Abstract
Opioid use disorder (OUD) in pregnant women has increased significantly in recent years. Maintaining these women on sublingual (SL) buprenorphine (BUP) is an evidence-based practice but BUP-SL is associated with several disadvantages that an extended-release (XR) BUP formulation could eliminate. The National Drug Abuse Treatment Clinical Trials Network (CTN) is conducting an intent-to-treat, two-arm, open-label, pragmatic randomized controlled trial, Medication treatment for Opioid-dependent expectant Mothers (MOMs), to compare mother and infant outcomes of pregnant women with OUD treated with BUP-XR, relative to BUP-SL. A second aim is to determine the relative economic value of utilizing BUP-XR. Approximately 300 pregnant women with an estimated gestational age (EGA) of 6–30 weeks, recruited from 12 sites, will be randomized in a 1:1 ratio to BUP-XR or BUP-SL, balancing on site, EGA, and BUP-SL status (taking/not taking) at the time of randomization. Participants will be provided with study medication and attend weekly medication visits through 12 months postpartum. Participants will be invited to participate in two sub-studies to evaluate the: 1) mechanisms by which BUP-XR may improve mother and infant outcomes; and 2) effects of prenatal exposure to BUP-XR versus BUP-SL on infant neurodevelopment. This paper describes the key design decisions for the main trial made during protocol development. This Investigational New Drug (IND) trial uniquely uses pragmatic features where feasible in order to maximize external validity, hence increasing the potential to inform clinical practice guidelines and address multiple knowledge gaps for treatment of this patient population.
Keywords: Opioid, Pregnant, Infant, Buprenorphine, Extended-release, Neurodevelopment
1. Introduction
The opioid-use epidemic in the U.S. has been associated with a significant increase in the prevalence of pregnant women with opioid use disorder (OUD) [[1], [2], [3], [4]] and infants diagnosed with neonatal opioid withdrawal syndrome (NOWS) [5,6]. NOWS results from a physical dependence to in-utero opioids and can cause prolonged delivery hospitalizations and increased health care costs [6,7]. National guidance recommends that pregnant women with OUD be maintained on methadone or sublingual buprenorphine (BUP-SL) [8]. Relative to methadone, BUP-SL offers the advantages of lower NOWS severity in infants [9] and greater convenience for pregnant women by removing the requirement of near-daily clinic visits for dosing [10]. BUP-SL limitations include risk for diversion [11], potential for non-adherence [11], poor retention [[12], [13], [14]], and daily peak-trough effects [15], with evidence that BUP-maintained pregnant women may be at sub-therapeutic doses for most of their dosing interval [16].
Extended-release (XR) formulations can address some of the disadvantages of BUP-SL including non-adherence [17,18], diversion [17] and potential peak-trough issues. In addition, the PK profile of BUP-XR may reduce NOWS severity relative to BUP-SL. Specifically, there is evidence that the peak-to-trough fluctuation of opioid-maintenance may increase NOWS severity [19]; BUP-XR, which has a lower peak and higher trough relative to BUP-SL, should, thus, result in lower NOWS severity. Moreover, higher infant norbuprenorphine levels are associated with greater NOWS severity [20]; first-pass metabolism of BUP to norbuprenorphine is 3–7 times lower for BUP-XR relative to BUP-SL [21] and thus it is predicted that both infant norbuprenorphine and NOWS severity will be lower with BUP-XR. A possible disadvantage of BUP-XR relative to BUP-SL is higher medication costs; however these costs may be justified by cost offsets, for example, those associated with reduced utilization of NOWS-related services. Another possible disadvantage of BUP-XR is that it includes different non-active ingredients than those included in BUP-SL and the potential impact of fetal exposure to BUP-XR has not yet been evaluated.
To evaluate the effects of BUP-XR, relative to BUP-SL, on mother and infant outcomes, the National Drug Abuse Treatment Clinical Trials Network (CTN) has developed the study: “Medication treatment for Opioid use disorder in expectant Mothers (MOMs): a pragmatic randomized trial comparing two buprenorphine formulations” (NCT03918850; IND# 140724). In addition to being the first trial to evaluate BUP-XR in pregnant women, its distinctive features include being one of the few multi-site randomized controlled trials (RCTs) of medication for OUD conducted with pregnant women and providing active treatment to women for 12 months postpartum. The trial is also unique in using pragmatic features where possible to improve real world applicability. To address important research gaps in a manner that will not overburden participants for the main study, participants will be invited, but not required, to participate in two sub-studies that will evaluate: 1) mechanisms by which BUP-XR may improve mother and infant outcomes (NCT03911466); and 2) the effects of prenatal exposure to BUP-XR versus BUP-SL on infant neurodevelopment (NCT03911739). This paper describes the key design considerations associated with the main study.
2. Research design and study organization
2.1. Research questions
The primary objective of the main trial is to evaluate the impact of treating OUD in pregnant women with BUP-XR, compared to BUP-SL, on mother and infant outcomes. It is hypothesized that the BUP-XR, relative to the BUP-SL, group will: 1) not have greater illicit opioid use during pregnancy (primary outcome, non-inferiority); 2) have lower infant NOWS severity (key secondary outcome, superiority); and 3) not have greater postpartum illicit opioid use (key secondary outcome, non-inferiority). Determining the economic value of BUP-XR, compared to BUP-SL, to treat OUD in pregnant women is a second study objective. It is predicted that a larger reduction in the utilization of high-cost healthcare services and increase in quality-adjusted life-years will result in BUP-XR being cost-effective relative to BUP-SL, from a healthcare sector perspective.
2.2. Research design
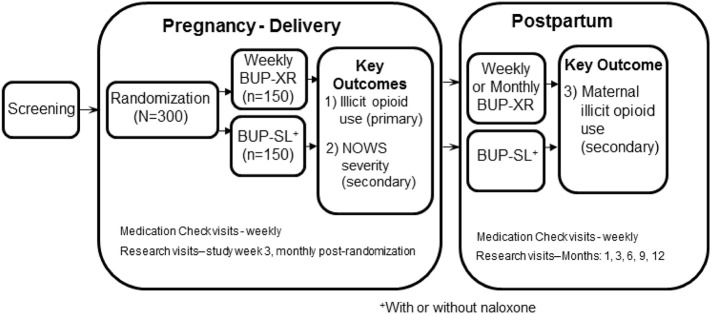
MOMs is a 12-site intent-to-treat, two-arm, open-label, non-inferiority, pragmatic randomized controlled trial in which 300 pregnant women with OUD will be randomized to BUP-XR or BUP-SL and treated through 12-months postpartum. The study schema is provided in Fig. 1 .
Fig. 1.
Study Schema.
MOMs can be compared with the only other multi-site RCT of medication for OUD conducted with a relatively large sample size of pregnant women: the MOTHER trial [22], which included 175 randomized participants. The MOTHER trial, which compared methadone and BUP-SL maintenance treatments, was an important clinical trial with a number of strengths. However, as noted by the investigators, MOTHER was a tightly controlled efficacy study that maximized internal validity at the expense of external validity [23], limiting the generalizability of the results to clinical practice. Given the dearth of evidence upon which to base clinical guidance documents [8], the present trial is designed to protect internal validity through the use of randomization but to otherwise favor external validity. As noted by Ford and Norrie [24], pragmatic trials are typically not pragmatic on all dimensions. MOMs follows the suggestion to utilize pragmatic features where feasible while still maintaining trial quality and the ability to answer the question of interest [24]. The rationales for key design decisions related to the main trial are provided below.
2.2.1. BUP-XR formulations: CAM2038 vs. Sublocade™
Two BUP-XR products have been developed recently (CAM2038 from Braeburn Pharmaceuticals and Sublocade™ from Indivior), both of which are subcutaneously injected and form a gel deposit that releases BUP at a steady rate for approximately one month; the CAM2038 product is also available as a weekly formulation. Sublocade™ received FDA approval in 2017. In 2018, the FDA determined that CAM2038 met all safety and efficacy standards necessary for approval but did not approve it for marketing due to exclusivity issues; CAM2038 will be available in the U.S. in December 2020 as Brixadi™. The monthly formulations of both Sublocade™ and CAM2038 contain N-methyl-2-pyrrolidone (NMP), an excipient which animal studies suggest could have adverse fetal-developmental effects. The weekly CAM2038 product does not include NMP and, thus, was selected as the BUP-XR medication for MOMs. Specifically, the weekly CAM2038 product will be utilized while participants are pregnant or breastfeeding and the monthly formulation will be utilized during the postpartum phase for women who are not breastfeeding. The weekly CAM2038 product includes three excipients: 1) phosphatidylcholine; 2) glycerol dioleate (GDO); and 3) ethanol.
The maximum potential exposure to the excipients for any participant/fetus during pregnancy and any participant/breastfeeding infant postpartum was calculated. Phosphatidylcholine may offer health benefits during pregnancy [25]; choline supplements of 450 mg/day during pregnancy have been recommended by the Institute of Medicine [26] and the American Medical Association [27]. The total possible maximum exposure during pregnancy would be 8.84 g, which is less than the 107.1 g that would be consumed by taking the recommended supplement of 450 mg/day. The total possible maximum exposure postpartum would be 13.52 g compared to 163.8 g if the 450 mg/day supplement were continued through one year postpartum. The second excipient, GDO, is a diglyceride that naturally occurs in human plasma. Diacylglycerol, of which GDO is a significant component, is widely used in food products (e.g., mayonnaise, salad dressings, margarine, icing, etc.) [28]. The total possible maximum exposure during pregnancy would be 8.84 g, which equates to less than one tablespoon. The total possible maximum exposure postpartum would be 13.52 g, which equates to approximately one tablespoon.
The third excipient, ethanol, when consumed in sufficient quantities by pregnant women can have a teratogenic effect [29]. The maximum weekly dose of BUP-XR (32 mg) contains 0.061 g of ethanol; a standard alcoholic drink in the US contains 14 g of ethanol [30]. The total possible maximum exposure to ethanol during pregnancy would be 2.074 g (i.e., <15% of the ethanol in a single standard drink). Although no level of alcohol exposure is considered safe during pregnancy, the potential substantial benefit of BUP-XR, relative to BUP-SL (e.g., superior PK profile, elimination of diversion potential, improved adherence) justifies any theoretical risk from this negligible amount of subcutaneously injected ethanol. While alcohol use is not encouraged in breastfeeding women, the CDC notes that moderate alcohol use (up to 1 standard drink per day) is not known to be harmful to the infant [31]. The maximum possible total postpartum exposure would be 3.172 g, which is <23% of the ethanol in a single standard drink; thus, the risk-benefit ratio for postpartum use is also justified.
2.2.2. BUP-SL formulations: BUP-SL and BUP/NX-SL
Participants randomized to BUP-SL will receive buprenorphine, without (BUP-SL) or with naloxone (BUP/NX-SL). The primary rationale for allowing both medications is to avoid discouraging site/patient participation by requiring the use of a medication that is inconsistent with site preference. Additionally, allowing for both medications is consistent with the goal of maintaining a pragmatic design where feasible. As noted by Nguyen and colleagues [32], BUP-SL is more commonly used in pregnant women based on the principal of limiting fetal exposure to additional compounds and the potential for induced withdrawal if BUP/NX-SL is injected, but BUP-SL is more likely to be diverted and misused than BUP/NX-SL. The existing literature on the relative safety of utilizing BUP/NX-SL during pregnancy is limited to retrospective chart reviews [[32], [33], [34], [35]], which generally have found no evidence of worse outcomes with BUP/NX-SL [36,37] with the exception of a recent study which found rates for prematurity and low birth weight that were higher than expected [32]. In addition to being retrospective, the study sample sizes have been limited (i.e., N = 10 [33], N = 30 [34], N = 7 [36], N = 26 [32], N = 31 [35]). It is anticipated that at least three of the approximately 12 sites will utilize BUP/NX-SL; hence, it is estimated that a minimum of 38 MOMs participants will be taking BUP/NX-SL. Exploratory analyses of safety measures comparing participants taking BUP-SL to those taking BUP/NX-SL during pregnancy will be performed, which has the potential to make an important contribution to the field.
2.2.3. Open-label vs. “double dummy” design
There is limited evidence on which to base clinical guidance documents for the treatment of OUD in pregnant women [8]. Consistent with the CTN mission, this trial is designed to compare the effectiveness of interventions as they would be used in the “real world” [38]. The present trial protects internal validity by using randomization but otherwise favors external validity. A “double dummy” design does not represent standard clinical practice and would artificially remove a key advantage of BUP-XR: avoiding daily self-administration; hence, MOMs is an open-label trial. The potential for bias resulting from the open-label design is minimized for many of the outcome measures, including the primary and key secondary outcomes, which utilize lab results and medical record abstraction.
2.2.4. Medication Check Visits vs. Research Visits
Pragmatic trials minimize research assessments since they can reduce the generalizability of the study results to real world practice. However, this goal must be balanced with the need to closely monitor safety given that MOMs is the first trial to evaluate the BUP-XR formulation in pregnant women. This balance will be achieved by including weekly Medication Check Visits, which include a minimal number of clinical and safety assessments and procedures, while limiting more intensive data collection to Research Visits, which will occur less frequently (see Fig. 1).
2.2.5. Non-inferiority primary analysis
BUP-XR may improve outcomes relative to BUP-SL due to its superior PK profile. However, to be consistent with the design of the CAM2038 Phase 3 trial [39], MOMs utilizes a non-inferiority design. A finding of non-inferiority would suggest that BUP-XR is a reasonable alternative to BUP-SL, thus expanding available treatment options. Such a finding is important because, at present, the number of BUP providers is insufficient to meet treatment needs particularly in rural areas [40]. A significant concern of clinicians who are unwilling to prescribe BUP-SL is the potential for diversion [41]; removal of this barrier would, thus, have the potential to increase the availability of treatment.
2.2.6. Standardization of NOWS scoring and treatment
Variability in delivery-hospital approach to NOWS could adversely impact the evaluation of effects of BUP treatment arm on NOWS severity, which is a key secondary measure. On the other hand, the primary outcome measure for MOMs is illicit opioid use during pregnancy, and attempting to standardize NOWS scoring/treatment at the delivery hospitals, which are not participating in the trial as study sites, would conflict with the overall goal of utilizing pragmatic features where feasible. To resolve this problem, a plan to decrease delivery hospital variability without requiring standardized scoring and treatment was recommended based on a review of the literature as well as consensus of the NOWS experts on the protocol development team. Participants are only eligible if they plan to deliver at a hospital that meets all of the following requirements: 1) has a written protocol for the management of NOWS (implementation of a standard protocol decreases length of opioid treatment days, infant length of stay (LOS), and use of adjunctive drug therapy [42,43]); 2) offers rooming-in while infants are being observed for NOWS (rooming-in is associated with decreased need for pharmacologic treatment for NOWS and shorter LOS [44]); and 3) does not send infants home on opioids for the treatment of NOWS (infant NOWS severity will be measured by total number of opioid treatment days as assessed by hospital medical record and therefore opioids provided at home would not be captured). The Better Outcomes through Research for Newborns (BORN) survey [45] will be used to assess if delivery hospitals meet this protocol-defined standardization requirement.
2.2.7. Postpartum phase
The postpartum period is a time when women are particularly vulnerable for relapse [8] and an increased risk of overdose during the postpartum period exists [46]; thus, providing effective treatment during this time is critical. The protocol team was also of the opinion that significant benefits would be gained by evaluating the full postpartum year to better understand substance use trajectories and treatment adherence, both of which could impact infant neurodevelopmental outcomes. Thus, the decision was made to extend the active treatment phase through 12 months postpartum.
2.3. Study setting
The MOMs study is being conducted by NIDA's CTN in “real world” clinical settings. The recommended model of care for pregnant women with OUD is one in which there is close collaboration between prenatal care and addiction treatment providers and, where possible, integrated treatment [8]. All MOMs sites use a collaborative care model but differ in the specifics of the care model used, which could affect outcomes. Because there is no published survey instrument for characterizing models of care for the management of pregnant women with OUD, study investigators developed an assessment, the Pregnancy and Addiction Services Assessment (PAASA), to characterize treatment at the MOMs sites so site characteristics can be used as covariates in statistical analyses.
2.4. Site selection process
Recruiting pregnant women with OUD for RCTs is challenging. To help ensure adequate recruitment, potential study sites were carefully screened to help ensure that they will have an adequate pool of potential participants. During the site selection process, the sites were asked to provide the number of pregnant women who started on BUP-SL at their site during the prior 6 months and the proportion of those women who delivered at a hospital meeting the protocol-defined standardization requirement (see Section 2.2). Sites that provide BUP to pregnant women in an office-based setting, offer BUP treatment following delivery for ≥12 months, and admit enough potentially eligible women to meet the target randomization rate (1.25 per month) were eligible for potential participation. Other criteria included having adequate medical staffing to safely and effectively conduct the MOMs trial, performance in prior clinical trials, site/patient diversity, and commitment from the Site Director, and staff. Site selection involved a 3-phase process: 1) a Brief Site Interest Survey was distributed to all CTN Node PIs, resulting in 25 sites being designated “Good Candidate” sites; 2) “Good Candidate” sites were invited to complete a full Site Selection Survey, along with a general invitation to the CTN at large; 3) of the 22 full Site Selection Surveys received, 17 sites were invited to participate in a telephone interview. Final site selection was determined by the study Executive Committee based on information obtained from the written surveys and telephone interviews. The most common reasons for site exclusion were: 1) inadequate enrollment of BUP-SL-maintained pregnant patients; 2) minimal clinical trials experience; and/or 3) minimal resources to implement the trial. The selection process resulted in 12 geographically diverse study sites: Addiction Recovery Services/Swedish Hospital (Seattle, WA), Boston Medical Center (Boston, MA), CODA, Inc. (Portland, OR), Gateway Community Services (Jacksonville, FL), HOPE Clinic/Massachusetts General Hospital (Boston, MA), Medical University of South Carolina (Charleston, SC), Milagro Clinic/University of New Mexico School of Medicine (Albuquerque, NM), Pregnancy Recovery Center/Magee Women's Hospital (Pittsburgh, PA), The Perinatal Addiction Clinic/University of Cincinnati Health (Cincinnati, OH), SUPeRAD/ University of Utah Health System (Salt Lake City, UT), Vanderbilt University Medical Center (Nashville, TN), and Zuckerberg San Francisco General Hospital (San Francisco, CA).
2.5. Study population
Pregnant patients who have an EGA of 6–30 weeks at randomization, and, in the judgment of the treating provider, are good candidates for BUP-maintenance treatment will be recruited from clinic intakes. Participants may be recruited from a variety of other sources, including advertising, but must have completed intake at a study site to be eligible for randomization. Recruitment advertisements will be approved by the Institutional Review Board (IRB). Efforts will be made to recruit a study sample that reflects, or exceeds, the proportion of minorities in treatment at the sites. The eligibility criteria listed in Table 1 were developed to be the least restrictive possible while ensuring participant safety in order to maximize the generalizability of study findings to clinical settings. In addition, where possible, the criteria reflect the eligibility criteria from the MOTHER study [22] in order to increase the comparability of the MOTHER and MOMs study samples.
Table 1.
Eligibility criteria for the MOMs study.
| Inclusion Criteria | |
|---|---|
| Potential participants must | |
| 1. | be 18–41 years of age |
| 2. | be pregnant with an EGA of 6–30 weeks at randomization, have evidence of a viable intrauterine pregnancy if EGA <12 weeks, and not be planning to terminate the pregnancy |
| 3. | have a single fetus pregnancy |
| 4. | meet DSM-5 criteria for moderate/severe OUD and be a good candidate for BUP maintenance and/or be currently prescribed BUP for the treatment of OUD |
| 5. | be willing to be randomized to BUP-XR or BUP-SL and to comply with study procedures |
| 6. | be planning to deliver at one of the hospitals for which the BORN survey was completed |
| 7. | be enrolled in outpatient addiction treatment at a participating site |
| 8. | be able to understand the study, and having understood, provide written informed consent in English |
| Exclusion Criteria | |
|---|---|
| Potential participants must not | |
| 1. | have a physiological dependence on alcohol or sedatives requiring medical detoxification |
| 2. | have a psychiatric condition that, in the judgment of the site medical clinician, would make study participation unsafe or which would make treatment compliance difficult |
| 3 | have a medical condition that, in the judgment of the site medical clinician, would make study participation unsafe or which would make treatment compliance difficult |
| 4. | be currently in jail, prison, or any inpatient overnight facility as required by a court of law or have pending legal action or other situation that, in the judgment of the site investigator, could prevent participation in the study or in any study activities |
| 5. | be currently receiving methadone or naltrexone treatment |
| 6. | be enrolled in or planning to enroll in treatment beyond clinically managed low-intensity residential services |
| 7. | be enrolled in or planning to enroll in: a) a trial testing medication for managing OUD during pregnancy; b) research testing an intervention for substance use disorder or NOWS in their infant unless they are willing to provide a release for the research records |
2.6. Randomization
A permuted block randomization approach will be used to balance on site, whether participants are on BUP-SL at the time of randomization (yes vs. no), and EGA at time of randomization (6–18 weeks vs. 19–30 weeks); EGA was used as a stratification variable in the MOTHER trial [23].
3. Study treatments
Study participants will be randomly assigned to receive either BUP-XR or BUP-SL. At present, states vary in the extent to which they cover the cost of BUP treatment for pregnant and postpartum women, with some states covering the cost through one year postpartum and others covering only pregnancy. MOMs will evaluate the effectiveness of BUP-XR, relative to BUP-SL, under a model in which states would universally cover the cost of BUP treatment through 12 months postpartum. Given the variability in state coverage, this could only be achieved by providing BUP-XR and BUP-SL at no cost to study participants. CAM2038 comes in several doses in both the once weekly and once every 4-week (monthly) formulations to allow for individualized medication plans (see Table 2 ). They are small volume injections that come in prefilled syringes with a safety device that can be stored unrefrigerated and administered subcutaneously with a thin needle. The target doses will be 24 mg for the weekly formulation and 96 mg for the monthly formulation. Sites will be provided with the BUP-SL product(s) that they request. Sites requesting the mono-BUP product will be provided with 2 mg and 8 mg BUP tablets. Sites requesting the combination product will be provided with buprenorphine/naloxone film in 4 mg/1 mg and 8 mg/2 mg BUP/naloxone doses. Sites may request both forms of BUP-SL (e.g., mono-BUP product for use during pregnancy and combination product for use during the postpartum phase). The target dose will be 16 mg daily, which is consistent with SAMHSA's recommended dose during pregnancy [8]. The actual XR- or SL-BUP doses may be lower or higher than the recommended target doses as determined by the prescribing clinician.
Table 2.
BUP-SL dose and approximate equivalent weekly and monthly BUP-XR injections.
| BUP-SL | BUP-XR weekly | BUP-XR monthly |
|---|---|---|
| ≤6 mg | 8 mg (0.16 mL) | – |
| 8–10 mg | 16 mg (0.32 mL) | 64 mg (0.18 mL) |
| 12–16 mg | 24 mg (0.48 mL) | 96 mg (0.27 mL) |
| 18–24 mg | 32 mg (0.64 mL) | 128 mg (0.36 mL) |
Note: For a BUP-SL dose >24 mg there is no equivalent BUP-XR dose available for MOMs.
Induction. BUP-XR. Participants already being treated with BUP-SL can be safely transitioned to the corresponding dose of BUP-XR (see Table 2) [47]. For participants not being treated with BUP-SL, sites will utilize the induction setting (e.g., outpatient, inpatient) they typically use for BUP-SL. The recommended weekly dose in patients not currently receiving BUP-SL at the time of randomization is 24 mg CAM2038 (weekly) titrated up over the first week of treatment as follows: 1) a test dose of transmucosal BUP 4 mg is administered when objective signs of mild to moderate withdrawal appear; 2) if the test dose is tolerated without precipitated withdrawal, the first dose of CAM2038 (weekly) 16 mg is administered; 3) an additional dose of 8 mg CAM2038 (weekly) is administered 3 days after the first dose to achieve the recommended 24 mg CAM2038 (weekly) dose. If needed, during this first week of treatment, an additional 8 mg dose of CAM2038 (weekly) is administered, waiting for at least 24 h after the previous injection, for a total weekly dose of 32 mg CAM2038 (weekly). During the postpartum period, eligible BUP-XR participants can be safely transitioned to the corresponding dose of monthly CAM2038 (see Table 2). BUP-SL. The study sites are clinical practices that regularly use BUP-SL to treat pregnant women with OUD following relevant clinical practice and state guidelines. The BUP-SL induction procedures typically used by the site will be utilized.
4. Assessments
Ideally, pragmatic trial measures would be obtained unobtrusively in order to reduce participant burden and to avoid research assessments and interactions that could impact outcomes and, thus, reduce the generalizability of the results to “real world” practice; however, some outcomes can only be obtained with participant input [24]. MOMs is designed to rely as much as possible on electronic health record (EHR) data and to collect data directly from participants only when measures reflect important outcomes. Table 3 displays the schedule of assessments by category (i.e., efficacy, safety, health economics) and whether they are collected during the weekly Medication Check Visits, Research Visits, or derived from the EHR.
Table 3.
Schedule of assessments by visit type and category.
| Assessment | Medication Check | Research Visit | EHR-derived |
|---|---|---|---|
| Efficacy | |||
| Urine drug screen | X | ||
| Infant NOWS-related outcomes | X | ||
| Medication Adherence | X | ||
| Opioid Craving Scale | X | ||
| Adequacy of Prenatal Care Utilization | X | ||
| Short Opiate Withdrawal Scale-Gossop | X | ||
| Discharge outcomes | X | ||
| Ages and Stages Questionnaire | Xa | ||
| Safety | |||
| Adverse Events/Serious Adverse Events | X | ||
| Injection Site Reaction Reporting Form | X | ||
| Hospital Anxiety and Depression Scale | X | ||
| Prior/Concomitant Meds | X | ||
| Opioid Overdose Tracking | X | ||
| Maternal Delivery Outcomes | X | ||
| Adverse fetal outcomes | Xb | ||
| Birth/Neonatal Outcomes | X | ||
| Infant sedation assessment | Xc | ||
| Health Economics | |||
| Patient-Reported Outcomes Measurement Information System | X | ||
| Non-study Medical and Other Services | X | ||
| Treatment Services Review | X | ||
Only at 6- and 12-month postpartum visits.
As needed.
Postpartum only.
4.1. Primary and key secondary outcome measures
Avoiding illicit opioid use is a key purpose for providing buprenorphine to pregnant women with OUD. While BUP-SL is effective in reducing illicit opioid use, the CAM2038 Phase 3 trial revealed that CAM2038 was superior to BUP/NX-SL on the proportion of illicit opioid-negative urine samples in non-pregnant adults [39]. MOMs will use proportion of illicit opioid-negative urine samples during pregnancy as the primary outcome. Urine samples will be collected at the weekly Medication Check Visits; sample validity will be checked with temperature monitoring and a commercially available adulterant test. In cases where the temperature reading/adulterant test indicates a non-valid sample, an attempt will be made to obtain a second urine sample. Samples will be shipped to a central lab for analysis using a rapid UDS system. Urine samples will be tested for: buprenorphine/ norbuprenorphine, fentanyl, cocaine, methamphetamine, amphetamine, opioids, marijuana, benzodiazepines, methylenedioxymethamphetamine (MDMA, Ecstasy), barbiturates, methadone, oxycodone, phencyclidine (PCP), cotinine (a biomarker of nicotine use), and ethyl glucuronide (a biomarker of alcohol consumption). For primary outcome scoring, missing urine samples will be imputed as positive for illicit opioids, which is consistent with the approach taken in the CAM2038 Phase 3 trial [39] and with the greater likelihood of illicit opioid use in patients not engaged in treatment [48,49]. The number of UDSs expected for each participant will differ based on the length of the pregnancy, thus the number of UDSs potentially imputed will also differ for each participant. The study team decided to forgo a self-report assessment of substance use in order to minimize assessment burden in this lengthy trial being conducted with a participant sample (i.e., pregnant and postpartum women) that may find longer visits challenging and that may be more prone to under-reporting substance use given the potential repercussions of mandatory reporting requirements present in many states.
The mother key secondary outcome measure is postpartum illicit opioid abstinence, which will be assessed in a similar fashion to illicit opioid abstinence during pregnancy. The infant key secondary outcome measure is NOWS severity assessed by total days of opioid treatment during the hospital stay, which is a definition that has been used in past research [42]. This outcome will be abstracted from the medical record.
4.2. Other secondary outcome measures
4.2.1. Maternal outcomes
Other maternal secondary outcomes include: 1) drug and alcohol abstinence assessed by UDS; 2) the Opioid Craving Scale [50]; 3) Kotelchuck's Adequacy of Prenatal Care Utilization index [51]; 4) the Short Opiate Withdrawal Scale-Gossop [52]; and 5) BUP Medication Adherence during pregnancy through 12 months postpartum. Adherence to BUP-XR will be based on study records. For BUP-XR, the receipt of a weekly injection will be scored as 7 days of adherence. For monthly injections, a participant will be considered as adherent for 28 days. Adherence to BUP-SL will be defined as: 1) study records showing that BUP-SL was dispensed to the participant; 2) self-reported adherence assessed at the weekly Medication Check Visits; and 3) UDSs positive for buprenorphine/norbuprenorphine.
4.2.2. Infant outcomes
Infant secondary outcomes include five additional NOWS-related outcomes: 1) use of opioid medication for NOWS symptoms (yes/no); if yes, medication used; 2) infant hospital LOS defined as the infant's age, in days, at discharge; 3) use of adjunct medications (e.g., phenobarbital, clonidine); 4) NOWS scoring assessment used and peak score if applicable; 5) an ICD-10 code indicative of NOWS (Yes/No). Other outcomes include discharge outcomes, including custody (e.g., mother, other relative, foster/adoptive family), medications at discharge (e.g., phenobarbital, clonidine), and whether or not there is an open case with child protective services (yes/no). The 6- and 12-month versions of the Ages and Stages Questionnaire, third edition (ASQ-3) [53] will be used to screen for developmental issues in the infants. The ASQ-3 is a validated, parent-administered screen used throughout the world [[53], [54], [55]] and deemed appropriate for assessing infants exposed to opioids in utero [8].
4.3. Safety measures
Safety measures collected during study visits (see Table 3) include: 1) adverse events assessments; 2) injection site examination for the BUP-XR participants; 3) the Hospital Anxiety and Depression Scale [56]; 4) prior/ concomitant medications assessments; 5) opioid overdose tracking assessed by self-report; 6) infant sedation (e.g., not waking for feeding, difficulty breathing, etc.) assessed by self-report from participants feeding their infants with breastmilk and/or formula. EHR-derived safety measures include: 1) adverse fetal outcomes; 2) maternal delivery outcomes, (e.g., delivery type, medical complications at delivery, etc.); 3) birth/neonatal outcomes including measures collected on all newborns (e.g., EGA, head circumference, weight and length, etc.); as well as adverse birth and neonatal outcomes (e.g., major birth defects, need for resuscitation, etc.).
4.4. Health economics
The main health economics outcome is the incremental cost-effectiveness ratio (ICER). Costs will be measured according to the healthcare sector perspective [57], which includes all formal (medical) costs incurred by the system on behalf of participants in each arm, and their infants. Healthcare service utilization will be measured with: 1) the Treatment Services Review [58]; and 2) the Non-study Medical and Other Services (NMOS) form, which assesses use of therapy for issues other than substance use disorder, out-of-pocket healthcare expenditures, and type of insurance (if any). Effectiveness measures will include quality-adjusted life-years (QALYs) and Abstinent Years, operationalized as the estimated proportion of the year that the participant was abstinent from opioids based on UDS results. Health-related quality of life will be measured using the Patient-Reported Outcomes Measurement Information System (PROMIS) Preference (PROPr) scoring system [[59], [60], [61], [62]].
5. Sample size estimation
The primary outcome, proportion of illicit opioid-negative urine samples during pregnancy, will be assessed at a 2.5% significance level for non-inferiority, as recommended by the FDA [63]. There is a dearth of research on the treatment of OUD in pregnant women and therefore no data upon which to base the non-inferiority margin for CTN-0080. However, the OPTIMA study, a non-inferiority trial comparing BUP/NX-SL to methadone in non-pregnant participants, is utilizing a primary outcome similar to the MOMs primary outcome (i.e., proportion of opioid-negative UDSs collected at the time of the weekly medication check with missing UDSs imputed as positive) [64]. The non-inferiority margin for the OPTIMA trial is 15%, which was selected based on a literature review and expert input [64]. Given the more vulnerable nature of the CTN-0080 patient population in which illicit opioid use impacts the health of not only the mother but also the infant, we selected the slightly more conservative margin of 11% (i.e., ∆ = 0.11). This margin reflects the allowance of slightly less effectiveness of BUP-XR given its superior PK profile and its elimination of the potential for diversion. The non-inferiority margin will be used for both the primary outcome, as well as the key secondary outcome of postpartum illicit opioid abstinence. The power analyses required an estimate of the percent of UDSs that would be illicit opioid-negative during pregnancy in the BUP-SL group. The mean selected for the sample size simulations was based on a study by Fischer and colleagues [65], which found that the median percentage of urine samples negative for illicit opioids during the entire course of pregnancy was 65% for BUP-SL participants.
A simulation study was conducted to assess study power. Given a non-inferiority margin of 0.11, the objective of the sample size simulations was to determine whether there would be sufficient power (≥80%) to detect that margin with a sample size of 300 participants under different assumptions regarding the impact of the three factors used in randomization (site, EGA and BUP-SL status at randomization). All simulated data assumed 1:1 random allocation between the treatment arms. The mean in the BUP-SL arm was assumed to be 0.65, and the mean in the BUP-XR arm was assumed to be 0.54 under the null, which corresponds to the specific non-inferiority margin of 0.11, and 0.65 under the alternative. The variance in both arms was fixed at 0.11, with contributions coming from the three random effects capturing randomization strata/factors and additional random error. The results of the simulations evaluating the power and type I error for two possible relationships between the randomization factors and outcome are given in Table 4 . Note that if 50% of the variance is due to random error, then the randomization factors are associated with outcome. On the other hand, if 100% of the variance of the outcome is random error, then the randomization factors are not associated with outcome. As anticipated, this latter situation has less power and a lower type I error rate. From Table 4 we see that even if the randomization factors are not associated with outcome, there will still be at least 80% power to detect a 0.11 non-inferiority margin with a 2.5% significance level and a sample size of 300.
Table 4.
Power for a non-inferiority margin of 11% and a significance level of 2.5%.
| Proportion of variance due to random error | Mean in BUP-XR Arm | Power (%) |
|---|---|---|
| 50% | 0.54 | 3.1% |
| 0.65 | 96.4% | |
| 100% | 0.54 | 1.6% |
| 0.65 | 82.5% |
6. Analytic plan
6.1. Primary and key secondary analyses
The same analytic approach will be used for the primary and mother key secondary outcome. The non-inferiority design yields the following hypotheses where μ A is the mean in arm A, and ∆ the non-inferiority margin:
To evaluate non-inferiority at the 2.5% significance level, the two-sided 95% confidence interval for the treatment effect, μ XR − μ SL, will be calculated using a mixed effects model where treatment arm is a fixed effect and the three randomization strata are random effects. If the lower limit of the confidence interval is greater than −0.11 (i.e., ∆ = 0.11), we will reject the null hypothesis and conclude non-inferiority of BUP-XR to BUP-SL. Only if the null hypothesis is rejected will superiority of BUP-XR to BUP-SL be considered. This will involve examining the two-sided 95% confidence interval for the treatment effect, μ XR − μ SL. If the lower limit is above zero, then we can conclude that BUP-XR is superior to BUP-SL. If this order of testing is followed, then there is no need to adjust for multiplicity [63,66]. In the event that the distributional assumptions involved in the mixed effects model are not met, which is likely, alternative non-parametric methods will be considered for this modelling such as quantile regression.
Total days of infant opioid treatment will be obtained from the medical record. There are extensive covariates to be included in modelling for the infant outcomes at the mother-, infant-, and delivery-hospital-level. Since this outcome measure is a count variable and there are covariates requiring adjustment, modelling will utilize Poisson regression. The treatment effect will be measured by the regression coefficient for treatment assignment in the model (H0: β = 0; H1: β ≠ 0).
As previously mentioned, missing UDS will be imputed as positive for illicit opioids for both the primary and key secondary mother outcomes. Different imputation methods, such as those loosening the missing at random assumption, may be considered to measure the sensitivity of the study results. Similar sensitivity analyses may also be conducted for the key secondary infant outcome.
6.2. Other secondary outcomes
For binary secondary outcomes, such as the proportion of infants requiring opioid medication, and the proportion with a medical chart ICD-10 code indicating NOWS, logistic regression, Pearson's χ 2 test of association, or another appropriate method will be used to assess the relationship with treatment assignment. Continuous outcomes, such as medication adherence, opioid craving during pregnancy and infant weight at delivery, will be modeled using ANOVA or an appropriate alternative (e.g., quantile regression) to evaluate the effectiveness of BUP-XR. Lastly, the number of opioid overdoses can be modeled as a count variable with possible zero-inflation using Poisson or Zero-Inflated Poisson regression.
6.3. Health economics-incremental cost-effectiveness ratios (ICERs)
Four ICERs will be calculated, since we are evaluating two measures of effectiveness (QALYs; Abstinent Years) over two time periods of interest (pregnancy and delivery; entire study period). Individual multivariable regressions, combined with the statistical method of recycled predictions, will be used to predict the mean value for each resource category and outcome, at each time period, by study arm. ICER confidence intervals will be estimated using nonparametric bootstrapping techniques within the multivariable framework, and acceptability curves will be constructed using parameters obtained from bootstrapping to illustrate the probability that BUP-XR is a good value relative to BUP-SL for different willingness-to-pay thresholds (i.e., cost-per-QALY and cost-per-Abstinent-Year). Acceptability curves will be developed regardless of the statistical significance for individual cost and effectiveness differences, given the increased power to detect a joint difference in costs and effects [67].
7. Oversight of data and safety
Oversight is provided by multiple boards in addition to the single IRB (University of Cincinnati). This protocol is being conducted under an US Food and Drug Administration Investigational New Drug Application as the study drug is not currently available commercially and has not been studied in this population. IND annual reports will be submitted to the FDA. The protocol also was reviewed and approved by the NIDA CTN Protocol Review Board with experts in maternal and neonatal care. Finally, an independent CTN Data and Safety Monitoring Board (DSMB) will examine accumulating data to assure protection of participants' safety while the study's scientific goals are being met. The DSMB recommends to the sponsor (NIDA) whether there is support for continuation of the trial, or evidence that study procedures should be changed, or if the trial should be halted, for reasons relating to the safety of the study participants, the efficacy of the treatment under study, or inadequate trial performance (e.g., poor recruitment).
8. Current status of the trial
Study recruitment was scheduled to start in April of 2020 but site initiation has been delayed by COVID-19.
9. Summary
There is a dearth of research upon which to base clinical guidance for the treatment of pregnant women with OUD [8]. The MOMs trial is an IND trial comparing the maternal and infant outcomes of pregnant women with OUD treated with BUP-XR, relative to BUP-SL that has been designed with pragmatic features and will be conducted in “real world” settings to ensure that the findings are generalizable to clinical settings. The trial is also unusual in following women for 12 months postpartum. This unique design will yield data to address a number of gaps in the care of OUD in pregnant women including mechanisms by which BUP treatment affects maternal and infant outcomes and the potential impact of BUP exposure on neurodevelopment. This trial holds great promise to provide an impactful advance in the treatment of pregnant and postpartum women for OUD and their children.
Role of the funding source
This work was supported by the National Institute on Drug Abuse, United States of America, National Drug Abuse Treatment Clinical Trials Network, National Institutes of Health, by grants: UG1DA013732 to the University of Cincinnati (Theresa Winhusen), UG1DA015831 (Roger Weiss and Kathleen Carroll), UG1DA015815 (James Sorensen and Todd Korthuis), UG1DA013720 (Jose Szapocznik, Daniel Feaster, and Lisa Metsch), UG1DA013727 (Kathleen Brady and Matthew Carpenter), UG1DA049436 (Jane Liebschutz and Judith Feinberg), UG1DA013714 (Dennis Donovan and Mary Hatch-Maillette), UG1DA049468 (Kimberly Page), UG1DA049444 (Adam Gordon, Gerald Cochran, and Jon-Kar Zubieta), UG1DA040317 (Li-Tzy Wu). The work was also supported by NIDA contracts to the Clinical Trials Network Clinical Coordinating Center (CCC) (HHSN271201500065C) and Clinical Trials Network Data and Statistics Center (DSC) (HHSN271201400028C). The Publications Committee of the National Drug Abuse Treatment Clinical Trials Network reviewed and gave approval for submission of this manuscript.
Declaration of Competing Interest
Dr. Lofwall reported receiving research funding from Braeburn Pharmaceuticals, Inc., consulting fees from Braeburn Pharmaceuticals, Inc. and Titan, and honorarium/travel expenses from Camurus. Dr. Murphy reports having consulted for Sandoz Inc. for work unrelated to the investigation reported here. Dr. Wexelblatt reported receiving consulting fees from Braeburn Pharmaceuticals, Inc. All other authors have no potential conflicts of interest to report.
References
- 1.Hayes M.J., Brown M.S. Epidemic of prescription opiate abuse and neonatal abstinence. JAMA. 2012;307(18):1974–1975. doi: 10.1001/jama.2012.4526. [DOI] [PubMed] [Google Scholar]
- 2.Jones H.E., Heil S.H., Baewert A., Arria A.M., Kaltenbach K., Martin P.R., Coyle M.G., Selby P., Stine S.M., Fischer G. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107:5–27. doi: 10.1111/j.1360-0443.2012.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda A., Bateman B.T., Clancy C.R., Creanga A.A., Leffert L.R. Opioid abuse and dependence during pregnancy temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 4.Patrick S.W., Schiff D.M. U.S.E. committee on substance, prevention, a public health response to opioid use in pregnancy. Pediatrics. 2017;139(3) doi: 10.1542/peds.2016-4070. [DOI] [PubMed] [Google Scholar]
- 5.Patrick S.W., Schumacher R.E., Benneyworth B.D., Krans E.E., McAllister J.M., Davis M.M. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. Jama. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 6.Patrick S.W., Davis M.M., Lehmann C.U., Cooper W.O. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J. Perinatol. 2015;35(8):650–655. doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandall S.R., Albin S., Gartner L.M., Lee K.S., Eidelman A., Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Hum. Dev. 1977;1(2):159–169. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- 8.Abuse Substance, Administration Mental Health Services. Department of Health and Human Services; Rockville, MD: 2018. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and their Infants. [Google Scholar]
- 9.Reddy U.M., Davis J.M., Ren Z., Greene M.F. N.A.S. Opioid Use in Pregnancy, S. Childhood Outcomes Workshop Invited, Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet. Gynecol. 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder C.M., Winhusen T. Pharmacological management of opioid use disorder in pregnant women. CNS Drugs. 2015;29(8):625–636. doi: 10.1007/s40263-015-0273-8. [DOI] [PubMed] [Google Scholar]
- 11.Lofwall M.R., Walsh S.L. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J. Addict. Med. 2014;8(5):315–326. doi: 10.1097/ADM.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hser Y.I., Saxon A.J., Huang D., Hasson A., Thomas C., Hillhouse M., Jacobs P., Teruya C., McLaughlin P., Wiest K., Cohen A., Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto H., Maskrey V., Swift L., Rumball D., Wagle A., Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J. Subst. Abus. Treat. 2010;39(4):340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Gryczynski J., Mitchell S.G., Jaffe J.H., Kelly S.M., Myers C.P., O’Grady K.E., Olsen Y.K., Schwartz R.P. Retention in methadone and buprenorphine treatment among African Americans. J. Subst. Abus. Treat. 2013;45(3):287–292. doi: 10.1016/j.jsat.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwald M.K., Comer S.D., Fiellin D.A. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11. doi: 10.1016/j.drugalcdep.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caritis S.N., Bastian J.R., Zhang H., Kalluri H., English D., England M., Bobby S., Venkataramanan R. An evidence-based recommendation to increase the dosing frequency of buprenorphine during pregnancy. Am. J. Obstet. Gynecol. 2017;217(4):459 e1–459 e6. doi: 10.1016/j.ajog.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compton W.M., Volkow N.D. Improving outcomes for persons with opioid use disorders: buprenorphine implants to improve adherence and access to care. JAMA. 2016;316(3):277–279. doi: 10.1001/jama.2016.8897. [DOI] [PubMed] [Google Scholar]
- 18.Iglay K., Cao X., Mavros P., Joshi K., Yu S., Tunceli K. Systematic literature review and meta-analysis of medication adherence with once-weekly versus once-daily therapy. Clin. Ther. 2015;37(8):1813–1821. doi: 10.1016/j.clinthera.2015.05.505. (e1) [DOI] [PubMed] [Google Scholar]
- 19.McCarthy J.J., Leamon M.H., Willits N.H., Salo R. The effect of methadone dose regimen on neonatal abstinence syndrome. J. Addict. Med. 2015;9(2):105–110. doi: 10.1097/ADM.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 20.Shah D., Brown S., Hagemeier N., Zheng S., Kyle A., Pryor J., Dankhara N., Singh P. Predictors of neonatal abstinence syndrome in buprenorphine exposed newborn: can cord blood buprenorphine metabolite levels help? SpringerPlus. 2016;5(1):854. doi: 10.1186/s40064-016-2576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braeburn Pharmaceuticals Inc. 2017. FDA Advisory Committee Meeting Briefing Document: CAM2038 (buprenorphine) subcutaneous injection.https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM582594.pdf (Accessed April 17 2018) [Google Scholar]
- 22.Jones H.E., Kaltenbach K., Heil S.H., Stine S.M., Coyle M.G., Arria A.M., O’Grady K.E., Selby P., Martin P.R., Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones H.E., Fischer G., Heil S.H., Kaltenbach K., Martin P.R., Coyle M.G., Selby P., Stine S.M., O’Grady K.E., Arria A.M. Maternal opioid treatment: human experimental research (MOTHER)-approach, issues and lessons learned. Addiction. 2012;107:28–35. doi: 10.1111/j.1360-0443.2012.04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford I., Norrie J. Pragmatic trials. N. Engl. J. Med. 2016;375(5):454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 25.Cheatham C.L., Goldman B.D., Fischer L.M., da Costa K.A., Reznick J.S., Zeisel S.H. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2012;96(6):1465–1472. doi: 10.3945/ajcn.112.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . National Academies Press; Washington, D.C.: 1998. Choline, Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; pp. 390–422. [PubMed] [Google Scholar]
- 27.American Medical Association PolicyFinder: Choline Supplementation in Prenatal Vitamins H-420.951. 2017. https://policysearch.ama-assn.org/policyfinder (Accessed January 15 2019)
- 28.Ferretti C.A., Spotti M.L., Di Cosimo J.I. Diglyceride-rich oils from glycerolysis of edible vegetable oils. Catal. Today. 2018;302:233–241. [Google Scholar]
- 29.Burd L., Blair J., Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J. Perinatol. 2012;32(9):652–659. doi: 10.1038/jp.2012.57. [DOI] [PubMed] [Google Scholar]
- 30.Kalinowski A., Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction. 2016;111(7):1293–1298. doi: 10.1111/add.13341. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Breastfeeding: Alcohol. 2018. https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/vaccinations-medications-drugs/alcohol.html (Accessed November 13 2018)
- 32.Nguyen L., Lander L.R., O’Grady K.E., Marshalek P.J., Schmidt A., Kelly A.K., Jones H.E. Treating women with opioid use disorder during pregnancy in Appalachia: initial neonatal outcomes following buprenorphine + naloxone exposure. Am. J. Addict. 2018;27(2):92–96. doi: 10.1111/ajad.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debelak K., Morrone W.R., O’Grady K.E., Jones H.E. Buprenorphine plus naloxone in the treatment of opioid dependence during pregnancy-initial patient care and outcome data. Am. J. Addict. 2013;22(3):252–254. doi: 10.1111/j.1521-0391.2012.12005.x. [DOI] [PubMed] [Google Scholar]
- 34.Dooley J., Gerber-Finn L., Antone I., Guilfoyle J., Blakelock B., Balfour-Boehm J., Hopman W.M., Jumah N., Kelly L. Buprenorphine-naloxone use in pregnancy for treatment of opioid dependence retrospective cohort study of 30 patients. Can. Fam. Physician. 2016;62(4):E194–E200. [Google Scholar]
- 35.Wiegand S.L., Stringer E.M., Stuebe A.M., Jones H., Seashore C., Thorp J. Buprenorphine and naloxone compared with methadone treatment in pregnancy. Obstet. Gynecol. 2015;125(2):363–368. doi: 10.1097/AOG.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 36.Jumah N.A., Edwards C., Balfour-Boehm J., Loewen K., Dooley J., Finn L.G., Kelly L. Observational study of the safety of buprenorphine plus naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6(10) doi: 10.1136/bmjopen-2016-011774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krsak M., Trowbridge P., Regan N., Freedman K.I. Buprenorphine with, or without, naloxone for pregnant women?–review of current evidence and practice in Massachusetts. J. Alcohol. Drug Depend. 2017;5(3) [Google Scholar]
- 38.Tai B., Straus M.M., Liu D., Sparenborg S., Jackson R., McCarty D. The first decade of the National Drug Abuse Treatment Clinical Trials Network: bridging the gap between research and practice to improve drug abuse treatment. J. Subst. Abus. Treat. 2010;38:S4–S13. doi: 10.1016/j.jsat.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lofwall M.R., Walsh S.L., Nunes E.V., Bailey G.L., Sigmon S.C., Kampman K.M., Frost M., Tiberg F., Linden M., Sheldon B., Oosman S., Peterson S., Chen M., Kim S. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern. Med. 2018;178(6):764–773. doi: 10.1001/jamainternmed.2018.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones E.B. Medication-assisted opioid treatment prescribers in federally qualified health Centers: capacity lags in rural areas. J. Rural Health. 2018;34(1):14–22. doi: 10.1111/jrh.12260. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Shorter D., Kosten T.R. Buprenorphine in the treatment of opioid addiction: opportunities, challenges and strategies. Expert. Opin. Pharmacother. 2014;15(15):2263–2275. doi: 10.1517/14656566.2014.955469. [DOI] [PubMed] [Google Scholar]
- 42.Hall E.S., Wexelblatt S.L., Crowley M., Grow J.L., Jasin L.R., Klebanoff M.A., McClead R.E., Meinzen-Derr J., Mohan V.K., Stein H., Walsh M.C., Consortium O. Implementation of a neonatal abstinence syndrome weaning protocol: a multicenter cohort study. Pediatrics. 2015;136(4):e803–e810. doi: 10.1542/peds.2015-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick S.W., Schumacher R.E., Horbar J.D., Buus-Frank M.E., Edwards E.M., Morrow K.A., Ferrelli K.R., Picarillo A.P., Gupta M., Soll R.F. Improving Care for Neonatal Abstinence Syndrome. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacMillan K.D.L., Rendon C.P., Verma K., Riblet N., Washer D.B., Volpe Holmes A. Association of rooming-in with outcomes for neonatal abstinence syndrome: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(4):345–351. doi: 10.1001/jamapediatrics.2017.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogen D.L., Whalen B.L., Kair L.R., Vining M., King B.A. Wide variation found in care of opioid-exposed newborns. Acad. Pediatr. 2017;17(4):374–380. doi: 10.1016/j.acap.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiff D.M., Nielsen T., Terplan M., Hood M., Bernson D., Diop H., Bharel M., Wilens T.E., LaRochelle M., Walley A.Y., Land T. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet. Gynecol. 2018;132(2):466–474. doi: 10.1097/AOG.0000000000002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frost M., Bailey G.L., Lintzeris N., Strang J., Dunlop A., Nunes E.V., Jansen J.B., Frey L.C., Weber B., Haber P., Oosman S., Kim S., Tiberg F. Long-term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult out-patients with opioid use disorder. Addiction. 2019;114(8):1416–1426. doi: 10.1111/add.14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss R.D., Potter J.S., Griffin M.L., Provost S.E., Fitzmaurice G.M., McDermott K.A., Srisarajivakul E.N., Dodd D.R., Dreifuss J.A., McHugh R.K., Carroll K.M. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Depend. 2015;150:112–119. doi: 10.1016/j.drugalcdep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hser Y.I., Evans E., Huang D., Weiss R., Saxon A., Carroll K.M., Woody G., Liu D., Wakim P., Matthews A.G., Hatch-Maillette M., Jelstrom E., Wiest K., McLaughlin P., Ling W. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHugh R.K., Fitzmaurice G.M., Carroll K.M., Griffin M.L., Hill K.P., Wasan A.D., Weiss R.D. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend. 2014;145:121–126. doi: 10.1016/j.drugalcdep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotelchuck M. The adequacy of prenatal care utilization index: its US distribution and association with low birthweight. Am. J. Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gossop M. The development of a short opiate withdrawal scale (SOWS) Addict. Behav. 1990;15(5):487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- 53.Squires J., Bricker D. Paul H. Brookes Publishing Co; Baltimore: 2009. Ages & Stages Questionnaires®, Third Edition (ASQ- 3TM). A Parent-Completed Child-Monitoring System. [Google Scholar]
- 54.Squires J., Bricker D., Potter L. Revision of a parent-completed development screening tool: ages and stages questionnaires. J. Pediatr. Psychol. 1997;22(3):313–328. doi: 10.1093/jpepsy/22.3.313. [DOI] [PubMed] [Google Scholar]
- 55.Singh A., Yeh C.J., Boone Blanchard S. Ages and stages questionnaire: a global screening scale. Boletin Medico Del Hospital Infantil de Mexico. 2017;74(1):5–12. doi: 10.1016/j.bmhimx.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 57.Neumann P.J., Sanders G.D., Russell L.B., Siegel J.E., Ganiats T.G. 2nd ed. Oxford University Press; New York: 2017. Cost Effectiveness in Health and Medicine. [Google Scholar]
- 58.Cacciola J.S., Alterman A.I., Lynch K.G., Martin J.M., Beauchamp M.L., McLellan A.T. Initial reliability and validity studies of the revised treatment services review (TSR-6) Drug Alcohol Depend. 2008;92(1–3):37–47. doi: 10.1016/j.drugalcdep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Amtmann D., Bode R., Buysse D., Choi S., Cook K., Devellis R., DeWalt D., Fries J.F., Gershon R., Hahn E.A., Lai J.S., Pilkonis P., Revicki D., Rose M., Weinfurt K., Hays R., P.C. Group The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.HealthMeasures PROMIS® (Patient-Reported Outcomes Measurement Information System) 2019. http://www.healthmeasures.net/explore-measurement-systems/promis (Accessed August 15 2019)
- 61.Dewitt B., Feeny D., Fischhoff B., Cella D., Hays R.D., Hess R., Pilkonis P.A., Revicki D.A., Roberts M.S., Tsevat J., Yu L., Hanmer J. Estimation of a preference-based summary score for the patient-reported outcomes measurement information system: the PROMIS((R))-preference (PROPr) scoring system. Med. Decision Making. 2018;38(6):683–698. doi: 10.1177/0272989X18776637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanmer J., Cella D., Feeny D., Fischhoff B., Hays R.D., Hess R., Pilkonis P.A., Revicki D., Roberts M., Tsevat J., Yu L. Selection of key health domains from PROMIS((R)) for a generic preference-based scoring system. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehab. 2017;26(12):3377–3385. doi: 10.1007/s11136-017-1686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.FDA Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research . U.S. Department of Health and Human Services; Silver Spring, MD: 2016. Non-Inferiority Clinical Trials to Establish Effectiveness: Guidance for Industry. [Google Scholar]
- 64.Socias M.E., Ahamad K., Le Foll B., Lim R., Bruneau J., Fischer B., Wild T.C., Wood E., Jutras-Aswad D. The OPTIMA study, buprenorphine/naloxone and methadone models of care for the treatment of prescription opioid use disorder: study design and rationale. Contemp. Clin. Trials. 2018;69:21–27. doi: 10.1016/j.cct.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer G., Ortner R., Rohrmeister K., Jagsch R., Baewert A., Langer M., Aschauer H. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 66.Committee for Proprietary Medicinal Products . 2000. Points to Consider on Switching Between Superiority and Non-inferiority, European Agency for the Evaluation of Medicinal Products, London. [Google Scholar]
- 67.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Oxford University Press; 2014. Economic Evaluation in Clinical Trials. [Google Scholar]