Abstract
In as few as 3 months, coronavirus disease 2019 (COVID-19) has spread and ravaged the world at an unprecedented speed in modern history, rivaling the 1918 flu pandemic. Severe acute respiratory syndrome coronavirus-2, the culprit virus, is highly contagious and stable in the environment and transmits predominantly among humans via the respiratory route. Accumulating evidence suggest that this virus, like many of its related viruses, may also be an enteric virus that can spread via the fecal–oral route. Such a hypothesis would also contribute to the rapidity and proliferation of this pandemic. Here we briefly summarize what is known about this family of viruses and literature basis of the hypothesis that severe acute respiratory syndrome coronavirus-2 is capable of infecting the gastrointestinal tract and shedding in the environment for potential human-to-human transmission.
Abbreviations used in this paper: ACE2, angiotensin I converting enzyme 2; CoV, coronavirus; COVID-19, coronavirus disease 2019; DPP4, dipeptidyl-peptidase 4; GI, gastrointestinal; MERS, Middle East respiratory syndrome; S, spike glycoprotein; SARS, severe acute respiratory syndrome; TMPRSS2, transmembrane serine protease 2
Coronaviruses (CoVs) are ubiquitous in nature and infect a wide range of animals, causing diseases involving the respiratory, gastrointestinal (GI), and neurological systems.1 Before 2000, only 2 species of CoVs (HCoV-229E and HCoV-OC43) were known to infect humans and cause mild respiratory illness.2 Two other species of human CoVs (HCoV-NL63 and HCoV-HKU1) were isolated in the early 2000s. Since then, the human race has encountered the following 3 novel CoV outbreaks: severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, Middle East respiratory syndrome (MERS)-CoV in 2012, and the ongoing SARS-CoV-2, all of which have jumped species from animals to humans and are associated with severe respiratory disease and high mortality. Zoonotic infections involving nonhuman species as intermediate hosts, such as invertebrate vectors, rodents, and bats, are common in nature.3 But cross-species jumps from animals to humans with altered tropism as a result of genetic alterations are less frequent. At present, it is not clear what accounts for this seemingly increased pace of cross-species transmission of such virulent pathogens that can cause devastating disease in humans. Manipulation of the environment by mankind, increased human–animal contacts, and globalization may have facilitated conditions for cross-species infection.4
At the time this review was completed (April 23, 2020), coronavirus disease 2019 (COVID-19) has emerged as a world pandemic. Globally SARS-CoV-2 has infected 2,667,532 people, of which 850,116 cases are identified in the United States alone. Currently, no clinically approved specific antivirals (except the recently demonstrated benefit of remdesivir), other therapeutic remedies, or vaccines are available for this disease. Although spread of the virus among humans is predominantly through respiratory droplets, questions remain regarding other potential modes of transmission that may contribute to the initial cross-species infection, a large number asymptomatic cases, and the rapid and unusual pattern of dissemination across the globe. In this review, we provide a summary of the molecular biology of the virus, evidence for its infection of cells within the gastrointestinal and hepatotropic/biliary tracts, and implications for potential fecal–oral transmission of the virus. For more extensive review of the virus and its associated diseases, we refer to other review articles and brief summaries.5, 6, 7, 8, 9
Molecular Biology and Tropism of Coronavirus
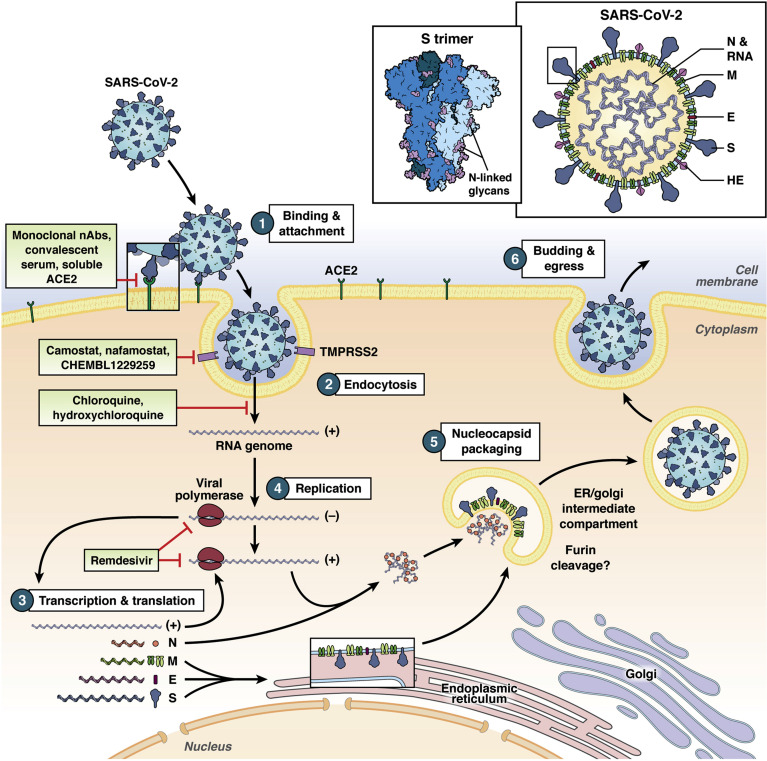
CoVs belong to the Coronaviridae family within the Nidovirales order. They are enveloped, nonsegmented, positive-sense RNA viruses with a large genome of approximately 30 kb. Figure 1 illustrates the schematic replication cycle of the virus. The initial attachment of the CoV to the host cell is mediated by interactions between the spike glycoprotein (S) and its cognate receptor. This molecular interaction is a major determinant of species, tissue, and cell tropism of a CoV. Many CoVs utilize cell-surface peptidases as their receptors, but the peptidase activity seems to be dispensable for viral entry.10 Many alphacoronaviruses use aminopeptidase N.11 , 12 In the case of SARS-CoV and SARS-CoV-2, angiotensin I converting enzyme 2 (ACE2) mediates entry into host cells,13, 14, 15 whereas dipeptidyl-peptidase 4 (DPP4) is the receptor for MERS-CoV.16 Of note, ACE2 is an X-linked gene and has sex-specific expression profiles17 that may contribute to the observed more severe clinical manifestations in males compared to females with COVID-19.18 Smokers and individuals with chronic obstructive pulmonary disease have higher ACE2 expression levels.19 Innate immune signaling such as interferon also seems to regulate ACE2 levels and thus susceptibility to SARS-CoV-2 infection.20 In the context of the GI tract, patients with enteric virus infections and other inflammatory conditions may have a different cytokine profile and thus distinct ACE2 levels in the gut. In addition, genetic polymorphisms in the ACE2 gene have been associated with diabetes and hypertension.21 , 22 Whether they are linked to clinical outcomes in COVID-19 patients remains to be tested and may shed light on the role of genetic predisposition to more severe diseases.
Figure 1.
A simplified diagram of the SARS-CoV-2 replication cycle (with potential pharmacological inhibitors under investigation depicted at respective steps). The virion and its associated viral proteins are shown schematically at the top. The structure of the S trimer is depicted. (1) Interaction between viral S protein and host ACE2 mediates virus binding to the host cell. (2) S protein is cleaved by host serine proteases, such as TMPRSS2, allowing the fusion of viral membrane with the host membrane and single-stranded RNA (ssRNA) (+) genome release into the cytoplasm. (3) Transcription and translation of viral proteins from genomic and subgenomic RNAs. (4) Replication occurs within the replicative membranous compartment, where and new ssRNA(+) are synthesized. (5) Virus assembly at the endoplasmic reticulum (ER), the intermediate compartments, and/or the Golgi complex. (6) Release of new virions by exocytosis. E, envelope protein; HE, hemagglutinin-esterase glycoprotein; M, membrane protein; N, nucleocapsid protein.
Interestingly, these viral receptors have been the targets of drug development for cardiac disease, hypertension, and diabetes, with ACE inhibitors blocking the renin-angiotensin-aldosterone system and gliptins inhibiting the DPP4 action to improve glucose control. While the enzymatic actions of these peptidases are dispensable for viral infection, these inhibitors can result in the up-regulation of the protein, offering an intriguing hypothesis that patients with hypertension and diabetes are often on these drugs and thus may be more susceptible to more severe COVID-19 disease.4
Multiple Cryo-EM structures of the recombinant S receptor binding domain and ACE2 complex have already been solved with an unprecedented speed.23, 24, 25, 26 These data indicate that the receptor-binding domain of S binds tightly to human and bat ACE2, suggestive of a zoonotic origin. A recent study demonstrated that the virus can indeed be transmitted to cats (including recent news of transmission to tigers in the Bronx Zoo) and ferrets, but not dogs, chickens, or pigs,24 although porcine ACE2 mediates viral entry in cell culture.14 This broad species tropism raises concerns of potential transmission from domestic pets to humans and vice versa. From a structural and immunogen design perspective, more information is needed regarding the native S protein trimeric state on virions, how the trimer interacts with ACE2, and how such interaction is disrupted by neutralizing antibodies. A recent publication on the crystal structure of an antibody in complex with the receptor-binding domain of the SARS-CoV-2 S protein provides important molecular insight into antibody recognition of the virus and a potential strategy for vaccine development.27 Besides ACE2, other potential entry factors, such as CD14728 and integrins,29 are currently under investigation.
After receptor engagement, SARS-CoV-2 gains access into the host cell. Like other human CoVs, this process is generally accomplished by acid-dependent proteolytic cleavage of S protein proteases such as cathepsins, which exposes the fusion domain of the S protein in the endosome,30 or by transmembrane serine protease 2 (TMPRSS2) at the plasma membrane.31 , 32 For MERS-CoV, furin-mediated cleavage and fusion also occurs during virus entry,33 and may be relevant for SARS-CoV-2.34 This step takes place before fusion of the viral and cellular membranes and is also a key determinant of tissue and species tropism of the virus.35 This acid-dependent process may explain the proposed efficacy of chloroquine or hydroxychloroquine, as a lysosomotropic agent, in the treatment of COVID-1936 (Figure 1). Recent publications suggest that, similar to SARS-CoV, trypsin and TMPRSS2 also prime SARS-CoV-2 S protein for efficient infection.15 , 37 Overexpression of TMPRSS2 in African green monkey Vero-E6 cells significantly enhanced SARS-CoV-2 infectivity.38 Serine protease inhibitor camostat blocked SARS-CoV-2 entry into host cells in a dose-dependent manner,15 making it and other similar inhibitors, such as nafamostat39 and Pharos compounds (CHEMBL1229259), candidate small-molecule inhibitors in the treatment of COVID-19 patients (Figure 1).
Besides the respiratory tract, including oral mucosa,40 the GI tract, in particular the small intestine, has high expression levels of ACE2 and TMPRSS2 in both humans41 and mice.42 Multiple data sets of single-cell RNA-sequencing analysis indicated that mature absorptive enterocytes from ileum and colon have high ACE2 messenger RNA expression levels.43, 44, 45 ACE2 protein levels have been validated by immunostaining in human small intestinal epithelium.46 On the luminal surface of intestinal epithelial cells, ACE2 associates with the neutral amino acid transporter B0AT1 and regulates intestinal microflora.47 , 48 SARS-CoV-2 infection of the GI tract, by altering the levels of ACE2 at the brush border, can cause microbial dysbiosis and inflammation. In addition, high ACE2 expression is also evident in cholangiocytes and, to a lesser extent, hepatocytes, and suggests possible hepatobiliary infection by SARS-CoV-2.49
Human and Animal Coronaviruses With Intestinal and Hepatic Involvement
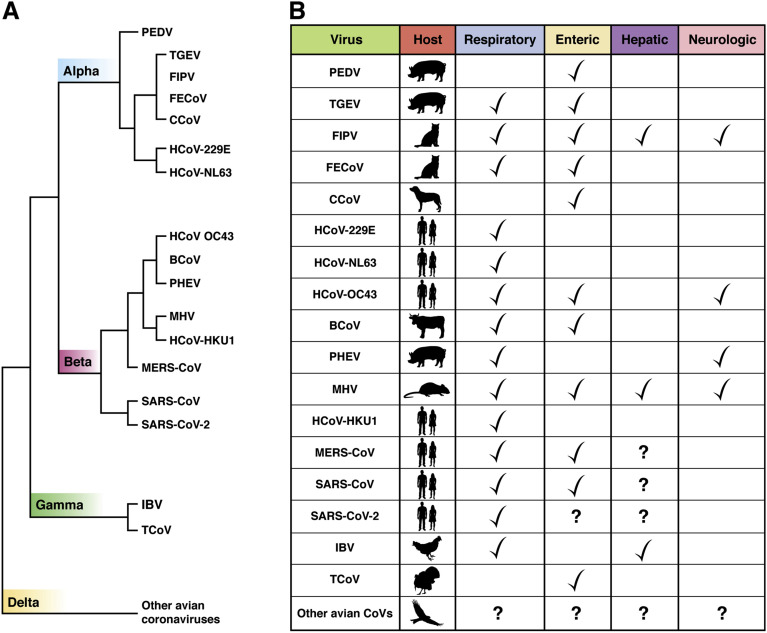
As major human pathogens of medical significance, CoVs cause a variety of diseases in animals.50 For instance, the prototypic mouse hepatitis virus infects the lung and spreads systemically to the GI tract, liver, and brain, causing gastroenteritis, hepatitis, and encephalitis. Transmissible gastroenteritis virus and porcine epidemic diarrhea virus cause severe gastroenteritis in young piglets, leading to significant morbidity and mortality. Avian infectious bronchitis virus is a major cause of economic loss within the poultry industry. Bat CoV infects the GI and respiratory tracts of the bats without apparent diseases. Feline enteric CoV causes a mild or asymptomatic infection in domestic cats. It is clear from the names of these animal CoVs that they are entero-pathogens and the primary symptoms point to an intestinal tropism. Figure 2 illustrates the species and tissue tropisms of the common human and animal CoVs and diseases they are known to cause.
Figure 2.
Coronaviruses and their associated diseases. (A) Common human and animal CoVs are shown in relative genetic distance by phylogenetic analysis (not drawn to scale). Coronaviruses can be divided into 4 genera: alpha, beta, gamma, and deltacoronaviruses (division shown on the branches of the phylogenetic tree). BCoV, bovine coronavirus; CCoV, canine coronavirus; FECoV, feline enteric coronavirus; FIPV, feline infectious peritonitis virus; IBV, infectious bronchititis virus; PEDV, porcine epidemic diarrhea virus; PHEV, porcine hemagglutinating encephalomyelititis virus; TCoV, turkey coronavirus; TGEV, transmissible gastroenteritis virus. HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2 are human CoVs. Hundreds of bat CoVs (not shown on the phylogenetic tree here) have been isolated and many of them are closely related to these human and animal CoVs, suggesting that bats are the original source of these viruses. SARS-CoV has been proposed to jump from bat to civet to human, SARS-CoV-2 from bat to pangolin to human, and MERS-CoV from bat to camel to human. The main hosts and involvement of organ systems of these CoVs100 are shown in (B).
The receptors of the human pathogens, HCoV-229E, SARS-CoV, and MERS-CoV, are aminopeptidase N (also known as CD13), ACE2, and DPP4 (also known as CD26), respectively, all brush-border enzymes highly expressed on the apical surface of mature enterocytes.51 GI involvements were frequently reported in both SARS-CoV and MERS-CoV infections. During the SARS outbreak, up to 76% of patients with SARS developed diarrhea, usually within the first week of illness.52 Intestinal biopsy demonstrated active SARS-CoV replication within both the small and large intestines and infectious virus was isolated from intestinal tissue but not fecal specimens.53 In 2012, during the MERS outbreak, one-quarter of patients with MERS-CoV reported GI symptoms, including diarrhea and abdominal pain, before the manifestation of respiratory symptoms54 and active shedding of viral RNA could be detected in the stool of these patients, although no infectious virus was recovered.55 MERS virus was shown to actively replicate in primary human intestinal enteroids and can be transmitted enterically to human DPP4 transgenic mice with replication in intestinal epithelium, enterocolitis, and subsequent spread to other organs.56
Frequent liver involvement has also been reported in SARS and MERS infections, mostly with mild to moderate elevations of aminotransferases (more than 2 times the upper limit of normal).57 Viral RNA and particles have been detected in the liver of SARS patients on autopsy.58 Overall, it is not clear whether liver injury is indeed the result of direct viral infection, inflammation-mediated damage, or drug-induced injury.
The majority of human enteric viruses, including rotaviruses, noroviruses, and astroviruses, are characterized by a nonenveloped, naked capsid,59 which presumably can tolerate digestive enzymes and the harsh environment of gastric fluids and bile in the duodenum. This naturally begs the question: how can enveloped CoVs survive the low pH of the stomach and withstand the detergent effect of bile salts in the small bowel? Known enteric CoVs, like transmissible gastroenteritis virus, can resist these harsh conditions by heavy glycosylation of S protein, evolving intrinsic resistance to low pH and digestive enzymes, and forming a tight complex with mucins.60 Both SARS-CoV and MERS-CoV are intrinsically capable of enduring harsh conditions: SARS-CoV is viable for up to 2 weeks after drying and up to 5 days in room temperature and low humidity.61 MERS-CoV appears to be similarly hardy.62 SARS-CoV RNA was detected in sewage of hospitals treating patients with SARS and the virus remained infectious up to 2 weeks in sewage water under experimental condition.63 At least 2 recent studies suggest that SARS-CoV-2 seems to have a remarkably similar stability in the environment.64 , 65
SARS-CoV-2: Another Enteric Coronavirus?
Similar to SARS-CoV and MERS-CoV, there is mounting evidence that SARS-CoV-2 infection also involves the GI tract. In early reports from the city of Wuhan, 2%–10% of patients with COVID-19 had GI symptoms, such as abdominal pain, diarrhea, nausea, or vomiting.66, 67, 68, 69, 70 A recent meta-analysis of >4000 East Asian patients with COVID-19 described up to 20% had GI symptoms and viral RNA was detected in the stool of almost 50% of patients.71 Notable GI symptoms had also been reported in several other studies and a meta-analysis, and can either precede or follow respiratory symptoms.72 , 73 In another cohort study that included more than 200 individuals, digestive symptoms were observed in 50.5% of patients, and those with GI symptoms took longer to be discharged from the hospital.74
In addition to clinical symptoms, SARS-CoV-2 RNA was detected in the endoscopic specimens of the esophagus, stomach, duodenum, and rectum from several patients.72 Substantial amounts of SARS-CoV-2 RNA have been consistently detected in stool specimens from COVID-19 patients.71 The first reported case of COVID-19 in the United States experienced diarrhea and tested positive for viral RNA in his feces but not serum.75 These results were subsequently confirmed by other studies. In some cases, by day 5 of admission, more anal swabs tested positive for viral RNA than oral swabs.76 Stool samples from patients tested positive somewhere between 36% and 53% of all confirmed cases.8 , 71 Prolonged presence of SARS-CoV-2 viral RNA was noted in fecal samples.77 , 78 The stool specimens of many patients remained positive after a negative nasopharyngeal swab test.79 Persistent fecal viral shedding was also observed in SARS-CoV-2–infected children.80 Although detection of high copy numbers of viral RNA in the stool does not equate to shedding of infectious viruses or transmission of the disease, these findings raise the possibility that SARS-CoV-2 may also be an enteric virus and can be transmitted via the fecal–oral route.
Vis-à-vis direct evidence of viral infection of gut tissues, SARS-CoV-2 antigen was positively stained in the intestinal epithelium of 1 COVID-19 patient78 and high viral loads were observed in the intestines of infected macaques.81 In 1 study, no infectious virus was successfully isolated from the feces of COVID-19 patients,82 but the cohort size is small and it is unclear how sensitive the cell assay system was. In a recently published ferret model, fecal shedding was seen in naïve animals in direct or indirect contact with the infected host.83 However, respiratory transmission was not specifically blocked, making it difficult to attribute the transmission to fecal–oral route.
Evidence for direct liver involvement by SARS-CoV-2 is less clear. Recent studies on COVID-19 patients from Asia showed the presence of liver injury, indicated by elevated aminotransferases, ranged from 15%–50% of the patients.66 , 67 , 70 But the elevations were mostly mild except for patients with more severe COVID-19,84 who might be experiencing drug-induced or sepsis/shock-related liver injury. Limited postmortem pathology of the liver of COVID-19 patients showed moderate microvesicular steatosis and mild lobular and portal activity, which are nonspecific changes.85 In a US-based study, serum aminotransferase levels were also not significantly altered in COVID-19 patients.86 Further studies are necessary to define whether SARS-CoV-2 is indeed a hepato- or cholangiotropic virus that can cause direct liver injury or be secreted into the bile.
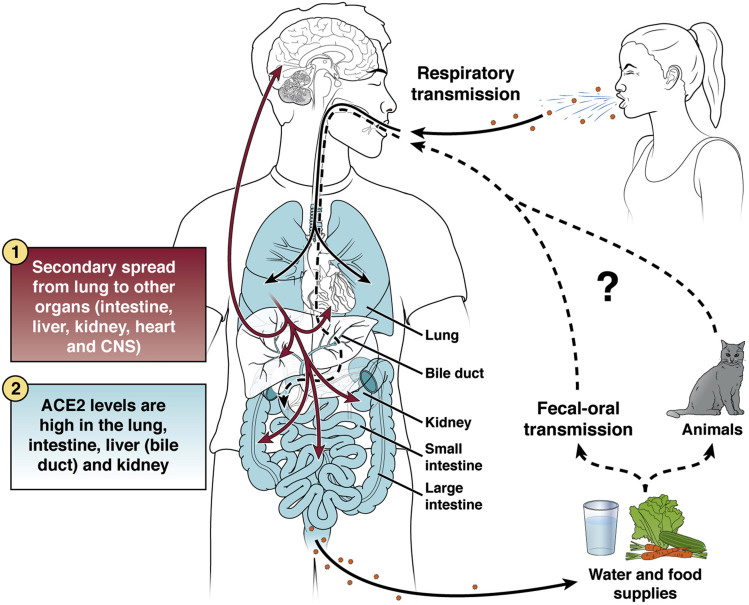
Many key questions remain before we can definitively address whether SARS-CoV-2 is an enteric virus that can be transmitted via fecal–oral route. Figure 3 illustrates the predominant respiratory route of transmission and potential fecal–oral spread of SARS-CoV-2 in human populations. Does SARS-CoV-2 actively infect human enteriods and intestinal epithelial cells in vivo? Are GI symptoms caused by viral replication in the enterocytes and its interaction with GI mucosal immunity? Are infectious viruses shed in the fecal samples and can they be recovered in a laboratory setting? If so, is the fecal viral load sufficiently high for human transmission? How long can the excreted virus persist in the environment, more specifically in the sewage system? Can fecally shedded virus infect animals that may serve as a reservoir for spread? During transmission, can gut be the first site of infection or does the virus spread to the gut from the respiratory or other tissues?
Figure 3.
Modes of transmission of SARS-CoV-2 in humans. The primary mode of human-to-human transmission is airborne for SARS-CoV-2. The virus likely first infects the respiratory epithelium and spreads to the rest of the body via circulation. Other potential organs of involvement include intestine, hepatobiliary system, heart, kidney, or central nervous system, many of which express high levels of ACE2, the main receptor for viral entry. Whether the virus can directly infect the intestine bypassing the respiratory system is unknown. Either way, the virus may infect, replicate, and shed from the enterocytes and possibly hepatocytes/cholangiocytes and be excreted as fecal materials into the environment, contaminating water and food supplies. Whether the virus can be transmitted directly to other humans via fecal–oral route or infect household pets, like cats, or wildlife first before passing to humans remain key questions.
Future Research Opportunities in COVID-19
From both clinical and public health standpoints, it is critical to fully understand SARS-CoV-2’s route of transmission. If high levels of infectious viruses are present in the intestinal lumen of infected patients, especially in asymptomatic patients, this poses risks to gastroenterologists,87 endoscopy personnel, and other patients during endoscopy and colonoscopy. For the general public, infectious viral particles in the feces shed by infected people, if aerosolized, have great implications in confined environments, such as cruise ships, hospitals, individual households, and densely populated housing, especially in regions with poor sanitation.
In basic research, much work is needed to examine the full extent of GI and liver aspects of COVID-19. Primary human intestinal epithelial cells, hepatocytes, or cholangiocytes, as well as donor-derived human intestinal enteroids88 and liver organoids, will be valuable models to study SARS-CoV-2 infectivity and replication. So far, there is 1 report of SARS-CoV-2 infection of cholangiocytes49 and the physiological relevance is unclear. If infection is observed in gut epithelial cells, it would be of great interest to understand the apical vs basolateral polarity of infection and secretion, whether and how the epithelium barrier integrity is disrupted, and whether M cells in the gut mediate systemic spread, like other enteric viruses.89 A genetically tractable animal model, for instance, several lines of transgenic mice that encode human ACE290, 91, 92, 93 will be important to dissect the relative contribution of different transmission routes. Murine models would also help investigate the disease in vivo, such as potential intestinal and/or hepatic injury, effects on various digestive functions and interactions with gut and systemic immunity. Other larger animal models, such as ferrets,83 , 94 cats,95 and macaques,81 , 83 , 95 all recently shown to be infectible by SARS-CoV-2 with similar disease, will also be valuable models to study the biology of infection and testing of vaccine and drug therapies. Finally, host innate immunity to SARS-CoV-2, including interferon signaling96 and the inflammasome-related cytokines,97 in particular those pathways at the mucosal surfaces,98 , 99 the short- and long-term adaptive immune responses against this virus, and how these antiviral activities vary in children, adults and the elderly, are largely unknown and fertile for future research.
Conclusions
As COVID-19 continues to have a devastating impact on countless people’s lives, we do not know with certainty what the future holds. Whether the virus will persist in human populations with recurrent bouts of outbreaks, like influenza or other emerging infections; attenuate after accumulating mutations to the likes of HCoV-OC43 and HCoV-229E common cold CoVs; or disappear after the primary outbreak, like SARS-CoV, remain vital questions for the coming year. Regardless of the answers, it is indisputable that future pandemic like this one will likely occur again. We are at the crossroads of science, medicine, and societal policies; our actions and commitments will profoundly shape the future of our world. For now, lessons learned from studying this virus, its infection modes, pathogenesis, and disease manifestations will not only be invaluable for developing effective vaccines and therapies against this emerging disease, but will also better prepare our world for future pandemics.
Acknowledgments
CRediT Authorship Contributions
Siyuan Ding, PhD (Writing – original draft: Equal; Writing – review & editing: Equal). T. Jake Liang, M.D. (Writing – original draft: Equal; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding S.D. is supported by National Institutes of Health (NIH) grants K99/R00 AI135031 and R01 AI150796, and T.J.L. by the Intramural Research Program of NIDDK, NIH.
Author names in bold designate shared co-first authorship.
References
- 1.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S.R. Forty years with coronaviruses. J Exp Med. 2020:217. doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plowright R.K., Parrish C.R., McCallum H. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson C.K., Hitchens P.L., Pandit P.S. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc Biol Sci. 2020;287:20192736. doi: 10.1098/rspb.2019.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 May;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020;17:259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y., Rong L., Nian W. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 10.Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmas B., Gelfi J., L'Haridon R. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeager C.L., Ashmun R.A., Williams R.K. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj V.S., Mou H., Smits S.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tukiainen T., Villani A.C., Yen A. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung J.M., Yang C.X., Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues [published online ahead of print April 27, 2020]. Cell 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed]
- 21.Lu N., Yang Y., Wang Y. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol Biol Rep. 2012;39:6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y.H., Li J.Y., Wang C. The ACE2 G8790A polymorphism: involvement in type 2 diabetes mellitus combined with cerebral stroke. J Clin Lab Anal. 2017;31 doi: 10.1002/jcla.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 24.Shang J., Ye G., Shi K. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine [published online ahead of print March 19, 2020]. Cell Mol Immunol 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed]
- 26.Yan R., Zhang Y., Li Y. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan M., Wu N.C., Zhu X. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Chen W, Zhou Y-S, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv Preprint 10.1101/2020.03.14.988345. [DOI]
- 29.Sigrist C.J., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.E., Li K., Barlan A. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc Natl Acad Sci U S A. 2016;113:12262–12267. doi: 10.1073/pnas.1608147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glowacka I., Bertram S., Muller M.A. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. 2013;87:12552–12561. doi: 10.1128/JVI.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bestle DH, Heindl MR, Limburg MR, et al. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-1 CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv Preprint 10.1101/2020.04.15.042085. [DOI]
- 35.Menachery V.D., Dinnon K.H., 3rd, Yount B.L., Jr. Trypsin treatment unlocks barrier for zoonotic bat coronavirus infection. J Virol. 2020;94(5) doi: 10.1128/JVI.01774-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19 [published online ahead of print March 10, 2020]. J Crit Care 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed]
- 37.Ou X., Liu Y., Lei X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuyama S., Nao N., Shirato K. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlen M., Fagerberg L., Hallstrom B.M. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 42.Wu C., Orozco C., Boyer J. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin J.C., Chang C., Boschetti G. Single-cell analysis of Crohn's disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508 e20. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smillie C.S., Biton M., Ordovas-Montanes J. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730 e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Song W., Wang J. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217(2) doi: 10.1084/jem.20191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamming I., Timens W., Bulthuis M.L. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camargo S.M., Singer D., Makrides V. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto T., Perlot T., Rehman A. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai XH, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv Preprint 10.1101/2020.02.03.931766. [DOI]
- 50.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai J.P. Distribution of brush-border membrane peptidases along the intestine of rabbits and rats: implication for site-specific delivery of peptide drugs. J Drug Target. 1993;1:231–236. doi: 10.3109/10611869308996080. [DOI] [PubMed] [Google Scholar]
- 52.Kwan A.C., Chau T.N., Tong W.L. Severe acute respiratory syndrome-related diarrhea. J Gastroenterol Hepatol. 2005;20:606–610. doi: 10.1111/j.1440-1746.2005.03775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung W.K., To K.F., Chan P.K. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corman V.M., Albarrak A.M., Omrani A.S. Viral shedding and antibody response in 37 patients with Middle East Respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J., Li C., Zhao G. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3 doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L., Liu J., Lu M. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q.L., Ding Y.Q., Hou J.L. [Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization] Di Yi Jun Yi Da Xue Xue Bao. 2003;23:1125–1127. [PubMed] [Google Scholar]
- 59.Bushman F.D., McCormick K., Sherrill-Mix S. Virus structures constrain transmission modes. Nat Microbiol. 2019;4:1778–1780. doi: 10.1038/s41564-019-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmes K.V. Enteric infections with coronaviruses and toroviruses. Novartis Found Symp. 2001;238:258–269. doi: 10.1002/0470846534.ch16. discussion 269–275. [DOI] [PubMed] [Google Scholar]
- 61.Chan K.H., Peiris J.S., Lam S.Y. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eur Surveill. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 63.Wang X.W., Li J.S., Guo T.K. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J Virol Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with sARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin A.W.H., Chu J.T.S., Perera M.R.A. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print February 7, 2020]. JAMA 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 71.Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis [published online ahead of print April 3, 2020]. Gastroenterology 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed]
- 72.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 73.D'Amico F, Baumgart DC, Danese S, et al. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management [published online ahead of print April 8, 2020]. Clin Gastroenterol Hepatol 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed]
- 74.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen C, Gao G, Xu Y, et al. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19 [published online ahead of print March 30, 2020]. Ann Intern Med 10.7326/M20-0991. [DOI] [PMC free article] [PubMed]
- 80.Xu Y., Li X., Zhu B., Liang H. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munster VJ, Feldmann F, Williamson BN, et al. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2 [published online ahead of print May 12, 2020]. Nature 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed]
- 82.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019 [published online ahead of print April 1, 2020]. Nature 10.1038/s41586-020-2196-x. [DOI] [PubMed]
- 83.Kim Y.I., Kim S.G., Kim S.M. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California [published online ahead of print April 10, 2020]. Gastroenterology 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed]
- 87.Iacucci M., Cannatelli R., Labarile N. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol Hepatol. 2020;5:598–606. doi: 10.1016/S2468-1253(20)30119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng N., Hu L., Ding S. Human VP8∗ mAbs neutralize rotavirus selectively in human intestinal epithelial cells. J Clin Invest. 2019;130:3839–3851. doi: 10.1172/JCI128382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding S, Song Y, Brulois KF, et al. Retinoic acid and lymphotoxin signaling promote differentiation of human intestinal M cells [published online ahead of print April 1, 2020]. Gastroenterology 10.1053/j.gastro.2020.03.053. [DOI] [PMC free article] [PubMed]
- 90.Menachery V.D., Yount B.L., Jr., Sims A.C. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A. 2016;113:3048–3053. doi: 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Netland J., Meyerholz D.K., Moore S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7675. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tseng C.T., Huang C., Newman P. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol. 2007;81:1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bao L, Deng W, Huang B. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice [published online ahead of print May 7, 2020]. Nature 10.1038/s41586-020-2312-y. [DOI] [PubMed]
- 94.Richard M, Kok A, de Meulder D, et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. bioRxiv Preprint 10.1101/2020.04.16.044503. [DOI] [PMC free article] [PubMed]
- 95.Shi J., Wen Z., Zhong G. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020 doi: 10.1126/science.abb7015. [published online ahead of print April 8, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kokugamage KGH, A. Schindewolf, C. Rajsbaum, R. Menachery, V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. bioRxiv Preprint 10.1101/2020.03.07.982264. [DOI]
- 97.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding S., Diep J., Feng N. STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection. Nat Commun. 2018;9:1485. doi: 10.1038/s41467-018-03782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu S., Ding S., Wang P. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masters P.S., Perlman S. Coronaviridae. In: Knipe D., Howley P., editors. Fields Virology. 6th ed. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 825–858. [Google Scholar]