs and neurogenesis. Inhibitory neurons have distinct laminar distributions of axonal and dendritic arborizations that influence dendritic, somatic, and distal compartments of principal cells to govern spiking, gate synaptic plasticity, and synchronize neuronal firing. Understanding how topographic organization and physiological patterns of IN activity relate to their roles in modulation of NSC homeostasis and neurogenesis will inform how hippocampal network activity calibrates levels of neurogenesis.
Glial niche actors: Astrocytes
Astrocytes are glial cell-types abundantly present in the DG neurogenic niche that provide both functional and structural support for NSCs and maturing adult-born DGCs. A pioneering in vitro co-culture study demonstrated that mature astrocytes from adult hippocampus, but not adult spinal cord, promote proliferation and neuronal fate specification of adult neural progenitors (Song et al., 2002). Based on co-culturing of neural stem cells with astrocytes or astrocyte conditioned media, the authors predicted roles for both astrocytic membrane-bound and diffusible factors in regulation of neurogenesis. Using cultured adult NSPCs, subsequent studies have identified pro-neurogenic (interleukin IL-1b and IL-6, FGF-2) and inhibitory neurogenic astrocytic derived factors (Decorin, IGFBP6)(Barkho et al., 2006; Kirby et al., 2013). Astrocytes release gliotransmitters (Araque et al., 2014) that may modulate NSC activation. Preliminary evidence from an in vitro study suggested that ATP derived from astrocytes promotes proliferation of adult hippocampal neural stem and progenitors cells (Cao et al., 2013). Although hippocampal astrocytes express diverse repertoire of membrane bound and secreted factors (Clarke et al., 2018; Hillen et al., 2018; Morel et al., 2017), many with potential roles in neurogenesis, a paucity of studies have examined their contributions to regulation of NSC homeostasis in vivo. One study showed that Ephrin-B2 expression is enriched in hilar astrocytes and found that viral downregulation of Ephrin-B2 in the DG decreased neuronal differentiation of progenitors without affecting progenitor and stem cell proliferation (Ashton et al., 2012). It is likely that transgenic lines (such as GLAST CreERT2)(Mori et al., 2006) used to manipulate RGLs may also affect astrocytic contributions to RGL homeostasis.
Astrocytes, as part of tripartite synapses that involve pre-synaptic terminals and post-synaptic specializations, are thought to regulate excitatory synapse formation and synaptic functions in the developing and adult brain (Araque et al., 2014; Christopherson et al., 2005) (Adamsky et al., 2018; Chung et al., 2015; Farhy-Tselnicker and Allen, 2018). Astrocytes secrete soluble factors such as thrombospondins, glypicans, chordin-like 1 and hevin that promote excitatory synapse formation, maturation and refinement during development (Allen et al., 2012; Blanco-Suarez et al., 2018; Farhy-Tselnicker et al., 2017; Kucukdereli et al., 2011). Additionally, astrocytes also release factors that antagonize synapse formation such as secreted protein acidic and rich in cysteine (SPARC) that inhibits Hevin (Kucukdereli et al., 2011). In addition to secreted factors, astrocytes also express fatty acid binding proteins that participate in uptake and transport of fatty acids and that may influence excitatory synapse formation (Ebrahimi et al., 2016). Interestingly, many of these factors such as hevin and Fabp7 are expressed at high levels in DG astrocytes (Mongredien et al., 2019) suggesting that they may continue to play a role in synapse maturation and refinement of adult-born DGCs. Direct evidence for a role of astrocytes in regulating maturation of adult-born DGCs comes from a study that deployed two conditional genetic approaches to block astrocytic vesicular release (Sultan et al., 2015). The authors found that inhibition of astrocytic vesicular release, decreased extra-cellular levels of D-Serine but not glutamate, glycine, or GABA, and reduced dendritic arbor complexity and length, and dendritic spine density of adult-born DGCs. These morphological changes were accompanied by reductions in functional excitatory synapses onto adult-born DGCs. Furthermore, exogenous D-serine administration rescued alterations in dendritic arbors, spine density and excitatory synapse to different degrees. Interestingly, preexisting spines of mature DGCs were not affected suggesting that astrocytic vesicular release influences the formation, rather than pruning, of dendritic spines. While the genetic manipulations used in this study affected astrocytic function and not astrocytic ensheathment of synapses, it is plausible that structural alterations of astrocytes may disrupt topographic control of dendritic spine formation and dendritic elaboration.
Astrocytes may also modulate DGC and IN activity to regulate NSC homeostasis and maturation of adult-born DGCs as described earlier (Figure 1, 2a). Astrocytes sense neural activity and modulate synaptic transmission and plasticity via release of gliotransmitters(Adamsky et al., 2018; Araque et al., 2014; Haydon, 2001). In the adult DG, astrocytes release glutamate that transiently strengthens excitatory synaptic transmission by activating NMDARs on their juxtaposed presynaptic partners (Jourdain et al., 2007). In addition, astrocytes may play a role in synchronizing network activity locally via spread of gliotransmitters (Araque et al., 2014)or distally through gap junction coupling with 100s of other astrocytes to regulate neurogenesis (Chai et al., 2017). Whether astrocytes are coupled to their ancestral NSCs is not known.
Glial niche actors: Microglia, the brain’s immune cells
Microglia are the brain’s macrophages that maintain neuronal homeostasis by scanning, surveillance, phagocytosis and eradication of apoptotic cells and debris (Figure 2a). Microglia have highly motile processes that can contact different cell types and can respond to changes in the local environment (Davalos et al., 2005; Nimmerjahn et al., 2005; Paris et al., 2018). Fate mapping studies have demonstrated that, in mice, microglia arise from the yolk sac progenitor cells that invade the brain during mid gestation and then differentiate into microglia (Ginhoux et al., 2010). In the mouse hippocampus, the peak of microglia density is reached around postnatal day 15 when it is inferred that microglia show heightened synaptic pruning activity (Paolicelli et al., 2011). In the adult DG, electron microscopy in combination with Iba1 immunohistochemistry has revealed microglia in hilus and molecular layers tightly apposed to DGC bodies (Ribak et al., 2009; Shapiro et al., 2009). Confocal imaging shows microglia distributed across molecular layers and surrounding NSPCs and DGCs (Mosher et al., 2012; Sierra et al., 2010). Microglia that populate the hippocampal neurogenic niche are highly proliferative and undergo rapid turnover (Askew et al., 2017). Single cell transcriptomics has revealed that microglia barely express genes associated with the canonical activation state of microglia, suggesting that in the adult DG, microglia exist in the M2 resting state and exert some of their functions in this state (Artegiani et al., 2017). Consistent with this idea, unchallenged ramified microglia were found to phagocytose late neural progenitors/early neuroblasts in the adult SGZ thereby, potentially regulating survival of newborn neurons (Sierra et al., 2010). Indeed, EM analysis detected processes of microglial cells surrounding DGCs at the hilar-GC layer suggestive of microglial dependent phagocytosis of DGCs (Ribak et al., 2009). Furthermore, partial genetic ablation of microglia in adult mice decreased numbers of adult-born neuroblasts (Kreisel et al., 2019).
Early studies suggested a role for activated microglia in regulation of neurogenesis in vivo (Ekdahl et al., 2003; Monje et al., 2003) and ex vivo(Butovsky et al., 2006) via secretion of inflammatory factors. Neuronally derived signals are thought to support resting and activated microglia states, each associated with distinct profiles of microglial secreted chemokines and pro- and anti- inflammatory cytokines (Kierdorf and Prinz, 2017). Neuronally derived fracktalkine acting on its receptor, CX3CR1, expressed in microglia is thought to promote adult hippocampal neurogenesis. Mice lacking CX3CR1 exhibit reduced proliferation and numbers of adult-born DGCs (Vukovic et al., 2012). Intracerebroventricular infusion of CX3CR1 blocking antibodies into adult rats increased hippocampal IL-1Beta levels and decreased progenitor proliferation (Bachstetter et al., 2011). Conversely, fracktalkine infusions in aged, but not adult, rats increased progenitor proliferation (Bachstetter et al., 2011). Ex vivo studies also support a role for CX3CL1-CX3CR1 signaling in increasing proliferation (Vukovic et al., 2012). In addition to fracktalkine, hippocampal neurons secrete many other factors that may potentially, modulate microglia. Overexpression of vascular endothelial growth factor A (VEGF-A, referred to as VEGF), a neuronally secreted angiogenic factor that signals through VEGFR1 and VEGFR2, in hippocampal excitatory neurons increased microglial proliferation and adult hippocampal neurogenesis. Whether microglia, rather than endothelial cells or neurons, mediate these VEGF dependent effects on neurogenesis is not clear (Kreisel et al., 2019). Although genetic targeting of pro-neurogenic factors within microglia has not been performed to date, an elegant study showed that conditional ablation of a core retromer transport protein (critical for endosomal membrane trafficking of transmembrane proteins) in microglia resulted in increased hippocampal microglial density (Appel et al., 2018). Interestingly, the number of immature DGCs was reduced because of increased NSPC proliferation and failure to exit cell cycle. It is plausible that deletion of the retromer protein impedes trafficking and secretion of specific transmembrane receptors in microglia that sense neurogenic signals. How is microglial activation correlated with both increased and decreased neurogenesis? One possibility is that distinct subpopulations of microglia are activated, each with opposing roles in promoting neurogenesis (Hammond et al., 2018; Masuda et al., 2019).
Neural progenitors may act as niche cells and directly modulate microglia independent of local neurons. Conditioned media obtained from early postnatal cortical neural progenitors increased microglial functions such as phagocytosis, chemotaxis and proliferation ex vivo and in the striatum (Mosher et al., 2012). The authors found that cortical NPC secreted VEGF was sufficient to regulate microglial functions in the striatum. Whether adult hippocampal NSPCs derived VEGF regulates microglia is yet to be determined (Kirby et al., 2015). If so, NPCs may participate in a regulatory feedback loop that recruits microglia to control NSPC activation and phagocytosis of early NPCs and neuroblasts.
Studies in the developing brain suggest a role for microglia in regulation of synaptic pruning via engulfment of presynaptic terminals and dendritic spines (Schafer et al., 2012) (Paolicelli et al., 2011; Tremblay et al., 2010; Weinhard et al., 2018). Neuronal activity appears to drive this process and microglia preferentially engulf less active presynaptic inputs in the visual system (Schafer et al., 2012; Tremblay et al., 2010). Not surprisingly, and much like other niche actors, microglia also regulates maturation of adult-born DGCs. Cx3cr1 null mice exhibit an increase in activated microglia in DG and elevated levels of pro-inflammatory cytokines in the hippocampus (Bolos et al., 2018). In addition, there was increased deposition of extracellular matrix proteoglycans some of which restrict IN plasticity and consequently, may affect IN-NSC and adult-born DGC communication (Bolos et al., 2018; Fawcett et al., 2019). Adult-born DGCs had less complex dendritic arbors, fewer dendritic spines and smaller mossy fiber terminals. Although the use of null mutants encumbers interpretation of these results, acute genetic deletion of retromer protein in microglia in adulthood also impaired dendritic arborization and decreased dendritic spine density of immature adult-born DGCs (Appel et al., 2018). Whether the dendritic spine alterations of adult-born DGCs are due to increased microglial dependent phagocytosis is debated. This is because a recent study found no evidence for microglial dependent phagocytosis of dendritic spines or functional synapses in CA1 (Paolicelli et al., 2011; Weinhard et al., 2018). Instead, the authors inferred that microglia participate in trogocytosis of axons and presynaptic terminals and generation of filopodia at preexisting mature spines (Figure 1, 2a).
The precise contributions of microglia to NSC homeostasis and maturation of adult-born DGCs necessitate more studies that acutely manipulate signaling pathways within these different cell-types. Interestingly, DG microglia may differ from CA1 microglia in a limited set of genes suggesting that they may preferentially exhibit a neurogenic program. Indeed, some of these genes such as Axl and others that encode lipid binding, signaling and transport proteins have been previously implicated in neurogenesis (Kreisel et al., 2019). Do signals from NSPCs and adult-born DGCs regulate distinct aspects of microglial functions? Membrane phospholipids are thought to represent one such signal that promotes engulfment by macrophages (Weinhard et al., 2018). Do microglia regulate all or only specific inputs and output connectivity (inhibitory interneurons vs CA3) of adult-born DGCs? Given the prominent roles of inhibitory interneurons in governing adult hippocampal neurogenesis, it will be important to interrogate whether microglia regulate inhibitory synapse numbers.
Extracellular Matrix: Attachment, Signaling and Stiffness
The ECM is a network of diverse glycoproteins (eg: Tenascin C), proteogylcans (bearing heparan sulfate, chondroitin sulfate, or dermatan sulfate side chains) and cell adhesion molecules that surrounds cells to provide a functional scaffold for maintaining signaling gradients and stiffness (Figure 2b). Key components of ECM such Reelin, Tenascins, laminins and their receptors, integrins, have well-characterized roles in regulating progenitor proliferation, neurite extension, apical radial glial morphology and neuronal differentiation during development (Long and Huttner, 2019). Consistently, ECM proteins such as Laminin signal through integrins expressed in NSPCs to regulate NSPC homeostasis (Porcheri et al., 2014). Integrins are important for attachment of apical radial glial endfeet to basement membranes and maintenance of characteristic bipolar morphology of radial glial cells in the neocortex (Radakovits et al., 2009). Although cytoskeletal proteins, adhesion molecules and ECM ligands that maintain RGL architecture in the adult DG have yet to be defined, their roles in regulating neurogenesis are emerging. The integrin-linked kinase is expressed throughout apical processes of NSCs and not in progenitors in the adult DG. Conditional deletion of integrin-linked kinase in adult NSPCs resulted in increased proliferation due to enhanced downstream c-Jun N-terminal protein kinase (JNK) activity (Porcheri et al., 2014). Conditional deletion of reelin signaling in adult DG NPCs enhanced gliogenesis, and disrupted dendritic development of immature adult-born DGCs. Specifically, immature adult-born DGCs had reduced apical dendritic complexity and aberrant ramification of basal hilar dendrites (Teixeira et al., 2012).
Although a large number of secreted factors (Notch, FGFs, Wnts, BMPs, Sonic hedgehog) have been identified as regulators of adult hippocampal neurogenesis, the precise distribution of these ligands within the niche is poorly understood. It is highly unlikely that ligands freely diffuse through the ECM but instead, localize to discrete regions to facilitate communication between niche cell-types and NSPCs (Figure 2b). Indeed, ultrastructural analysis of the subepenymal layer in the anterior horn of the lateral ventricles has revealed extracellular matrix (ECM) projections called fractones because of their fractal structure that contact astrocytes, microglial cells, and NSPCs (Mercier, 2016; Mercier et al., 2002) Fractones are rich in N sulfate heparan sulfate proteoglycans that trap FGF2 to create pockets of NSPC proliferation (Kerever et al., 2007). While structural fractone analogs are not observed in the DG, it is plausible that functional ECM analogs might exist in the DG niche to localize ligands.
Ultrastructural analysis reveals tight packing of different niche cell-types and suggests a role for ECM in regulating mechanotransduction in NSCs. In vivo, atomic force microscopy measurements reveal a gradient of increasing stiffness from the SGZ upwards through the granule cell layer (Luque et al., 2016). Additionally, cultured adult NSPCs increase their own intrinsic mechanical properties in response to increased ECM stiffness (Rammensee et al., 2017). The lineage sensitivity of adult NSPCs to ECM stiffness is manifest during the first 12–36 hours ex vivo. Candidates for mechanosensors include the stretch activated cation channel, Piezo 1, whose activity is modulated by ECM stiffness in cultured human fetal cortical NSPCs (Pathak et al., 2014). Importantly, Piezo 1 is necessary for mechanically induced currents in hNSPCs (Pathak et al., 2014). Several intra cellular mechanisms have been invoked to convey ECM stiffness dependent signal transduction to bias lineage specification ex vivo. One group found a putative role for YAP (Yes Associated Protein)-Beta Catenin interaction and mobilization of Rho GTPAses (Keung et al., 2011; Rammensee et al., 2017) whereas another study showed that Piezo1 activity was necessary for nuclear localization of Yap1(Pathak et al., 2014). Whether Yap’s nuclear and cytoplasmic roles and Rho GTPAses also mediate ECM stiffness dependent mechanotransduction in adult DG NSPCs in vivo is to be determined.
Neural stem cells: Masters of their own destiny
Growing evidence suggest a role for stem cells as niche actors that regulate their own homeostasis and influence differentiation of their progeny (Figure 2b). In the subventricular zone, NSCs and progenitors produce an endozepine called diazepam binding inhibitor that inhibits GABA-A receptors and whose proteolytic product blocks the differentiation promoting effects of neuroblast derived GABA(Alfonso et al., 2012). As such, NSCs and progenitors maintain a proliferative state by antagonizing signals from their descendants (Alfonso et al., 2012). In the adult hippocampus, NSPCs regulate their own activation and quiescence through distinct autocrine and paracrine mechanisms. One study showed using VEGF-GFP reporter mice and ex vivo cultures that NSPCs are an important source of VEGF in the SGZ. Conditional ablation of VEGF in Nestin+ cells led to a transient increase in NSC proliferation and subsequent partial depletion. This autocrine/paracrine effect was mediated by VEGFR2 expressed in NSPCs (Kirby et al., 2015). Quiescent NSCs also exhibit elevated levels of Mfge8 (milk-fat globuleepidermal growth factor EGF factor 8) that promotes quiescence through b1integrin receptors expressed on NSCs (Zhou et al., 2018). Thus, NSCs may regulate themselves via autocrine and paracrine mechanisms.
Because receptors for many NSC derived ligands such as VEGF are also expressed in their progeny, neuroblasts and immature DGCs, and other niche actors (like microglia as discussed earlier), NSCs may regulate multiple stages of maturation of adult-born DGCs directly or indirectly through modulation of niche actors. For example, the NSC secreted factor pleiotrophin was shown to act on ALK receptors expressed on immature adult-born DGCs and influence their dendritic complexity, dendritic spine density and afferent connectivity (Tang et al., 2019). NSCs may also modify their own ECM milleu by synthesizing and depositing proteoglycans around them as suggested by ex vivo studies (Tham et al., 2010). How do NSCs secrete factors to self-regulate or modulate their niche? Embryonic cortical radial glial cells exhibit local translation of nuclear exported transcripts encoding signaling factors at their endfeet (Pilaz et al., 2016). NSCs may utilize a similar mechanism to secrete factors on demand via their endfeet-like processes (Figure 2b).
Systemic niche factors
RGLs sense and respond to blood-born circulatory factors through their apical processes that wrap around blood vessels. Heterochronic parabiosis studies that entail surgical joining of circulatory systems of young and aged mice have led to the identification of pro- and anti-neurogenic soluble factors (Villeda et al., 2011; Villeda et al., 2014). Other studies have identified pro-neurogenic plasma factors but it is not clear if these factors traverse the blood brain barrier or are secreted by hippocampal neurons to exert their pro-neurogenic effects (Katsimpardi et al., 2014; Moon et al., 2016; Moon et al., 2019). Several immune system chemokines have been identified that negatively regulate NSPC proliferation in the adult DG (Lee et al., 2013; Smith et al., 2015). With aging, immune T cells are found to localize in the SGZ and may potentially, impede proliferation akin to that seen in the SVZ (Dulken et al., 2019).
NSCs also express steroid hormone receptors that enable responsiveness to circulating glucocorticoids that promote quiescence and constrain dendritic complexity and dendritic spine density (Fitzsimons et al., 2013; Schouten et al., 2019). Whether a reverse dialogue between NSCs and peripheral tissues and blood exists remains largely unexplored. It is plausible that RGLs secrete factors that are taken up by blood to influence peripheral tissue homeostasis. Interestingly, patches of SGZ are thought to be hypoxic (Chatzi et al., 2016). However, it is not known whether the blood vessels that contact RGL processes is highly oxygenated. Further studies will inform how systemic factors and low oxygen act in concert to regulate activation-quiescence decisions of NSCs.
Competition in the niche
The signaling mechanisms described support a framework for thinking about how competition contributes to homeostasis in the adult hippocampal neurogenic lineage. Limitations in access to niche actors such as endothelial cells, astrocytes and microglia may result in competition within NSCs by influencing activation-quiescence decisions (Figure 2a). NSCs may use on-demand translation mechanisms at their end-feet like processes to signal in autocrine or paracrine mode and advance self-survival vs. that of neighboring NSCs (Bonaguidi et al., 2012; Garcia et al., 2004; Goncalves et al., 2016b; Pilz et al., 2018; Seri et al., 2001) (Figure 2b). Unlike granule cells in the olfactory bulb (Platel et al., 2019), adult-born DGCs compete with pre-existing mature DGCs for perforant path inputs (McAvoy et al., 2016; Miller and Sahay, 2019; Tashiro et al., 2006; Toni et al., 2007) (Krzisch et al., 2016; McAvoy et al., 2016). Partial genetic elimination of dendritic spines in mature DGCs or increasing dendritic spines in immature abDGCs enhanced neuronal integration of immature abDGCs. Neural activity may directly influence this competition or indirectly via regulation of niche actors such as astrocytes and microglia. Astrocytes and microglia may promote formation of dendritic spines by inducing filopodia (Figure 1).
Cross-talk: Who is listening to whom?
The rich repertoire of signaling mechanisms within the niche described in previous sections begins to edify how participation of different niche actors is coordinated or orchestrated to maintain NSC homeostasis and regulate adult hippocampal neurogenesis. Given evolution’s parsimony, it is most likely that signaling mechanisms coordinate activity of multiple niche actors much like a conductor in an orchestra. Within this framework, DGCs or local inhibitory neurons may secrete ligands in response to neural activity that in turn, act simultaneously on astrocytes, microglia and endothelial cells to recruit their functions (Figure 3a). As discussed in previous sections, evidence from different studies suggest that a single secreted factor (such as VEGF, Notch, Wnts, BMPs) acts on cognate receptors in distinct niche cell-types to mobilize their activities. An important next step is to concurrently evaluate within the same study how secreted factors co-ordinate mobilization of different niche cell-types to regulate neurogenesis. An alternative mode of cross-talk involves niche actors subscribing to privileged lines of communication such that NSCs or abDGCs receive signals solely from one cell-type (Figure 3b). A third mode invokes communication across a cascade of niche actors such that signals are sequentially transmitted to ultimately reach NSCs or abDGCs (Figure 3c). For example, a DGC-PV-endothelial cell axis has been implicated in mediating experience dependent regulation of neurogenesis. Specifically, activity levels of DGCs may regulate blood flow through secretion of neuronal NOS from local PV interneurons in the DG. nNOS elicits release of IGF from blood vessels to promote neurogenesis (Shen et al., 2019). As we glean more insights from studies on different signaling mechanisms within the niche, the prevalence of each of these modes of cross-talk will become clearer.
Figure 3. Inter-cellular cross talk in the niche.
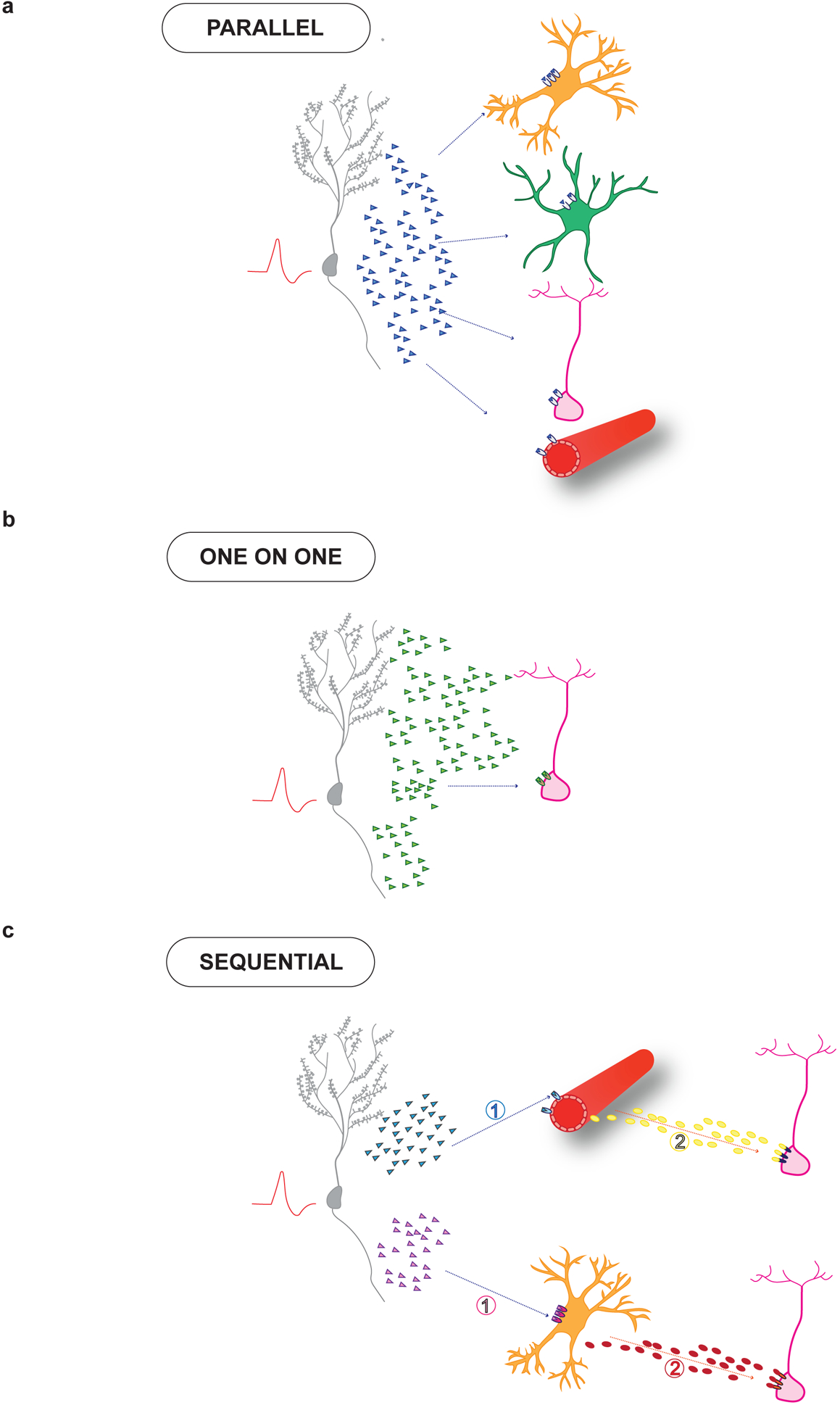
Neuronal activity may recruit multiple modes of signaling to coordinate activity of diverse niche actors and NSCs. a. Parallel mode: A single niche derived signal (light blue) may act simultaneously on cognate receptors present on astrocytes (green), microglia (orange), endothelial cells (red) and NSCs (pink). b. One-on-one mode: NSCs may receive a signal from only one cell-type. c. Sequential mode: This mode invokes communication across a cascade of niche actors such that signals are sequentially transmitted to ultimately reach NSCs. See text for details.
Signal Integration
The diversity of niche actors suggests that NSCs filter and integrate a wide-range of signals to make activation and division decisions. However, signal integration in adult NSCs is poorly understood. The identification of master transcription factors (TFs) and epigenetic factors involved in NSC underlying activation-quiescence and lineage choice will permit inference of how niche signaling mechanisms are linked to regulation of gene expression (Andersen et al., 2014; Beckervordersandforth et al., 2015; Hsieh, 2012; Hsieh and Zhao, 2016; Urban and Guillemot, 2014; Urban et al., 2016). A small number of TFs have been identified that couple maintenance of NSC quiescence with repression of asymmetric stem cell renewal (Gao et al., 2011; Jones et al., 2015; Mukherjee et al., 2016; Zhang et al., 2019). Specifically, conditional loss of TFs such as REST results in activation of NSCs and increased asymmetric stem cell divisions thereby producing more neurons (Gao et al., 2011; Mukherjee et al., 2016). It is likely that other as yet unidentified TFs couple NSC quiescence with repression of asymmetric and symmetric stem cell renewal (Figure 4a). Expression of master TFs may require convergence of a range of inputs (BMP signaling, Wnt signaling, Glucortocoids, Calcium signaling effectors) onto cis-regulatory elements as suggested for regulation of key meristem regulatory genes in plant stem cells (Janocha and Lohmann, 2018) (Figure 4b, top). Different signaling pathways may act co-operatively or antagonistically at the level of regulation of expression of master TFs. Alternatively, signaling pathways recruit distinct co-activators that bias master TF chromatin occupancy at different target gene promoters (Figure 4b, middle).
Figure 4. Signal integration in NSCs.
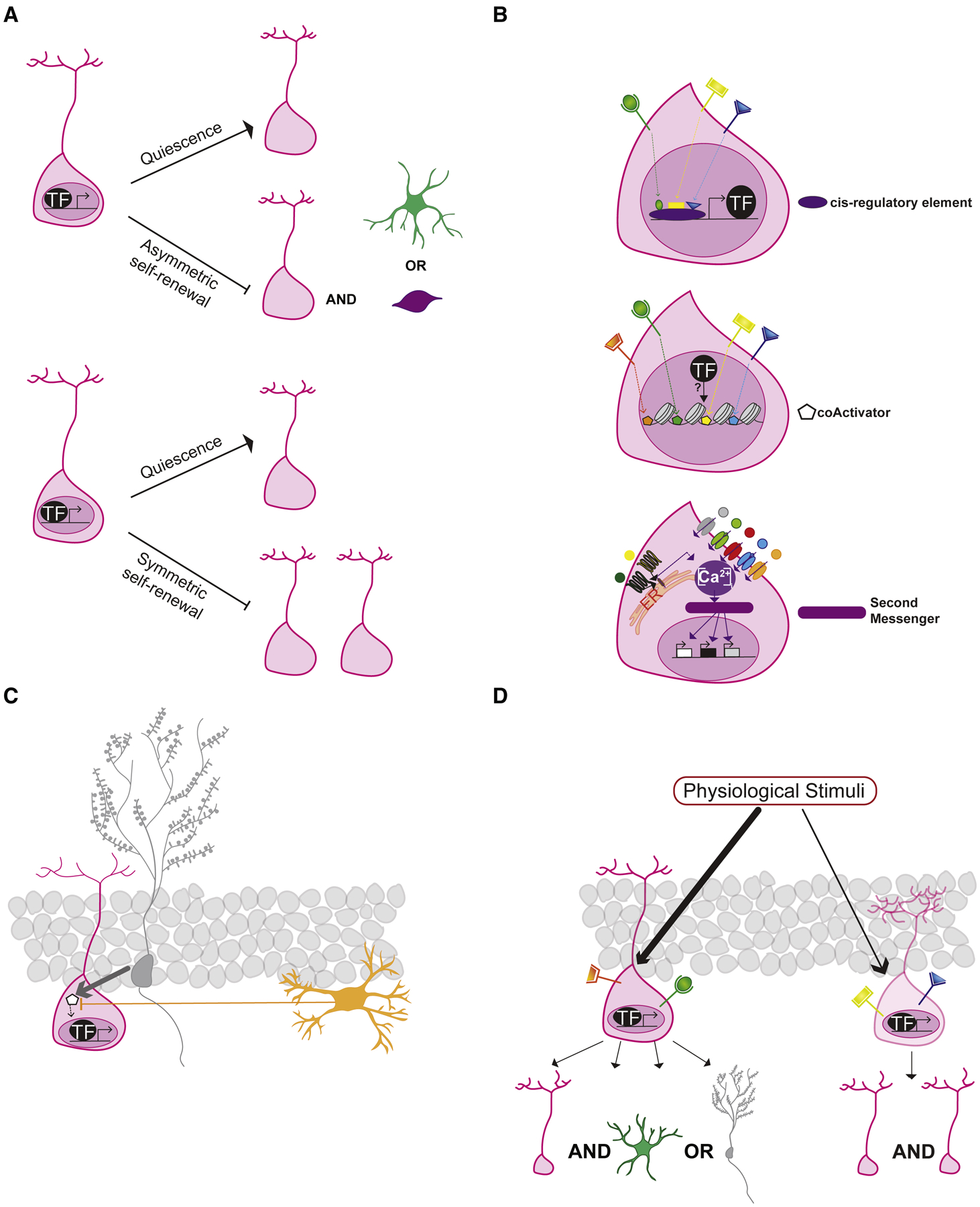
NSCs filter and integrate a wide-range of signals to make activation and division decisions. a. Master TFs may couple maintenance of NSC quiescence with repression of asymmetric stem cell renewal or symmetric stem cell renewal. b. Distinct physiological signals may converge onto the same cis regulatory element (purple) to regulate expression of master transcription factors (black) and influence the expression of target genes (white) involved in NSC quiescence-activation, fate choice and self-renewal (top). Distinct signals may recruit different coactivators (orange, green, yellow, blue) that bias master TF occupancy (black circle) at target gene promoters resulting in differential expression of genes (middle). Levels of intracellular calcium may mediate different biological responses in NSCs. Ca2+ influx into the cytoplasm from the extracellular environment (grey, green, red blue and orange receptors) and from the endoplasmic reticulum [G protein coupled receptors (dark green and yellow) and ER (light orange)] contribute to intracellular levels of calcium that, through the activation of second messengers, can mediate distinct cellular behaviors (bottom). c. The spatial organization of the niche can influence signal integration in NSCs. For example, a signal released by a DGC (grey) proximal to the NSC (pink) outcompetes an antagonistic signal released by distally located microglia (orange). d. Phenotypic and functional diversity of NSCs in the niche may support distinct fate choices i.e. bias towards asymmetric neurogenic or astrogenic stem cell renewal or symmetric stem cell renewal. See text for details.
Signal integration may also occur sequentially across a multi-layered network where each successive layer integrates and transduces fewer signals to converge onto one or few regulatory factors. Ligand-receptor signaling (eg: BMPs) duration and strength mediated by one niche actor maybe modulated by antagonists (eg: noggin) governing ligand availability produced by another niche cell-type (Warmflash et al., 2012). Niche architecture may also dictate topography of ligand distribution in the milieu of NSCs such that some ligands may produce graded responses in NSCs (Figure 4c). Superficial layers maybe populated by multi-signal integrators such as the mammalian target of rapamycin (mTOR) (Amiri et al., 2012; Bonaguidi et al., 2011; Zhou et al., 2018) (Yu and Cui, 2016) that recruit effectors in deeper layers to drive gene expression regulating stem cell state. Co-activators of gene expression such as SMADs may integrate multiple signaling pathways such as BMP, WNT and GSK3 to transcriptionally regulate gene expression in stem cells (Luo, 2017). Modulation of intracellular calcium dynamics in superficial layers may afford integration of different mitogenic signaling pathways including Notch to influence stem cell proliferation as shown in the intestine (Deng et al., 2015). Studies in the drosophila wing imaginal disc elegantly convey how various morphogens affect spatiotemporal patterning of intracellular calcium and how variations in calcium signaling result in a range of distinct biological readouts (Brodskiy et al., 2019). Although adult DG NSCs also exhibit elevation in calcium transients in response to pro-proliferative signals (Itou et al., 2011), much less is known about how different signaling pathways affect calcium levels in NSCs (Figure 4b, bottom). Future studies should determine how a multi-layered architecture such as that described here supports signal integration in adult NSCs.
The induction of expression of master TFs is reflective of signal integration. Dynamic regulation of levels of master TFs may permit efficient coupling of re-entry into quiescence with symmetric and asymmetric stem cell renewal (Figure 4a). Prior signaling events may alter epigenetic landscape of regulatory elements of master TFs and consequently, bias NSCs responses in the future. Finally, functional heterogeneity of NSCs may permit differential mobilization or recruitment to physiological signals (Bonaguidi et al., 2016; Gebara et al., 2016; Pilz et al., 2018) (Figure 4d).
Outlook
Studies in rodents, non-human primates and humans suggest that adult hippocampal neurogenesis is conserved across mammals(Boldrini et al., 2018; Eriksson et al., 1998; Gould et al., 1999; Knoth et al., 2010; Moreno-Jimenez et al., 2019; Spalding et al., 2013) and this unique form of circuit plasticity maybe altered in different disease states(Boldrini et al., 2012; Moreno-Jimenez et al., 2019; Tobin et al., 2019; Yun et al., 2016). However, a recent study (Sorrells et al., 2018) has warranted further investigation into quantification of adult-born DGCs generated in the human hippocampus across the lifespan. As new efforts continue to address this challenge, insights gleaned from systematic analysis and comparison of the DG and SVZ niches in adulthood and during development in multiple species (Bjornsson et al., 2015; Gotz et al., 2016; Obernier and Alvarez-Buylla, 2019; Paul et al., 2017) may guide strategies to boost neurogenesis, reactivate dormant NSCs or expand the NSC pool to optimize DG functions in memory and regulation of emotion (Anacker and Hen, 2017; McAvoy and Sahay, 2017; Miller and Sahay, 2019; Snyder, 2019; Toda et al., 2019; Tuncdemir et al., 2019). Targeting cell-autonomous mechanisms within NSCs or abDGCs are unlikely to prove effective on their own in promoting integration of abDGCs into an unhealthy niche. Instead, multipronged efforts that repair disease associated alterations in the niche, are necessary to promote neurogenesis (Choi et al., 2018). For example, targeting reactive astrocytes and inflammatory microglia and maintaining healthy vasculature in Alzheimer’s disease and aging is critical to support integration and maturation of abDGCs. Because transcriptional and epigenetic dependent maturation of abDGCs is intimately dependent on signals from the mileau, it is plausible that targeting non-cell autonomous mechanisms (niche actors, input connectivity) may partially compensate the impact of disease mutations on physiology and function of abDGCs. Parabiosis studies support this idea of restoring vitality to adult hippocampal neurogenesis in aged rodents (Fan et al., 2017). Additionally, the use of tissue scaffolds that mimic stiffness of neonatal environments to revert mechanical and chemical signaling may restore NSPC plasticity as shown recently for adult OPCs (Segel et al., 2019). Ultimately, a deeper understanding of communication, cross talk and signal integration in the niche will inspire novel approaches to stimulate neurogenesis to restore cognitive functions in diseases characterized by impaired hippocampal functions.
Acknowledgements
We thank members of Sahay lab for discussions. C.V. is supported by a NARSAD Young Investigator Award. A.S. acknowledges support from the NIH-R01MH104175, NIH-R01AG048908, NIH-1R01MH111729, James and Audrey Foster MGH Research Scholar Award, the Ellison Medical Foundation New Scholar in Aging, the Whitehall Foundation, an Inscopix Decode award, a NARSAD Independent Investigator Award, Ellison Family Philanthropic support, the Blue Guitar Fund, a Harvard Neurodiscovery Center-MADRC Center Pilot Grant award, Alzheimer’s Association Research Grant, a Harvard Stem Cell Institute Development grant and HSCI seed grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial and non-financial interests.
References
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, et al. (2018). Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71 e14. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, and Monyer H (2012). Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10, 76–87. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, and Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez DD, Giacomini D, Yang SM, Trinchero MF, Temprana SG, Buttner KA, Beltramone N, and Schinder AF (2016). A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 354, 459–465. [DOI] [PubMed] [Google Scholar]
- Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, McKay RM, and Parada LF (2012). Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci 32, 5880–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, and Hen R (2017). Adult hippocampal neurogenesis and cognitive flexibility -linking memory and mood. Nat Rev Neurosci 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Goritz C, Frisen J, et al. (2014). A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel JR, Ye S, Tang F, Sun D, Zhang H, Mei L, and Xiong WC (2018). Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice. J Neurosci 38, 5949–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, and Volterra A (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, Lyubimova A, Muraro M, van Es JH, van Oudenaarden A, and Clevers H (2017). A Single-Cell RNA Sequencing Study Reveals Cellular and Molecular Dynamics of the Hippocampal Neurogenic Niche. Cell reports 21, 3271–3284. [DOI] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, and Schaffer DV (2012). Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci 15, 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, et al. (2017). Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell reports 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, et al. (2011). Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging 32, 2030–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Asrican B, Li W, Gu B, Wen Z, Lim SA, Haniff I, Ramakrishnan C, Deisseroth K, Philpot B, et al. (2017). Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis. Cell Stem Cell 21, 604–617 e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, and Zhao X (2006). Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem cells and development 15, 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Zhang CL, and Lie DC (2015). Transcription-Factor-Dependent Control of Adult Hippocampal Neurogenesis. Cold Spring Harbor perspectives in biology 7, a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, and Gaiarsa JL (1997). GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci 20, 523–529. [DOI] [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Gobel J, Yang SM, Conzelmann KK, Schinder AF, Gotz M, et al. (2015). A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 85, 710–717. [DOI] [PubMed] [Google Scholar]
- Bjornsson CS, Apostolopoulou M, Tian Y, and Temple S (2015). It takes a village: constructing the neurogenic niche. Dev Cell 32, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Suarez E, Liu TF, Kopelevich A, and Allen NJ (2018). Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 100, 1116–1132 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, et al. (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, and Arango V (2012). Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72, 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos M, Perea JR, Terreros-Roncal J, Pallas-Bazarra N, Jurado-Arjona J, Avila J, and Llorens-Martin M (2018). Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav Immun 68, 76–89. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Song J, Ming GL, and Song H (2012). A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol 22, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Stadel RP, Berg DA, Sun J, Ming GL, and Song H (2016). Diversity of Neural Precursors in the Adult Mammalian Brain. Cold Spring Harbor perspectives in biology 8, a018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, and Song H (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun SM, Pilz GA, Machado RA, Moss J, Becher B, Toni N, and Jessberger S (2015). Programming Hippocampal Neural Stem/Progenitor Cells into Oligodendrocytes Enhances Remyelination in the Adult Brain after Injury. Cell reports 11, 1679–1685. [DOI] [PubMed] [Google Scholar]
- Brodskiy PA, Wu Q, Soundarrajan DK, Huizar FJ, Chen J, Liang P, Narciso C, Levis MK, Arredondo-Walsh N, Chen DZ, et al. (2019). Decoding Calcium Signaling Dynamics during Drosophila Wing Disc Development. Biophysical journal 116, 725–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker SM, Gobeske KT, Chen J, Peng CY, and Kessler JA (2017). Hippocampal bone morphogenetic protein signaling mediates behavioral effects of antidepressant treatment. Mol Psychiatry 22, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, and Schwartz M (2006). Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31, 149–160. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, et al. (2013). Astrocytic adenosine 5’-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 31, 1633–1643. [DOI] [PubMed] [Google Scholar]
- Caputi A, Melzer S, Michael M, and Monyer H (2013). The long and short of GABAergic neurons. Curr Opin Neurobiol 23, 179–186. [DOI] [PubMed] [Google Scholar]
- Catavero C, Bao H, and Song J (2018). Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell and tissue research 371, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, et al. (2017). Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, and Overstreet-Wadiche LS (2013). GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 33, 6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Zhang Y, Shen R, Westbrook GL, and Goodman RH (2016). Transcriptional Profiling of Newly Generated Dentate Granule Cells Using TU Tagging Reveals Pattern Shifts in Gene Expression during Circuit Integration. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, et al. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, and Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, and Eroglu C (2015). Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harbor perspectives in biology 7, a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, and Barres BA (2018). Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115, E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope EC, and Gould E (2019). Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 24, 690–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, and Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Deng H, Gerencser AA, and Jasper H (2015). Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature 528, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Gotz M, and Berninger B (2013). Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci USA 110, E1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni CV, Panichi R, Aimone JB, Kuo CT, Wadiche JI, and Overstreet-Wadiche L (2016). Low excitatory innervation balances high intrinsic excitability of immature dentate neurons. Nature communications 7, 11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Pan YB, Wu XR, He LN, Liu XD, Feng DF, Xu TL, Sun S, and Xu NJ (2019). A neuronal molecular switch through cell-cell contact that regulates quiescent neural stem cells. Sci Adv 5, eaav4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, and Hen R (2011). Experience dictates stem cell fate in the adult hippocampus. Neuron 70, 908–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, George BM, Boutet SC, Hebestreit K, Pluvinage JV, et al. (2019). Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi M, Yamamoto Y, Sharifi K, Kida H, Kagawa Y, Yasumoto Y, Islam A, Miyazaki H, Shimamoto C, Maekawa M, et al. (2016). Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia 64, 48–62. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, and Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100, 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vazquez ME, Switzer RC, Chamberland DW, Nick H, Levine HG, Scarpa PJ, Enikolopov G, and Steindler DA (2008). Quiescent adult neural stem cells are exceptionally sensitive to cosmic radiation. Exp Neurol 210, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Zhang R, and Taylor V (2018). Notch and Neurogenesis. Advances in experimental medicine and biology 1066, 223–234. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, and Gage FH (1998). Neurogenesis in the adult human hippocampus. Nat Med 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, and Schinder AF (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25, 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wheatley EG, and Villeda SA (2017). Mechanisms of Hippocampal Aging and the Potential for Rejuvenation. Annu Rev Neurosci 40, 251–272. [DOI] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, and Allen NJ (2018). Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural development 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, van Casteren ACM, Lee A, Chang VT, Aricescu AR, and Allen NJ (2017). Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron 96, 428–445 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, et al. (2008). Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci USA 105, 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Oohashi T, and Pizzorusso T (2019). The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20, 451–465. [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T, Schouten TG, Saaltink DJ, Dijkmans T, Steindler DA, et al. (2013). Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry 18, 993–1005. [DOI] [PubMed] [Google Scholar]
- Freund TF (2003). Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci 26, 489–495. [DOI] [PubMed] [Google Scholar]
- Freund TF, and Antal M (1988). GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336, 170–173. [DOI] [PubMed] [Google Scholar]
- Freund TF, and Buzsaki G (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, and Ray J (1995). Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA 92, 11879–11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ding P, Nashaat M, Yuan L, Ma J, Hammer RE, and Hsieh J (2011). The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci 31, 9772–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, and Sofroniew MV (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, and Song H (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Bonaguidi MA, Beckervordersandforth R, Sultan S, Udry F, Gijs PJ, Lie DC, Ming GL, Song H, and Toni N (2016). Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells 34, 997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, and Kessler JA (2009). BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS ONE 4, e7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, Tran T, Chang T, and Gage FH (2016a). In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci 19, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Schafer ST, and Gage FH (2016b). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. [DOI] [PubMed] [Google Scholar]
- Gotz M, Nakafuku M, and Petrik D (2016). Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences. Cold Spring Harbor perspectives in biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, and Fuchs E (1999). Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96, 5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, and Sahay A (2017). Neural Circuits Serve as Periscopes for NSCs. Cell Stem Cell 21, 557–559. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang L, Christopher DM, Teng ZQ, Fausett SR, Liu C, George OL, Klingensmith J, Jin P, and Zhao X (2011). RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron 70, 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, et al. (2018). Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Calvo CF, Kang TH, Baker KL, Park JH, Parras C, Levittas M, Birba U, Pibouin-Fragner L, Fragner P, et al. (2015). Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell reports 10, 1158–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG (2001). GLIA: listening and talking to the synapse. Nat Rev Neurosci 2, 185–193. [DOI] [PubMed] [Google Scholar]
- Heigele S, Sultan S, Toni N, and Bischofberger J (2016). Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nat Neurosci 19, 263–270. [DOI] [PubMed] [Google Scholar]
- Hillen AEJ, Burbach JPH, and Hol EM (2018). Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog Neurobiol 165.–, 66–86. [DOI] [PubMed] [Google Scholar]
- Hsieh J (2012). Orchestrating transcriptional control of adult neurogenesis. Genes Dev 26, 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, and Zhao X (2016). Genetics and Epigenetics in Adult Neurogenesis. Cold Spring Harbor perspectives in biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou Y, Nochi R, Kuribayashi H, Saito Y, and Hisatsune T (2011). Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 21, 446–459. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, and Lie DC (2009). GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci 29, 7966–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, et al. (2013). Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janocha D, and Lohmann JU (2018). From signals to stem cells and back again. Curr Opin Plant Biol 45, 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Saric N, Russell JP, Andoniadou CL, Scambler PJ, and Basson MA (2015). CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult hippocampus. Stem Cells 33, 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, and Volterra A (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10, 331–339. [DOI] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, and Rubin LL (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, and Gage FH (1998). Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18, 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, and Mercier F (2007). Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25, 2146–2157. [DOI] [PubMed] [Google Scholar]
- Keung AJ, de Juan-Pardo EM, Schaffer DV, and Kumar S (2011). Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29, 1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, and Prinz M (2017). Microglia in steady state. J Clin Invest 127, 3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, and Wyss-Coray T (2015). Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci USA 112, 4128–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, and Kaufer D (2013). Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife 2, e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, and Somogyi P (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, and Kempermann G (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5, e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Wolf B, Keshet E, and Licht T (2019). Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia 67, 594–618. [DOI] [PubMed] [Google Scholar]
- Krzisch M, Fulling C, Jabinet L, Armida J, Gebara E, Casse F, Habbas S, Volterra A, Hornung JP, and Toni N (2016). Synaptic Adhesion Molecules Regulate the Integration of New Granule Neurons in the Postnatal Mouse Hippocampus and their Impact on Spatial Memory. Cereb Cortex. [DOI] [PubMed] [Google Scholar]
- Krzisch M, Temprana SG, Mongiat LA, Armida J, Schmutz V, Virtanen MA, Kocher-Braissant J, Kraftsik R, Vutskits L, Conzelmann KK, et al. (2015). Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct Funct 220, 2027–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA 108, E440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, and Ge S (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15, 399–405, S391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Haditsch U, Cord BJ, Guzman R, Kim SJ, Boettcher C, Priller J, Ormerod BK, and Palmer TD (2013). Absence of CCL2 is sufficient to restore hippocampal neurogenesis following cranial irradiation. Brain Behav Immun 30, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Tronel S, Montaron MF, Fabre A, Dugast E, and Abrous DN (2012). Long-lasting plasticity of hippocampal adult-born neurons. J Neurosci 32, 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Stam FJ, Aimone JB, Goulding M, Callaway EM, and Gage FH (2013). Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus. Proc Natl Acad Sci USA 110, 9106–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375. [DOI] [PubMed] [Google Scholar]
- Long KR, and Huttner WB (2019). How the extracellular matrix shapes neural development. Open Biol 9, 180216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Anacker C, Burghardt NS, Khandaker H, Andreu V, Millette A, Leary P, Ravenelle R, Jimenez JC, Mastrodonato A, et al. (2019). Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 364, 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K (2017). Signaling Cross Talk between TGF-beta/Smad and Other Signaling Pathways. Cold Spring Harbor perspectives in biology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque T, Kang MS, Schaffer DV, and Kumar S (2016). Microelastic mapping of the rat dentate gyrus. R Soc Open Sci 3, 150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, and Song H (2009). Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, and Schinder AF (2012). Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Dieni CV, Wadiche JI, and Overstreet-Wadiche L (2011). Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci 14, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Wadiche JI, and Overstreet-Wadiche LS (2009). Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci 29, 15063–15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Sankowski R, Staszewski O, Bottcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. [DOI] [PubMed] [Google Scholar]
- McAvoy KM, and Sahay A (2017). Targeting Adult Neurogenesis to Optimize Hippocampal Circuits in Aging. Neurotherapeutics 14, 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy KM, Scobie KN, Berger S, Russo C, Guo N, Decharatanachart P, Vega-Ramirez H, Miake-Lye S, Whalen M, Nelson M, et al. (2016). Modulating Neuronal Competition Dynamics in the Dentate Gyrus to Rejuvenate Aging Memory Circuits. Neuron 91, 1356–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F (2016). Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cellular and molecular life sciences : CMLS 73, 4661–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, and Hatton GI (2002). Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451, 170–188. [DOI] [PubMed] [Google Scholar]
- Miller SM, and Sahay A (2019). Functions of adult-born neurons in hippocampal memory interference and indexing. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89. [DOI] [PubMed] [Google Scholar]
- Mirescu C, and Gould E (2006). Stress and adult neurogenesis. Hippocampus 16, 233–238. [DOI] [PubMed] [Google Scholar]
- Mongredien R, Erdozain AM, Dumas S, Cutando L, Del Moral AN, Puighermanal E, Rezai Amin S, Giros B, Valjent E, Meana JJ, et al. (2019). Cartography of hevin-expressing cells in the adult brain reveals prominent expression in astrocytes and parvalbumin neurons. Brain Struct Funct 224, 1219–1244. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, and Palmer TD (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765. [DOI] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, et al. (2016). Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab 24, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HY, Javadi S, Stremlau M, Yoon KJ, Becker B, Kang SU, Zhao X, and van Praag H (2019). Conditioned media from AICAR-treated skeletal muscle cells increases neuronal differentiation of adult neural progenitor cells. Neuropharmacology 145, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, and Yang Y (2017). Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci 37, 8706–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, and Llorens-Martin M (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, and Gotz M (2006). Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia 54, 21–34. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, and Wyss-Coray T (2012). Neural progenitor cells regulate microglia functions and activity. Nat Neurosci 15, 1485–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Gebara E, Bushong EA, Sanchez-Pascual I, O’Laoi R, El M’Ghari I, Kocher-Braissant J, Ellisman MH, and Toni N (2016). Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA 113, E2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Brulet R, Zhang L, and Hsieh J (2016). REST regulation of gene networks in adult neural stem cells. Nature communications 7, 13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Obernier K, and Alvarez-Buylla A (2019). Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, and Westbrook GL (2006). Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci 26, 2326–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, and Gage FH (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425, 479–494. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Paris I, Savage JC, Escobar L, Abiega O, Gagnon S, Hui CW, Tremblay ME, Sierra A, and Valero J (2018). ProMoIJ: A new tool for automatic three-dimensional analysis of microglial process motility. Glia 66, 828–845. [DOI] [PubMed] [Google Scholar]
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, and Tombola F (2014). Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA 111, 16148–16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Chaker Z, and Doetsch F (2017). Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356, 1383–1386. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, and McBain CJ (2017). Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, and Schinder AF (2011). The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31, 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz LJ, Lennox AL, Rouanet JP, and Silver DL (2016). Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain. Curr Biol 26, 3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz GA, Bottes S, Betizeau M, Jorg DJ, Carta S, Simons BD, Helmchen F, and Jessberger S (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science 359, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Angelova A, Bugeon S, Wallace J, Ganay T, Chudotvorova I, Deloulme JC, Beclin C, Tiveron MC, Core N, et al. (2019). Neuronal integration in the adult mouse olfactory bulb is a non-selective addition process. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheri C, Suter U, and Jessberger S (2014). Dissecting integrin-dependent regulation of neural stem cell proliferation in the adult brain. J Neurosci 34, 5222–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, and Muller U (2009). Regulation of radial glial survival by signals from the meninges. J Neurosci 29, 7694–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee S, Kang MS, Georgiou K, Kumar S, and Schaffer DV (2017). Dynamics of Mechanosensitive Neural Stem Cell Differentiation. Stem Cells 35, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzel R, Sadek AR, Chang CH, Gray WP, Seifert G, and Steinhauser C (2013). Polarized distribution of AMPA, but not GABAA , receptors in radial glia-like cells of the adult dentate gyrus. Glia 61, 1146–1154. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Shapiro LA, Perez ZD, and Spigelman I (2009). Microglia-associated granule cell death in the normal adult dentate gyrus. Brain Struct Funct 214, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salib M, Joshi A, Katona L, Howarth M, Micklem BR, Somogyi P, and Viney TJ (2019). GABAergic Medial Septal Neurons with Low-Rhythmic Firing Innervating the Dentate Gyrus and Hippocampal Area CA3. J Neurosci 39, 4527–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, and Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, and Myers CE. (2012). Hilar mossy cells of the dentate gyrus: a historical perspective. Frontiers in neural circuits 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten M, Bielefeld P, Garcia-Corzo L, Passchier EMJ, Gradari S, Jungenitz T, Pons-Espinal M, Gebara E, Martin-Suarez S, Lucassen PJ, et al. (2019). Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, et al. (2019). Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenfaden R, Desoeuvre A, Bosio A, Virard I, and Cremer H (2006). Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci 32, 187–198. [DOI] [PubMed] [Google Scholar]
- Semerci F, Choi WT, Bajic A, Thakkar A, Encinas JM, Depreux F, Segil N, Groves AK, and Maletic-Savatic M (2017). Lunatic fringe-mediated Notch signaling regulates adult hippocampal neural stem cell maintenance. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, and Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21, 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Perez ZD, Foresti ML, Arisi GM, and Ribak CE (2009). Morphological and ultrastructural features of Iba1-immunolabeled microglial cells in the hippocampal dentate gyrus. Brain Res 1266, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wang D, Wang X, Gupta S, Ayloo B, Wu S, Prasad P, Xiong Q, Xia J, and Ge S (2019). Neurovascular Coupling in the Dentate Gyrus Regulates Adult Hippocampal Neurogenesis. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, and Maletic-Savatic M (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, et al. (2015). beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med 21, 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS (2019). Recalibrating the Relevance of Adult Neurogenesis. Trends Neurosci 42, 164–178. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, and Cameron HA (2009). The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 19, 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, and Gage FH (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. [DOI] [PubMed] [Google Scholar]
- Song J, Olsen RH, Sun J, Ming GL, and Song H (2016). Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis. Cold Spring Harbor perspectives in biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, et al. (2013). Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 16, 1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489, 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S, Li L, Moss J, Petrelli F, Casse F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, et al. (2015). Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron 88, 957–972. [DOI] [PubMed] [Google Scholar]
- Sun GJ, Sailor KA, Mahmood QA, Chavali N, Christian KM, Song H, and Ming GL (2013). Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J Neurosci 33, 11400–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo GG, Du X, Oijala M, Varga C, Parent JM, and Soltesz I (2017). Extended Interneuronal Network of the Dentate Gyrus. Cell reports 20, 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Wang M, Wang P, Wang L, Wu Q, and Guo W (2019). Neural Stem Cells Behave as a Functional Niche for the Maturation of Newborn Neurons through the Secretion of PTN. Neuron 101, 32–44e36. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, and Gage FH (2006). NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929–933. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Kron MM, Masachs N, Zhang H, Lagace DC, Martinez A, Reillo I, Duan X, Bosch C, Pujadas L, et al. (2012). Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J Neurosci 32, 12051–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham M, Ramasamy S, Gan HT, Ramachandran A, Poonepalli A, Yu YH, and Ahmed S (2010). CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS ONE 5, e15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, et al. (2019). Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 24, 974–982 e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Parylak SL, Linker SB, and Gage FH (2019). The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 24, 67–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, and Schinder AF (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, and Schinder AF (2015). Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus. Cold Spring Harbor perspectives in biology 8, a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, and Gage FH (2007). Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10, 727–734. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, and Hisatsune T (2005). GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, and Majewska AK. (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncdemir SN, Lacefield CO, and Hen R (2019). Contributions of adult neurogenesis to dentate gyrus network activity and computations. Behav Brain Res 374, 112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, and Guillemot F (2014). Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 8, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, van den Berg DL, Forget A, Andersen J, Demmers JA, Hunt C, Ayrault O, and Guillemot F (2016). Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 353, 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, and Gage FH (2000). Neural consequences of environmental enrichment. Nat Rev Neurosci 1, 191–198. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, and van Praag H (2012). Monosynaptic inputs to new neurons in the dentate gyrus. Nature communications 3, 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, and van Praag H (2013). All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Current topics in behavioral neurosciences 15, 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, and Bartlett PF (2012). Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci 32, 6435–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, and Kettenmann H (2005). A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci 29, 181–189. [DOI] [PubMed] [Google Scholar]
- Warmflash A, Zhang Q, Sorre B, Vonica A, Siggia ED, and Brivanlou AH (2012). Dynamics of TGF-beta signaling reveal adaptive and pulsatile behaviors reflected in the nuclear localization of transcription factor Smad4. Proc Natl Acad Sci USA 109, E1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, et al. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature communications 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Larimer P, Gao Y, and Strowbridge BW (2007). Semilunar granule cells: glutamatergic neurons in the rat dentate gyrus with axon collaterals in the inner molecular layer. J Neurosci 27, 13756–13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CY, Asrican B, Moss J, Quintanilla LJ, He T, Mao X, Casse F, Gebara E, Bao H, Lu W, et al. (2018). Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron 99, 493–510 e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, and Cui W (2016). Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143, 3050–3060. [DOI] [PubMed] [Google Scholar]
- Yun S, Reynolds RP, Masiulis I, and Eisch AJ (2016). Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat Med 22, 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Boareto M, Engler A, Louvi A, Giachino C, Iber D, and Taylor V (2019). Id4 Downstream of Notch2 Maintains Neural Stem Cell Quiescence in the Adult Hippocampus. Cell reports 28, 1485–1498 e1486. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG Jr., Ming GL, and Gage FH (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bond AM, Shade JE, Zhu Y, Davis CO, Wang X, Su Y, Yoon KJ, Phan AT, Chen WJ, et al. (2018). Autocrine Mfge8 Signaling Prevents Developmental Exhaustion of the Adult Neural Stem Cell Pool. Cell Stem Cell 23, 444–452 e444. [DOI] [PMC free article] [PubMed] [Google Scholar]


