Abstract
Background and Aims
The abrupt outbreak of the novel coronavirus disease 2019 and its rapid spread over many healthcare systems throughout the world has led to a shortage in personal protective equipment (PPE), which cannot be solved by reducing their use or by increasing production. It is thus necessary to promote PPE rational use, highlighting possible differences in terms of efficacy and promoting an effective technique to reuse them.
Methods
A literature search was performed on PubMed, Scopus, Cochrane database, and Google Scholar, and from the 25 top cited articles, 15 were selected for relevance and impact.
Results
Most studies on previous respiratory virus epidemics to date suggest surgical masks are not inferior compared with N95 respirators in terms of protective efficacy among healthcare workers. Therefore, the use of N95 respirators should be limited to high-risk situations. Concerning respirator reuse, highly energetic, short-wave, ultraviolet germicidal irradiation (UVGI) at 254 nm was determined to decontaminate N95 respirators from viral respiratory agents, but UVGI requires careful consideration of the type of respirator and of the biologic target.
Conclusions
Rational use and successful reuse of respirators can help in the shortage of PPE during a pandemic. Further studies testing UVGI and other decontamination techniques are an unmet need. The definitive answer to pandemic issues can be found in artificial intelligence and deep learning. These groundbreaking modalities could help in identifying high-risk patients and in suggesting appropriate types and use of PPE.
Abbreviations: COVID-19, coronavirus disease 2019; PAPR, powered air-purifying respirator; PPE, personal protective equipment; RCT, randomized controlled trial; UVGI, ultraviolet germicidal irradiation
Graphical abstract
The severe acute respiratory syndrome–coronavirus 2 outbreak abruptly resulted in the novel coronavirus disease 2019 (COVID-19) pandemic, almost leading to the collapse of many healthcare systems in the world that were overwhelmed with potentially infectious patients seeking testing and care. In the attempt to prevent the spread of a viral infection to and from healthcare workers, the health community generally relies on the efficacy of personal protective equipment (PPE): gloves, masks, respirators, goggles, face shields, and gowns. PPE, once omnipresent and easily available in the hospital environment, is now scarce and precious. This situation, driven not only by the number of COVID-19 cases but also by misinformation, panic buying, and stockpiling during a pandemic, is of tremendous concern especially for the health community that is at greatest risk for exposure. Digestive endoscopists are among those at highest risk to infection because of aerosolization during procedures. Reducing the use of PPE through the postponement of elective and nonurgent outpatient clinical procedures does not sufficiently compensate for the scarcity of these goods. Moreover, an increase in the production of PPE would require a time interval that many health systems cannot afford given the speed of the spread of the infection.
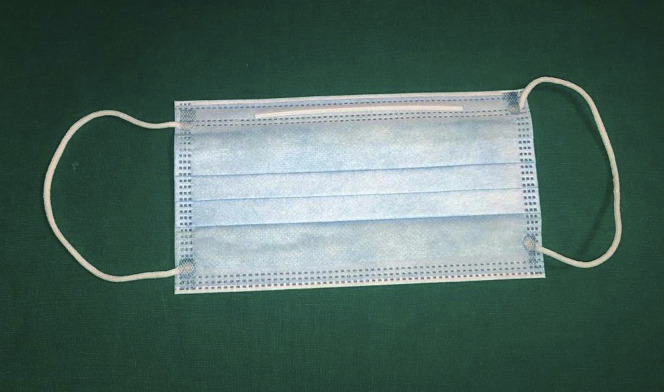
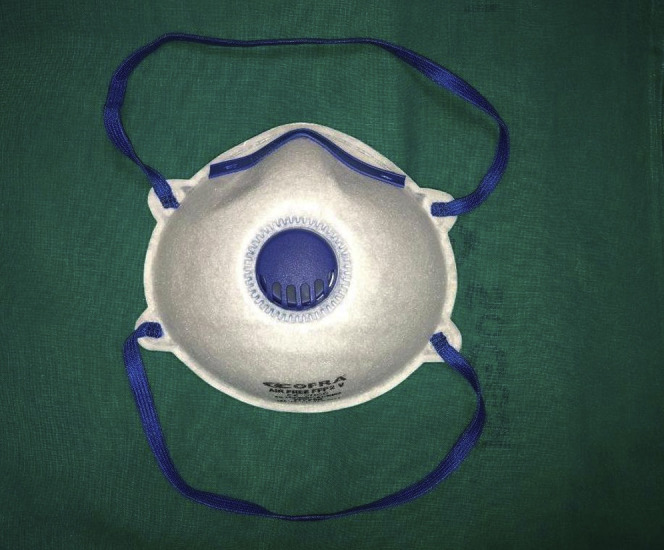
Among the various types of PPE, surgical masks, N95 respirators, and powered air-purifying respirators (PAPRs) are currently mostly used. Surgical masks (Fig. 1 ) are usually loose-fitting and disposable; they create a physical barrier between the mouth and nose of the wearer and potential contaminants in the immediate environment and vary by quality and levels of protection. N95 respirators (Fig. 2 ) have the advantage of blocking at least 95% of aerosol (<5 μm) and droplet-size (5-50 μm) particles. Their use requires an initial and periodic fit testing, and they are associated with poor tolerance by users because of breathing resistance and heat. N95 respirators correspond to European filtering face piece 2 standard (Fig. 3 ), which have at least a 94% filter capacity. PAPRs (Fig. 4 ) are battery-powered blowers that provide positive airflow through a filter. The type of filter is dictated by the amount of airborne contaminant exposure; they provide head and neck protection and do not require fit testing, especially if they do not have a tight-fitting face piece. However, PAPRs are usually associated with increased perception of eye dryness and are by far the most expensive PPE against respiratory infections.
Figure 1.
Surgical mask.
Figure 2.
N95 respirator.
Figure 3.
Filtering face piece 2 standard.
Figure 4.
Powered air-purifying respirator (Dräger X-plore 8000; Drägerwerk AG & Co KgaA, Lübeck, Germany).
In meeting the challenge of the PPE shortage, it is necessary to underline possible differences in terms of efficacy in preventing viral transmission among the currently most-used PPE to facilitate their rational use. The scarcity of PPE could also be mitigated by identifying an effective reuse technique. The primary aims of this review are therefore to summarize the protective efficacy of masks and respirators in preventing the spread of respiratory infections and to propose a proper biologic decontamination process to reuse respirators.
Methods
A literature search was conducted on PubMed, Scopus, Cochrane database, and Google Scholar using the terms “surgical masks,” “masks,” “N95 respirators,” “powered air-purifying respirators,” “respirators,” “respiratory viruses,” “respirators/PPE reuse,” “respirators/PPE disinfection,” and “healthcare workers.” From the 25 top-cited articles, 15 were selected for relevance and impact.
Results
Protective efficacy of healthcare workers comparing masks
According to a Cochrane-approved systematic review on physical interventions to prevent respiratory virus transmission conducted in 2011, surgical masks and N95 respirators are the most consistent and comprehensive supportive measures adopted among healthcare workers. The highest quality, cluster-randomized controlled trials (RCTs) included in this systematic review proved that N95 respirators are noninferior to surgical masks in terms of efficacy in preventing viral transmission.1
What is reported by this review is in accordance with a classic, highly cited, case-control study carried out in Hong Kong during the severe acute respiratory syndrome in 2003 that evaluated personal protective practices (handwashing, wearing paper masks, surgical masks or N95 respirators, gloves, and gowns). A multivariate logistic regression conducted to analyze the impact of each protective measure demonstrated that N95 respirators and surgical masks have similar protective effect.2
The equivalence in terms of efficacy between the 2 types of PPE considered was similarly demonstrated in the specific case of influenza. A quantitative assessment of the efficacy of surgical masks and N95 respirators to filter influenza virus in patients with acute influenza infection carried out in 2009 demonstrated that N95 respirators and surgical masks were equally able to prevent the spread of reverse transcription polymerase chain reaction–detectable virus when worn correctly by 9 patients with laboratory-confirmed acute influenza.3 In the same way, in 2009 an RCT comparing the efficacy of surgical masks versus N95 respirators among emergency department nurses during an influenza outbreak demonstrated the equal efficacy between the 2 practices (absolute risk difference, −.73%; 95% confidence interval [CI], −8.8% to 7.3%; P > .05).4 The latest RCT available focusing on influenza prevention among 2371 randomized healthcare workers published in September 2019 confirms that surgical masks and N95 respirators do not have significant differences in terms of laboratory-confirmed influenza infection prevention (difference, 1.0%; 95% CI, −.5% to 2.5%; P > .05).5
A systematic review and meta-analysis of observational studies and RCTs published in 2017 confirmed both PPE to be effective in protecting against severe acute respiratory syndrome (odds ratios, .13 [95% CI, .03-.62] for surgical masks and .12 [95% CI, .06-.26] for N95 respirators). Corresponding to previous reports, N95 respirators did not confer superior protection against viral infections or influenza-like illness compared with surgical masks, but they were demonstrated for the first time to be more effective in protecting against general clinical respiratory illness (relative risk [RR], .47; 95% CI, .36-.62) and laboratory-confirmed bacterial illness (RR, .46; 95% CI, .34-.62).6
Similar evidence was derived from the most recent version of a systematic review and meta-analysis updated in February 2020. Among a total of 6 RCTs involving 9171 participants, no statistically significant differences in preventing laboratory-confirmed influenza (RR, 1.09; 95% CI, .92-1.28; P > .05), laboratory-confirmed respiratory viral infections (RR, .89; 95% CI, .70-1.11), laboratory-confirmed respiratory infection (RR, .74; 95% CI, .42-1.29), and influenza-like illness (RR, .61; 95% CI, .33-1.14) were reported using surgical masks or N95 respirators. N95 respirators proved to have a protective effect against laboratory-confirmed bacterial colonization (RR, .58; 95% CI, .43-.78).7 , 8
The use of PAPRs was mainly proposed during the outbreak of ebola,9 and, bearing in mind that their greater level of respiratory protection than N95 masks has not been subjected to rigorous scientific investigation, their use is generally recommended in situations in which a live airborne virus is being handled.10
Scientific methods for respirators reuse
Disposable filtering face-piece respirators are not approved for routine decontamination and reuse as standard of care, but a possible strategy in a PPE shortage during specific emergency situations is to reuse them after a proper biologic decontamination process to render infectious material inactive. It is very important that the treatment does not deteriorate the respirator material, thus decreasing its filtering power against respiratory infectious species, or release any toxic residues on the respirator surface. PPE reuse can be realized exclusively by the original healthcare worker. The Centers for Disease Control and Prevention reported that decontamination methods such as autoclave, 160°C dry heat, 70% isopropyl alcohol, and soap and water cause significant respirator filter degradation, which consequently allows excessive particle penetration levels.11
An observational study conducted in 2009 during an influenza pandemic compared several ways of decontaminating N95 respirators: ultraviolet germicidal irradiation (UVGI), ethylene oxide, vaporized hydrogen peroxide, microwave oven irradiation, and bleach.12 These decontaminating processes were evaluated for changes in N95 respirator physical appearance, odor, and laboratory performance (filter aerosol penetration and filter airflow resistance); however, this study did not assess the efficiency of the decontamination methods to inactivate viable microorganisms. The decontamination methods using microwave oven irradiation and bleach were determined to be the least desirable among the 5 methods tested because of excessive degradation of the respirator surface and unpleasant odor, respectively. UVGI, ethylene oxide, and vaporized hydrogen peroxide were found to be the most promising decontamination methods for respiratory viral agent elimination and respirator integrity maintenance; however, concerns were raised about the throughput capabilities for ethylene oxide and vaporized hydrogen peroxide.
Three decontamination methods against H5N1 influenza virus were similarly compared in another observational study carried out in 2011: UVGI, microwave-generated steam, and moist heat were compared when used to purify N95 respirators from viral contamination. A highly energetic, short-wave UVGI at 254 nm was demonstrated to be the most effective method in quantitatively reducing reverse transcription polymerase chain reaction viral RNA on N95 respirator surfaces.13
An experimental study conducted in 2015 confirmed that 254-nm UVGI was efficient in decontaminating N95 respirators from viral respiratory agents; the authors evaluated the effect of UVGI not only in terms of filtration performance, but also in terms of structural integrity. UVGI was performed in conditions of controlled humidity and temperature in a custom-made chamber (91 cm × 31 cm × 64 cm high) fitted with two 15-W, T-150, 254-nm ultraviolet C lamps and lined with black felt to minimize reflections. UVGI did not substantially affect the filtration performance or the flow resistance at doses up to 950 J/cm2. Reduction in structural integrity was reported only for higher doses of UVGI.14
Supplementary evidence derived from another experimental study performed in 2018 showed UVGI efficiency on influenza-contaminated N95 respirators.15 N95 respirator samples contaminated with H1N1 influenza were treated for approximately 60 to 70 seconds with approximate UVGI irradiance of 17 mW/cm2 for a total of dose of about 1 J/cm2. All contaminated and treated surfaces were cut out and virus was extracted; viable influenza was quantified using a median tissue culture infectious dose assay. Significant reductions (≥3 log) in influenza viability were observed on the respirators’ surfaces.
Discussion
Most studies on previous respiratory virus epidemics to date suggest similar efficacy of surgical masks to N95 respirators. A strong protective effect of both masks has in fact been demonstrated, especially when used in combination with other protective measures of hand washing, eye protection, gowns, and gloves. International organizations, first and foremost the World Health Organization, do however recommend healthcare workers to use N95 respirators in high-risk situations such as aerosol-generating procedures. In specific emergency situations such as the COVID-19 pandemic, the use of N95 respirators should be restricted among the general public and non–high-risk medical staff in favor of high-risk healthcare workers. The use of PAPRs should be limited to high-risk healthcare personnel dealing with airborne virus outbreaks. To avoid the excessive waste of PPE, the same respirator can be worn while caring for multiple patients who have the same diagnosis without removing it; respirators maintain their protection when used for extended periods. However, using 1 respirator for longer than 4 hours can lead to discomfort and should be avoided.16
As far as respirator reuse is concerned, overall, UVGI is widely known as an effective and useful decontaminative technique. Its virucidal mechanism was proficiently applied to determine N95 respirator decontamination from viral respiratory agents. The highly energetic, short-wave UVGI at 254 nm was demonstrated to be especially effective in reducing reverse transcription polymerase chain reaction influenza RNA, but there are some critical points that need attention. First, an insufficient UVGI dose cannot reach all internal surfaces of respirators and, consequently, can leave active infectious material. On the other hand, an excessive UVGI dose can partially affect the structural integrity of respirators and lower their filtration performance. Furthermore, each PPE can tolerate a maximum number of disinfection cycles depending on its design and type of components; valves, for instance, technically cannot be sterilized with UVGI.
Conclusions and Perspectives
In the midst of the COVID-19 pandemic, it is essential to avoid excessive consumption of PPE and to implement their rational use. Surgical masks and N95 respirators have been demonstrated to be equally efficient in protecting healthcare workers from respiratory viral infection; however, N95 respirators should be used in high-risk situations. Another possible strategy to tackle the PPE shortage is to reuse them after a proper biologic decontamination process. The UVGI method proved to be a valid alternative to decontaminate N95 respirators, but it requires careful consideration in the type of respirator and the biologic target. Further studies testing this technique on different models are an unmet need. The definitive answer to these problems could be found in artificial intelligence and deep learning: These groundbreaking modalities are rapidly growing worldwide, and their application in a pandemic could help to identify high-risk patients and situations and suggest appropriate use and types of PPE, thus saving lives. Although we have faced a pandemic almost every 10 years, to date the problem of shortages in PPE and their inappropriate use has not been solved. Victims of this are first and foremost healthcare workers. The time has come for the historical lessons of previous pandemics to be learned.
Footnotes
DISCLOSURE: The following authors disclosed financial relationships: I. Boškoski: Consultant for Apollo Endosurgery, Cook Medical, and Boston Scientific; board member for Endo Tools; research grant recipient fromApollo Endosurgery; food and beverage compensation from Apollo Endosurgery, Cook Medical, Boston Scientific, and Endo Tools. M. B. Wallace: Consultant for Virgo Inc, Cosmo/Aries Pharmaceuticals, Anx Robotica, Covidien, GI Supply, Boston Scientific, and Microtek; research grant recipient fromGI Supply,Fujifilm,Boston Scientific,Olympus,Ninepoint Medical, and Cosmo/Aries Pharmaceuticals; stockholder in Virgo Inc; food and beverage compensation from Synergy Pharmaceuticals, Cook Medical, and Boston Scientific. G. Costamagna: Consultant for and food and beverage compensation from Cook Medical, Boston Scientific, and Olympus. All other authors disclosed no financial relationships.
References
- 1.Jefferson T., Del Mar C.B., Dooley L. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011 Jul;6(7):CD006207. doi: 10.1002/14651858.CD006207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seto W., Tsang D., Yung R. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson D.F., Druce J.D., Birch C. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 4.Loeb M., Dafoe N., Mahony J. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 5.Radonovich L.J., Simberkoff M.S., Bessesen M.T. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offeddu V., Yung C.F., Low M.S.F. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.D., MacDougall C.C., Johnstone J. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ. 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Y., Hu T., Liu L. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. Epub. 2020 Mar 13 doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts V. To PAPR or not to PAPR? Can J Respir Ther. 2014;50:87–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Roberge R.J. Evaluation of the rationale for concurrent use of N95 filtering facepiece respirators with loose-fitting powered air-purifying respirators during aerosol-generating medical procedures. Am J Infect Control. 2008;36:135–141. doi: 10.1016/j.ajic.2007.04.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at:
- 12.Viscusi D.J., Bergman M.S., Eimer B.C. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lore M.B., Heimbuch B.K., Brown T.L. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 14.Lindsley W.G., Martin S.B., Thewlis R.E. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills D., Harnish D.A., Lawrence C. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization W.H. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance, 19 March 2020. https://apps.who.int/iris/handle/10665/331498 Available at: