Abstract
Strategies to improve the onset of protective immunity induced by vaccination against respiratory pathogens may have a significant impact on health of newly received beef calves. The objective was to determine if the use of injectable trace minerals (ITM; Se, Zn, Cu, and Mn) concurrent with a modified-live virus (MLV) vaccine enhances the immune response and onset of protection in beef calves challenged with BVDV2 five days after vaccination. Forty-five calves were randomly assigned to one of three groups (15/group): VAC + ITM, received MLV-vaccine and ITM (Multimin®90) subcutaneously (SC); VAC + SAL, received the same vaccine and saline SC; or UNVAC, unvaccinated. Five days after vaccination (d.0), calves were challenged with BVDV2 strain 890. Health status was evaluated and blood samples were collected for leukocyte counts, BVDV1 and 2 serum neutralizing antibodies (SNA), BVDV-PCR, and percentage of CD4+, CD8+, WC1+ and CD25+ T-cells. VAC + ITM had lower health scores than UNVAC (d.8 and 9). VAC + ITM had higher BVDV1 & 2 SNA titers than VAC + SAL and UNVAC on d.21 and 28. Lymphocyte counts decreased in UNVAC but not in VAC + ITM or VAC + SAL (d.3 to 11). CD4+ T-cells significantly decreased in UNVAC and VAC + SAL (d.3). VAC + ITM had higher percentage of CD4+ T-cells than UNVAC (d.3 and 7). VAC + ITM had lower percentage of activated CD4+ and CD8+ T-cells than UNVAC (d.7). In summary, vaccination induced a rapid protection against BVDV2 infection. Administration of ITM was associated with increased SNA response to BVDV1 & 2, enhanced health status, mitigation of CD4+ T-cells decrease, and reduction of T-cell activation in calves challenged with BVDV2 five days after immunization. These results support the strategic use of ITM concurrent with vaccination, especially when a rapid protection is needed in newly received beef calves.
Keywords: Trace minerals, Beef calves, BVDV, Modified-live virus vaccine, Protection
1. Introduction
Bovine respiratory disease (BRD) is the most economically important illness of beef cattle in North America (Taylor et al., 2010; Griffin, 1997; USDA APHIS, 2010). The infectious agents most commonly identified in BRD include Bovine viral diarrhea virus (BVDV), Bovine respiratory syncytial virus (BRSV), Bovine herpes virus 1 (BHV1), Parainfluenza 3 virus (PI3V), Bovine coronavirus (BCoV), Pasteurella multocida, Mannheimia haemolytica, Mycoplasma bovis, and Histophilus somni. Acute BVDV infections cause immunosuppression, which potentiates infections by other agents involved in BRD (Rosenquist et al., 1970; Fulton et al., 2000). Prevention and control of BVDV is based on biosecurity to avoid the introduction and spread of the virus in the herd, diagnosis and culling of persistently infected animals, allied with vaccination (Brock, 2004; Walz et al., 2010).
Vaccination with attenuated or inactivated vaccines is an efficient tool to prevent and lessen the impact of BVDV infections (Dean and Leyh, 1999; Newcomer et al., 2017). Despite the commercial availability of several highly efficacious vaccines in the USA, a significant number of producers do not vaccinate their calves against BVDV or other BRD pathogens. The efficacy of vaccination to prevent BVDV infection can be assessed by the vaccine’s capacity to induce robust humoral and cell-mediated immune responses able to control virus replication, viremia and shedding (Rhodes et al., 1999; Reber et al., 2006). Another crucial aspect when assessing the efficacy of BRD vaccines is the rapidity in inducing an adequate immune response to protect cattle against acute infections and disease (Palomares et al., 2012). This trait is especially important in stockyards and feedlots, where continuous addition of highly stressed cattle with unknown vaccination history and infectious status rises the risk of infections and clinical disease resulting in high morbidity and mortality rates and significant economic losses.
Previous studies characterized the onset of protection induced by modified-live virus (MLV) vaccines administered 7, 5, or 3 days before BVDV challenge with high or low virulence BVDV strains (Brock et al., 2007; Palomares et al., 2012). In both trials, calves vaccinated 5 and 7 days before BVDV challenge were protected from clinical disease, viremia, leukopenia, and virus shedding compared with unvaccinated calves (Brock et al., 2007; Palomares et al., 2012). Those trials were performed under controlled experimental conditions with low stress level and infectious pressure ensuring appropriate animal comfort and welfare. Stressors imposed on calves, such as abrupt weaning, lack of pre-conditioning, weather extremes, irregular vaccination, long transportation, comingling and frequent location and diet changes can compromise the immune function and the response to vaccination due to oxidative stress, affecting animal health and performance. Moreover, the current global pressure for reducing antibiotic use in food animal production creates the need for vaccination protocols that induce enhanced protection and limit disease transmission more efficiently.
Numerous studies have proven the effects of trace minerals such as selenium (Se), zinc (Zn), copper (Cu), and manganese (Mn) on immunity (Chirase et al., 1994; Percival, 1998; Underwood and Suttle, 1999), health and growth performance of cattle (Spears and Kegley, 2002). The impact of these minerals on the immune function has been associated with their role in the structure and function of antioxidant enzymes (e.g. superoxide dismutase, glutathione peroxidase), nucleic acid replication and transcription, mitochondrial metabolic cascades, among others (Tomlinson et al., 2008; Haase and Rink, 2014); resulting in improvement of neutrophil migration and phagocytic function, lymphocyte proliferation and antibody production (Pinna et al., 2002; Tomlinson et al., 2008; Arthington and Havenga, 2012; Haase and Rink, 2014; Bonaventura et al., 2015). The use of trace mineral supplementation may become particularly important in highly stressed cattle, such as newly received feeder calves (Duff and Galyean, 2007). Administration of injectable trace minerals (ITM) has shown beneficial effects on antibody production and cell mediated immune response to BRD vaccines in beef (Arthington and Havenga, 2012; Roberts et al., 2016) and dairy cattle (Palomares et al., 2016; Bittar et al., 2018a). A recent study developed by our group demonstrated that the use of ITM concomitant with an MLV-BRD vaccine in dairy calves resulted in earlier and greater serum neutralizing antibody titers to BVDV1 and Mannheimia haemolytica, and more rapid and stronger mononuclear cell proliferation upon stimulation with BVDV1, BRSV and Pasteurella multocida than the control calves (Palomares et al., 2016; Bittar et al., 2018a). After this study, there was a question as to whether the use of ITM could also favor the induction of a more rapid and improved immune response and protection shortly after vaccination in situations where a fast protection is required, such as that of newly received calves vaccinated at arrival and shortly exposed to BRD pathogens. Therefore, in the present study we hypothesized that administration of ITM at the time of MLV-BRD vaccination would benefit the immune response and protection in newly received BVDV-naïve beef calves experimentally challenged with BVDV five days after vaccination. The objective of this study was to determine if the use of ITM supplementation (containing Se, Zn, Cu, and Mn) concurrent with an MLV vaccine is able to enhance the immune response and onset of protection against an experimental BVDV2 infection in newly received BVDV-naïve beef calves challenged 5 days after vaccination.
2. Methods
2.1. Calves husbandry, vaccination, and treatments
The study was done at the University of Georgia (UGA) Oconee Farm (Watkinsville-GA) from May through June 2016. The research protocol was approved by the University of Georgia, Institutional Animal Care and Use Committee (UGA-AUP# A2014 02-005-Y3 A8). This study was performed using 45 weaned Angus and Angus-crossbred calves (7 months old) purchased from a commercial ranch in Calhoun, GA. The calves were BVDV-naïve confirmed via standard virus neutralization test for serum neutralizing antibody (SNA) titers against BVDV1 and 2, and ear notch biopsy for immunohistochemistry (BVDV antigen) done at the University of Georgia, Athens Veterinary Diagnostic Laboratory (Athens, GA). The calves and their dams were not vaccinated with BRD vaccines on the farm of origin before the beginning of the study in order to maintain the calves’ BVDV-naïve status. In addition, the calves and their dams were kept in an isolation pasture away from the main herd from birth to weaning to avoid contact with MLV from vaccinated cattle. At the farm of origin, the calves grazed rye grass (Lolium hybridum) with free access to bermuda grass hay (Cynodon dactylon), and water ad libitum.
On day -7, calves were transported for approximately 8 h from the farm of origin in Calhoun, GA to the Oconee Farm of the College of Veterinary Medicine at University of Georgia (UGA) at Watkinsville, GA. On the experimental farm, the calves grazed fescue grass (Festuca arundinacea) with free access to bermuda grass hay (Cynodon dactylon), and water ad libitum. In addition, calves were supplemented (2.5 Kgs per calf/day) with a commercial ration that contained trace minerals (Cattleman’s Special Beef; Godfreys Warehouse; Madison-GA) offered in two meals. Additional oral mineral supplementation was not provided during this study.
On day -5, calves were randomly assigned to one of the three groups: VAC + ITM (n = 15): calves received 2 mL of a 5-way MLV vaccine containing BHV1, BVDV1 and 2, BRSV, PI3V (Express 5®, Boehringer Ingelheim, Vetmedica, St. Joseph, MO) subcutaneously (SC) and a dose of injectable trace minerals (ITM, 1 mL/45 Kg of body weight SC; Multimin® 90, Multimin USA Inc, Fort Collins, CO); VAC + SAL (n = 15): calves received a dose of the same MLV vaccine as the previous group and an injection of sterile saline (1 mL/45 Kg SC; Vetone Sterile Saline®; Nova-Tech Inc., Grand Island, NE); or UNVAC (n = 15): calves were not vaccinated or received neither ITM nor saline. Administration of ITM provided 15, 60, 10 and 5 mg/mL of Cu, Zn, Mn, and Se, respectively. All injections were performed accordingly with the guidelines of the Beef Quality Assurance Program (Beef Quality Assurance 2010®; Centennial, CO). Calves in UNVAC were isolated in a pasture separated from the vaccinated calves until the day of challenge, in order to prevent infection with vaccine virus shed by calves in VAC + ITM and VAC + SAL.
2.2. Bovine viral diarrhea virus intranasal challenge
Five days after vaccination and treatment administration, all calves were intranasally challenged with a noncytopathic (ncp) BVDV2 isolate (strain 890). The BVDV2 strain 890 was originally acquired from the APHIS Center for Veterinary Biologics in 1989 (Ames, IA), where it was subjected to propagation in Madin-Darby Bovine Kidney (MDBK) cells to keep a viable stock. The stock BVDV2 strain 890 utilized in this trial was biologically cloned using successive passages as previously described (Bittar et al., 2018b). This strain has been previously proven to successfully induce lymphopenia and neutrophilia with mild to moderate clinical disease in non-vaccinated calves (Walz et al., 2001; Bittar et al., 2018b).
The inoculum comprised an infected cell culture supernatant containing 1 × 105 50 % tissue culture infectious dose (TCID50) per mL of noncytopathic BVDV2 strain 890. The BVDV isolate was grown in monolayers of MDBK cells using Dulbecco’s Modified eagle’s medium (DMEM®; Cellgro, Manassas, VA) supplemented with 10 % equine serum (HyClone™ Donor Equine Serum U.S.; Fischer Scientific; Pittsburgh, PA), 1% l-alanyl-l-glutamine (Corning® glutagro 100X Liquid™, 200 mM; Corning Cellgro; Manassas, VA), anti-fungal (Amphotericin B Liquid ®; Corning; Manassas, VA) and antibiotic (Penicillin-Streptomycin Solution 100X®; Corning; Manassas, VA) as previously described (Bittar et al., 2018b). The inocula remained frozen at −80 °C until the day of challenge. One hour before challenge, the frozen inoculum was thawed. Then, 5 mL of the BVDV2 inoculum were aliquoted into individual 12 mL sterile syringes, transported to the farm held in ice until use. The challenge was done by intranasal aerosolization of 5 mL of inoculum (2.5 mL in each nostril) with a 10 cm long tip-fenestrated cannula coupled to a 12 mL syringe for each individual animal, as previously described (Bittar et al., 2018b). After challenge all calves comingled in a 10-acre pasture with adequate shade, during the rest of the experimental period. A sample (5 mL) of the inoculum was transported on ice to the lab to determine the virus concentration (TCID50/mL) after challenges were finished.
2.3. Health status evaluation
Calves’ health status comprising rectal temperature, hydration, attitude, eyes and nasal discharge and fecal consistency, was assessed using the University of Wisconsin’s scoring system (http://www.vetmed.wisc.edu/dms/fapm/fapmtools/8calf/calf_health_scoring_chart.pdf). Clinical examination was performed on each calf on study days -6, -5, 0, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 18 and 21 relative to the day of BVDV challenge. However, rectal temperatures were measured only on study days -6, -5, 0, 3, 5, 6, 7, 8, 9, 11, 14 and 18. Clinical signs were assessed for each variable (hydration status, nasal secretion, head-ears [attitude], feces, eyes, and cough) on a scale from 0 to 3, with 0 representing a lack of clinical signs and 3 representing severe clinical signs. The daily sum health score was the total sum of each individual clinical sign, and was calculated by adding all individual scores of the six clinical variables, as previously described (Bittar et al., 2018b). Three experienced veterinarians, who were not aware of calves’ treatment allocation, assessed clinical signs and health scores.
2.4. Sample collection and processing
Blood samples were collected from the jugular vein using an 18 gauge x 2.5-cm single sample needle (Vacuette®; Nipro Medical Industries Ltd., Gunma, Japan) into vacuum tubes (Vacutainer®, BD Diagnosis, Franklin Lakes, NJ) with and without anticoagulant to obtain whole blood and serum, respectively. Blood samples were collected on study days -14, -6, -5, 0, 3, 5, 6, 7, 8, 9, 11, 14, 18, 21 and 28. Blood collection was done into four individual 8.5 mL glass tubes for buffy coat separation (BD Vacutainer ACD Solution A REF364606®; BD Diagnosis, Franklin Lakes, NJ), and into one 2 mL polypropylene tube for leukocyte and platelet count (BD Vacutainer K2 EDTA 3.6 mg REF367841®; BD Diagnosis, Franklin Lakes, NJ). In addition, blood samples were collected into two individual 10 mL glass tubes without anticoagulant (BD Vacutainer Serum®; BD Diagnosis, Franklin Lakes, NJ) for serum mineral concentration, and serum neutralizing antibody (SNA) titers to BVDV1 and 2.
The blood samples containing ACD Solution A were processed for buffy coat isolation as previously described (Harpin et al., 1999) and freshly used for staining for flow cytometry analysis or stored at −80 °C for further real time quantitative reverse transcription polymerase chain reaction (qRT-PCR) for BVDV1 and 2 nucleic acid detection. Clotted blood samples were centrifuged (900x g for 15 min) for serum separation, and serum samples were stored at −80 °C for determination of SNA titers against BVDV1 and 2.
Nasal swab samples were collected from each calf for BVDV nucleic acid detection using qRT-PCR on study days 0, 5 and 7. An individual cotton swab was inserted in each nostril scraping the nasal mucosa, and placed in a tube containing 3 mL of PBS with penicillin-streptomycin and amphotericin, and then transported to the laboratory for processing as previously described (Bittar et al., 2018b). Samples were mixed using a vortex; then swabs were removed and the solution was filtered and stored at −80 °C for subsequent qRT-PCR.
Trace mineral status in liver biopsy samples (15 mg of liver tissue) was assessed from each calf on days -14, and 21 relative to the day of vaccination. Liver biopsies were collected using sterile surgical tru-cut semi-automatic biopsy needles (14 g and four inches long) with the assistance of ultrasonography (Ibex®Lite, E.I. Imaging, Loveland, CO) for liver visualization and local anesthesia (Lidocaine injectable; Aspen Veterinary Resources, Ltd. Liberty, MO). Mineral content in liver samples was determined at the Diagnostic Center for Population and Animal Health at Michigan State University, Lansing, MI.
2.5. Serum neutralizing antibody (SNA) titers
Blood samples for serum neutralizing antibodies (SNA) titers against BVDV1 and 2 were collected on days -14, -5, 0, 7, 14, 21 and 28. Serum neutralizing antibody titers against BVDV1 and 2 were determined at the University of Georgia Athens Veterinary Diagnostic Laboratory (Athens, GA) via a standard virus neutralization protocol as previously described (Bittar et al., 2018b). In summary, after heat inactivation of serum samples (56 °C for 30 min), serum samples were serially diluted with DMEM into a 2-fold dilution series, beginning at 1:2 in 96-well cell culture plates. An equal volume of the appropriate cytopathic BVDV1 and 2 was added to each well (25 μL of DMEM with approximately 100 TCID50 of the respective viruses strain) making the starting serum dilution of 1:4. After incubation (5% CO2 at 37 °C for 1 h) MDBK cell suspension was added to each well (150 μL suspension with approximately 1.8 × 104 cells in DMEM containing 10 % fetal calf serum [FCS]).
The cell monolayer was examined with an inverted microscope for signs of virus-specific cytopathic effects after plates were incubated for 4 days (5% CO2 at 37 °C). The SNA titer for each sample was reported as the reciprocal of the highest dilution of serum that completely inhibited virus-induced cytopathic effects.
2.6. White blood cell and platelet counts
Blood samples for total white blood cells, lymphocyte, granulocyte, monocyte and platelet counts were collected on days -14, -6, -5, 0, 3, 5, 6, 7, 8, 9, 11, 14 and 18. Tubes with uncoagulated blood samples containing EDTA were kept cooled in ice, and posteriorly transported to the Department of Veterinary Pathology of the University of Georgia (UGA) in Athens, GA for complete blood count (CBC). Blood samples were then kept in room temperature on an orbital shaker to be stirred for 10 min before analysis. Total leukocyte, platelet and differential leukocyte counts for each sample were determined by use of an automatic cell counter (HESKA® CBC-Diff, Vet Hematology System, Des Moines, IA).
2.7. Expression of CD4, CD8, CD25 and WC1 T-cells in peripheral leukocytes by flow cytometry
Blood samples for T-cell phenotyping (CD4, CD8, and WC1) including CD25 expression were collected on days -6, 0, 3, 7, 14 and 18. For each blood sample a buffy coat was prepared. The leukocytes were assessed for cell viability (exclusion in 0.04 % trypan blue) and cell number using a hemocytometer chamber. The cells were suspended to a concentration of approximately 6 × 106 cells per mL. The samples all had 85 % viability or greater. Each sample was diluted in 3 mL PBS-1X in individual pre-labeled tubes (12 × 75 mm polystyrene. Falcon, BD Biosciences, San José, CA). The cells were washed twice by centrifugation (300 × g for 5 min). The supernatant was discarded after each wash. The cells were suspended in 1.5 mL of FACS buffer (PBS with 0.5 % bovine serum albumin, and 0.1 % sodium azide) and mixed uniformly by vortexing. For staining, 100 μL of cells suspension (approximately 3 × 106 cells) were added per well to a 96-well round bottom microtiter plate. Plates were centrifuged (300 x g for 5 min) and snapped sharply to remove the supernatant. Each plate was vortexed with lid on to suspend the cells in the residual volume. At this point, 20 μL of each antibody tested (at the minimum saturating concentration) was added to the desired wells, and wells that were dual stained (combination of CD25 with either CD4, CD8 or WC1). The primary antibodies were mouse anti-bovine CD4 isotype IgG2a antibody at a 1:5 dilution (clone CC8, MCA1653 F, BioRad, Hercules, CA), mouse anti-bovine CD8 isotype IgG2a antibody at a 1:5 dilution (clone CC63, MCA837 F, BioRad, Hercules, CA), mouse anti-bovine WC1 (gamma-delta T cell) isotype IgG2a antibody at a 1:5 dilution (clone CC15, MCA838 F, BioRad, Hercules, CA), and mouse anti-bovine CD25 isotype IgG1 antibody at a 1:7 dilution (clone IL-A111, MCA2430PE). FACS buffer was added to wells to function as an unstained control. A combination of CD25 and each of the other T-cell (CD4, CD8 and WC1) antibodies were used to stain the cells. Plates were incubated (4 °C for 45 min). Following staining, the plates were washed three times with FACS buffer (300 × g for 5 min). At the end of each wash, the plates were snapped sharply to remove the supernatant. Each plate was vortexed with lid on to suspend the cells in the residual volume. After the last wash, the supernatant was discarded and the cells were suspended in FACS fix (4 % formalin in FACS buffer). Each well received 100 μL of FACS fix. The plates were incubated overnight (18−20 hours) at 4 °C. Well contents were transferred into a pre-labeled 1.5 mL microcentrifuge tubes (MCT) containing 300 μL of FACS buffer. After dilution of FACS buffer, samples were stored for flow cytometry analysis at 4 °C. All samples were assessed within one week of fixation. Flow cytometry was done using a BD Accuri C6 first generation cytometer and C-flow plus software (BD Biosciences, Franklin Lakes, NJ). Samples were gated using FALS and 90LS two parameter histograms to identify the lymphocyte, monocyte and granulocyte cells in the sample based on previous validation with highly enriched populations of each. Compensation to minimize cross talk between FL1 and FL2 signal was established using single color samples at minimum saturating concentration to eliminate green in orange and orange in green color bleed. The positive and negative windows for FL1 and FL2 single color analysis, and FL1-FL2 dual parameter histograms were established in preliminary trials using staphylococcal enterotoxin B stimulated buffy coat cells under the same staining conditions and set in a template for flow collection. Approximately 10,000 events were recorded in the lymphocyte gate for each sample. Following data collection, the lymphocyte gate was confirmed using the list mode data for each sample to assure that the same population was measured for each sample across the total experiment. The C-flow plus software was used to generate values for percent positive cells for each marker, and for CD25 positive cells within each marker. In addition, values for the mean fluorescent intensity were generated from the analysis gates for each marker and for the CD25 population within each marker for all samples in the trial.
2.8. BVDV nucleic acid detection by qRT-PCR on peripheral leukocytes and nasal swab samples
Buffy coat and nasal swab samples that had been stored at −80 °C were thawed at room temperature for BVDV nucleic acid detection. Total RNA was extracted from buffy coats and nasal swab solutions using RNeasy Mini Kit (QIAGEN, Germantown, MD). Total RNA samples were submitted to the Athens Veterinary Diagnostic Laboratory where a BVDV1 and 2 multiplex qRT-PCR was performed as previously described (Letellier and Kerkhofs, 2003). Total RNA samples were reverse transcribed in the presence of hexanucleotides. One microliter of the resulting cDNAs were used for real-time PCR analysis (Letellier and Kerkhofs, 2003). Based on conserved regions of the 5’ UTR of BVDV1 and 2, the primer pair F2: 5’ CTCGAGATGCCATGTGGAC 3’ (position 224–242 of the NADL sequence) and PESTR: 5’ CTCCATGTGCCATGTACAGCA 3’ (position 391–371 of the NADL sequence) were used. Two probes differing by three nucleotides were utilized: the 5’ FAM CAGCCTGATAGGGTGCTGCAGAGGC TAMRA 3’ probe was specific of BVDV1 whereas the 5’ VIC CACAGCCTGATAGGGTGTAGCAGAGACCTG TAMRA 3’ was used for the detection of BVDV2. cDNA samples were amplified by qRT -PCR using the following conditions: 10’ 95 °C and 45 cycles 15” 95 °C and 1’ 60 °C. Samples with a qRT-PCR result equal or less than the threshold cycle of 35, were considered positive for the presence of BVDV nucleic acids. Only results of BVDV2 detection by qRT-PCR are reported in the present manuscript.
2.9. Statistical analysis
Data were analyzed using the Statistical Analysis System (SAS® version 9.3; SAS Institute, Cary, NC, USA). Statistical assumptions of normality and constant variance were assessed through Shapiro Wilk’s and Levene’s tests, respectively. A logarithmic base 2 transformation was applied to the antibody titers for each group on days -14, -5, 0, 7, 14, 21, and 28 after BVDV infection. Means daily sum health score, liver trace mineral concentration, rectal temperature, leukocyte and platelet counts, SNA titers, and percentage of T-cells phenotypes were compared among treatment groups by using repeated-measures analysis Proc-GLIMMIX model using calf as random effect, and group and time as fixed effects. Tukey test was used to adjust for multiple comparisons. The variables with significantly different group means on day -5 were used as covariate in the statistical model to minimize the differences among groups at the beginning of the study. Results of qRT-PCR for BVDV2 detection on buffy coat samples and nasal swab specimens were analyzed by use of a frequency procedure and compared by use of a χ2 test. For all analyses, values of P ≤ 0.05 were considered significant, and 0.05 < P ≤ 0.10 was considered a tendency. All results are shown as least squared means (LSM) obtained from the statistical software SAS after analysis.
3. Results
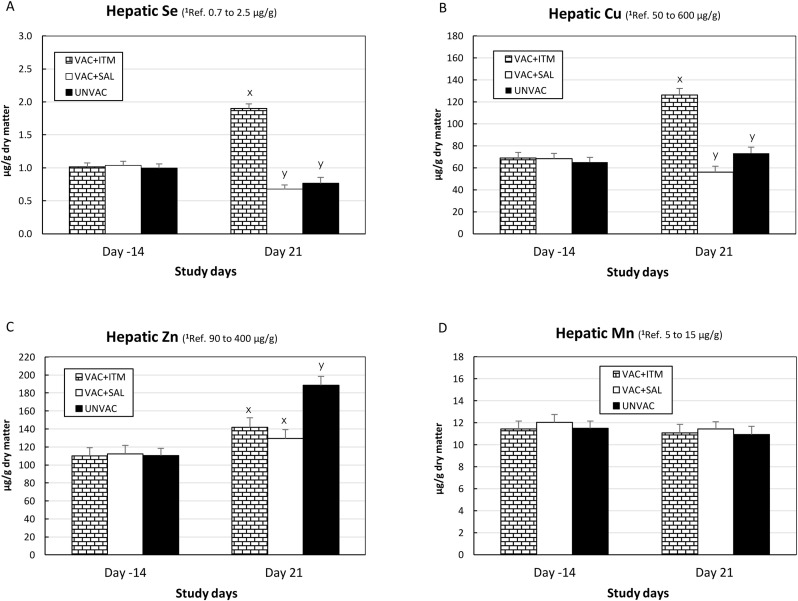
Calves used in the study did not show any inflammatory reaction at the injection site after treatment with ITM. The hepatic trace mineral concentrations (Se, Cu, Zn and Mn) before starting the study (day -14) were comparable in all groups (P > 0.05; Fig. 1 A–D) and within normal reference ranges (Herdt and Hoff, 2011). Injectable trace mineral administration was associated with increased hepatic Se (P < 0.001; Fig. 1A) and Cu (Fig. 1B; P < 0.01) concentrations in the VAC + ITM group compared with VAC + SAL and UNVAC groups. Hepatic Zn concentrations increased in all three groups on day 21 compared with day -14, and it was higher in the UNVAC group compared with both vaccinated groups (P = 0.04; Fig. 1C). There were no differences in hepatic Mn concentrations on day 21 among groups or relative to baseline levels on day -14 (Fig. 1D).
Fig. 1.
(A to D). Liver concentrations of Se (A), Cu (B), Zn (C) and Mn (D) for beef calves treated with injectable trace minerals (VAC + ITM) or not (VAC + SAL), concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) on study day -5 and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the standard error of the mean (SEM). 1 Hepatic trace mineral concentrations references values based on Herdt and Hoff, 2011. x, y Significant difference between groups (P < 0.01 for Se and Cu; and P < 0.05 for Zn).
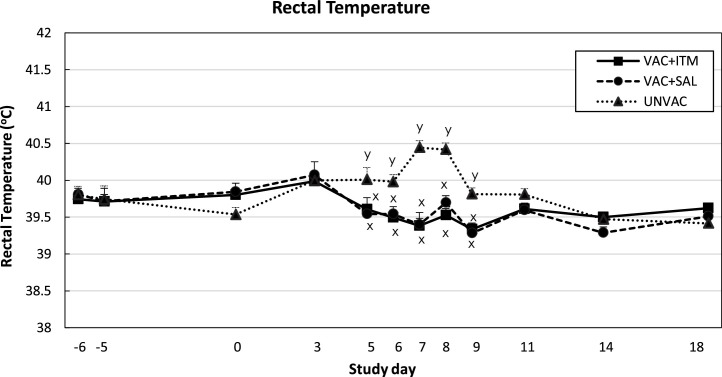
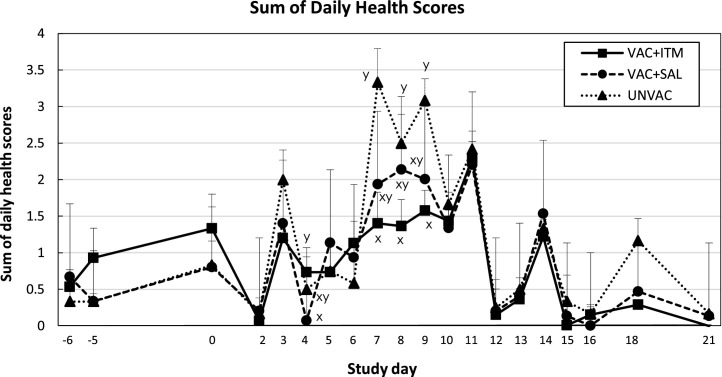
Rectal temperatures increased in the UNVAC group after BVDV2 challenge, peaking by days 7 and 8 (Fig. 2 ). Unvaccinated calves had significantly higher rectal temperature than both vaccinated groups on days 5–9 after BVDV challenge (P < 0.05). There was no difference in rectal temperature between vaccinated groups during the experimental period. Sum daily health scores increased on days 3, 7, 8 and 9 post BVDV challenge in the UNVAC group (Fig. 3 ). Calves receiving ITM had lower health scores than UNVAC calves on days 7–9 (P < 0.05). During this period, VAC + SAL group had intermediate health scores, which were not significantly different from either VAC + ITM or UNVAC groups (P > 0.05). Calves in VAC + ITM group had lower head-ears (attitude) scores compared to UNVAC (day 7) and VAC + SAL group (days 8, 9 and 11; P < 0.05. Data not shown).
Fig. 2.
Least square means (LSM) and standard error of the mean (SEM) for rectal temperature of beef calves treated (VAC + ITM) or not (VAC + SAL) with injectable trace minerals (ITM) concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) at study day -5, and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the SEM. x, y Significant difference between groups (P < 0.05).
Fig. 3.
Least square means (LSM) and standard error of the mean (SEM) for sum of daily health scores of beef calves treated (VAC + ITM) or not (VAC + SAL) with injectable trace minerals (ITM) concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) on study day -5 and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the SEM. x, y Significant difference between groups (P < 0.05).
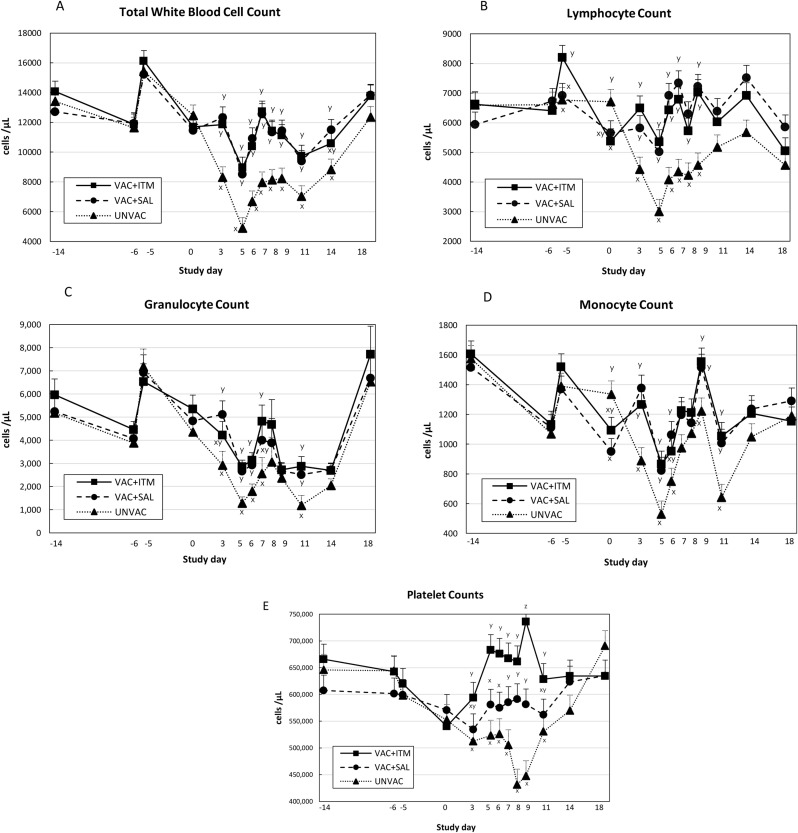
Mean total leukocytes count significantly decreased in the UNVAC group from day 0 to day 5 (approx. 66 %. Fig. 4 A) and remained low until day 11. These values were significantly lower than those in the vaccinated groups (P < 0.05). Vaccinated groups (treated with ITM or not) had similar mean leukocytes count along the study. Mean lymphocyte, granulocyte and monocyte counts significantly decreased in the UNVAC group between days 3 and 7 after BVDV challenge (P < 0.05; Fig. 4B–D), which was statistically different from vaccinated groups on the same days. Calves in both vaccinated groups had a slight decrease in lymphocyte, granulocyte and monocyte counts after vaccination and again on days 5 and 6 after BVDV challenge, with no significant differences between groups (Fig. 4B–D). Platelets count significantly declined after BVDV challenge in the UNVAC calves until days 8 and 9 (Fig. 4E). Differently, platelet counts increased in the VAC + ITM group after day 3, while they remained unaffected in the VAC + SAL group. Vaccinated calves that were treated with ITM had significantly higher platelet count compared to VAC + SAL (days 5, 6, and 9; P < 0.05) and UNVAC calves (days 3, 5, 6, 7, 8, 9, and 11; P < 0.05. Fig. 4E).
Fig. 4.
(A to E). Least square means (LSM) and standard error of the mean (SEM) for total white blood cells (A), lymphocyte (B), granulocyte (C), monocyte (D) and platelet (E) counts of beef calves treated (VAC + ITM) or not (VAC + SAL) with injectable trace minerals (ITM) concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) on study day -5, and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the SEM. x, y, z Significant difference between groups by each study day (P < 0.05).
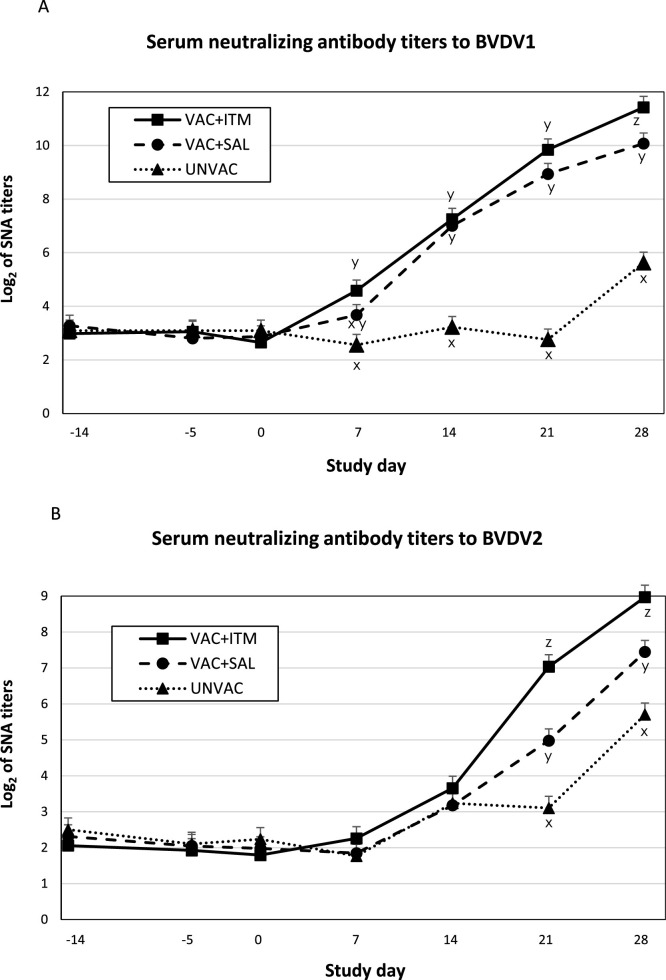
Serum neutralizing antibody (SNA) titers against BVDV1 and BVDV2 were low (<1:8) before challenge (on days -14, -5, and 0) in all groups (Fig. 5 A and B). SNA titers against BVDV1 dramatically increased (>4 log2) within two weeks after BVDV challenge in both vaccinated groups (Fig. 5A). Calves in VAC + ITM had higher BVDV1 SNA titers on day 28 compared with the VAC + SAL group (P < 0.05). Unvaccinated calves had lower SNA titers against BVDV1 after challenge than both vaccinated groups (P < 0.05). There was a moderate increase (approx. 2.5 log2) in BVDV1 SNA titers only on day 28 post challenge in the UNVAC group (Fig. 5A). Moreover, SNA titers against BVDV2 dramatically increased (> 5 log2) in VAC + ITM and VAC + SAL groups by days 21 and 28, respectively (Fig. 5B). Calves in the VAC + ITM group had higher BVDV2 SNA titers compared to the other groups on days 21 and 28 (P < 0.05). Calves in the UNVAC group had also a rise of BVDV2 SNA titers (approx. 3.5 log2) by day 28 after BVDV challenge, which were significantly lower than those in VAC + ITM and VAC + SAL groups (P < 0.05).
Fig. 5.
(A and B). Least square means (LSM) and standard error of the mean (SEM) for serum neutralizing antibody titers to BVDV-1 (A) and to BVDV-2 (B) for beef calves treated (VAC + ITM) or not (VAC + SAL) with injectable trace minerals (ITM) concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) at study day -5 and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the SEM. x, y, z Significant difference between groups by each study day (P < 0.05).
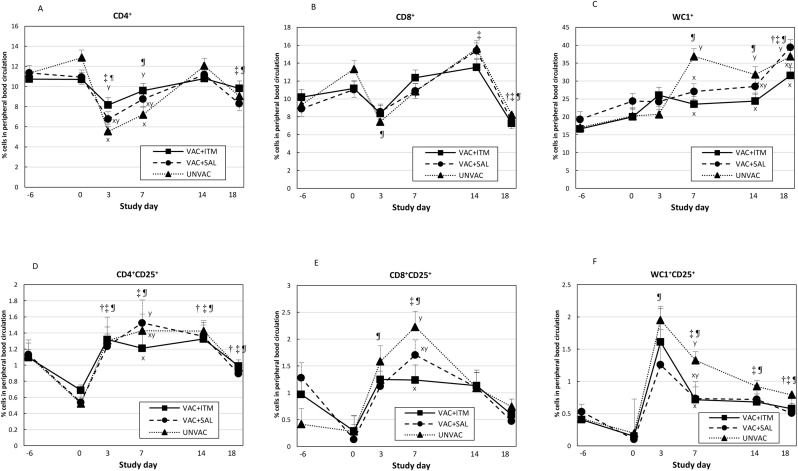
The percentage of CD4+ T-cells significantly decreased in the UNVAC and VAC + SAL groups on day 3 after BVDV2 challenge (P < 0.001; Fig. 6 A). On days 3 and 7, the VAC + ITM group had higher number of CD4+ T-cells than the UNVAC group. During this period, calves in the VAC + SAL group had intermediate values of CD4+ T-cells compared with the other groups. Infection with BVDV2 also caused a significant reduction in the percentage of CD8+ T-cells in the UNVAC calves by 3 days after BVDV challenge (P < 0.001. Fig. 6B). Vaccinated calves (treated with ITM or not) had a slight decline in the percentage of CD8+ T cells after BVDV2 challenge, which rebounded to initial values by day 7 post BVDV challenge. There were no statistical differences in the percentage of CD8+ T-cells among groups during the study (P > 0.05). However, values of CD8+ T cells tended to be numerically greater in the VAC + ITM treated calves on day 7 post BVDV challenge when compared with the other groups. Circulating activated T lymphocytes (CD4+CD25+ and CD8+CD25+) consistently increased in UNVAC and VAC + SAL calves on days 3, 7 (peak), and 14 (only for CD4+CD25+) after BVDV2 challenge (P < 0.05; Fig. 6D & E, respectively), but not in the VAC + ITM calves, in which activated T cells increased on day 3, but kept a plateau until day 14 post challenge. On day 7, calves treated with ITM had significantly lower percentages of CD4+CD25+ T-cells than the VAC + SAL group (P < 0.05; Fig. 6D) and CD8+CD25+ T cells than the UNVAC group (P < 0.05; Fig. 6E). Percentage of WC1+ T-cells significantly increased in the UNVAC group on days 7, 14 and 18 after BVDV2 infection (Fig. 6C). On day 18, calves in VAC + ITM and VAC + SAL also had a significant increase in WC1+ T lymphocytes. Between days 7 and 18 after BVDV2 challenge, the VAC + ITM group had significantly lower percentage of WC1+ T-cells compared to the UNVAC calves (P < 0.05). Activated WC1 T-cells (WC1+CD25+ T lymphocytes) significantly increased in all groups on day 3 (P < 0.05; Fig. 6F). Moreover, unvaccinated calves maintained high WC1+CD25+ T-cells lymphocytes levels until day 18 post BVDV2 challenge, while vaccinated calves had a decay in the percentage of WC1+CD25+ T-cells from day 7 to day 18 (Fig. 6F). On day 7, ITM-treated calves had significantly lower percentage of WC1+CD25+ T cells compared with the unvaccinated calves (P < 0.05).
Fig. 6.
(A to F). Least square means (LSM) and standard error of the mean (SEM) for percentage of CD4+ (A), CD8+ (B), WC1+ (C), CD4+CD25+ (D), CD8+CD25+ (E), and WC1+CD25+ (F) T-cell phenotype in peripheral blood circulation of beef calves treated (VAC + ITM) or not (VAC + SAL) with injectable trace minerals (ITM) concurrently with MLV-BRD vaccination, or calves not vaccinated or treated (UNVAC). Calves received vaccination and treatment (ITM or saline) on study day -5, and were individually challenged with ncp BVDV2 (strain 890) five days later (day 0). Errors bars represent the SEM. x, y Significant difference between groups on each study day (P < 0.05). † Significant difference relative to day 0 for VAC + ITM (P < 0.05). ‡ Significant difference relative to day 0 for the VAC + SAL group (P < 0.05). ¶ Significant difference relative to day 0 for UNVAC (P < 0.05).
All calves had negative PCR results for BVDV2 in nasal swabs or buffy coat samples on day 0 before BVDV2 challenge (Table 1 ). Fourteen of 15 calves (93.3 %) in the UNVAC group had at least one BVDV2 positive buffy coat sample on days 5 and 7. The percentage of BVDV2 positive buffy coat samples on days 5 and 7 was significantly greater in the UNVAC group compared with the vaccinated groups (P < 0.05; Table 1). Only one calf in VAC + ITM and one calf in VAC + SAL had a BVDV2 positive buffy coat sample on day 5 and 7 post challenge, respectively. Moreover, 46.7 % (7 out of 15) of the calves in the UNVAC group had at least one BVDV positive PCR result from nasal swab samples on days 5 and 7 post challenge, which was greater than the frequency observed in the vaccinated calves. There was no difference in the proportion of BVDV positive nasal swab specimens between both vaccinated groups.
Table 1.
Percentage (number) of positive results for BVDV2 via qRT-PCR from buffy coat and nasal swab samples for beef calves treated with injectable trace minerals (VAC + ITM; n = 15) or not (VAC + SAL; n = 15) concurrently with MLV-BRD vaccination, or calves neither treated nor vaccinated (UNVAC; n = 15). All calves were individually challenged with BVDV2 strain 890 five days after vaccination and treatment. 1Represents the total number of calves with at least one positive BVDV PCR result in all days out of 15 calves in each group (if a calf tested positive in more than one day, it was counted only once).* Significant difference (P < 0.05).
| Day | Buffy Coat |
Nasal Swabs |
||||
|---|---|---|---|---|---|---|
| VAC + ITM (n = 15) | VAC + SAL (n = 15) | UNVAC (n = 15) | VAC + ITM (n = 15) | VAC + SAL (n = 15) | UNVAC (n = 15) | |
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 5 | 6.7 % (1) | 0 (0) | 33.3 % (5)* | 13.3 % (2) | 13.3 % (2) | 33.3 % (5) |
| 7 | 0 (0) | 6.7 % (1) | 73.4 % (11)* | 13.3 % (2) | 6.7 % (1) | 20 % (3) |
| Cumulativea | 6.7 % (1) | 6.7 % (1) | 93.3 % (14)* | 26.7 % (4) | 13.3 % (2) | 46.7 % (7) |
Represents the total number of calves with at least one positive BVDV PCR result in all days out of 15 calves in each group (if a calf tested positive in more than one day, it was counted only once).
4. Discussion
In the current study, vaccination provided rapid protection from experimental infection with BVDV2 in newly-received beef calves. The efficacy of vaccination was demonstrated by lesser health scores (associated to improved health status according to the evaluation system used in this study), adequate leukocytes counts (absence of leukopenia and lymphopenia), lower proportion of BVDV2 positive nasal swab and buffy coat samples, and greater percentages of CD4+ T-cells in the vaccinated groups when compared to the control calves. These unvaccinated animals succumbed to mild clinical disease, had significant leukopenia, thrombocytopenia, viremia and virus shedding and a notable drop in CD4+ and CD8+ T-cells, caused by the moderately virulent BVDV2 strain 890. Moreover, administration of ITM resulted in increased hepatic concentrations of Se and Cu, and provided additional benefits of stronger immune response and protection after BVDV challenge (greater number of CD4+ T-cells, higher BVDV1 and 2 SNA titers, and lesser health scores) compared with the other groups. Hepatic Zn concentration was significantly higher in the unvaccinated group compared with the vaccinated calves. The reasons for this difference are unknown, but could be associated with higher level of stress (caused by BVDV infection) in the control calves, as previously reported (Nockels et al., 1993).
The experimental approach of performing vaccination and then virus challenge a few days after arrival was used to evaluate the onset of protection by attempting to mimic a common scenario of BVDV exposure upon arrival and commingling with cattle of unknown vaccination history. A rapid onset of protection induced by primary vaccination in susceptible calves with unknown immune status would be highly beneficial to beef cattle production, where high BRD-risk calves are subjected to management practices that involve significant stress including sudden weaning, transportation, comingling, and abrupt changes in diets. Previous studies evaluated the rapidity of the development of protection induced by MLV vaccination in beef calves challenged with low or high virulence BVDV at 3, 5 or 7 days after primary vaccination (Brock et al., 2007; Palomares et al., 2012). These studies demonstrated rapid protection against BVDV acute infection when vaccination was given 5 or 7 days before challenge. However, a high percentage of calves challenged 3 days after vaccination shed BVDV. At least in the context of BVDV prevention, our results and those of previous studies (Brock et al., 2007; Palomares et al., 2012), showing adequate immune response and rapid protection elicited by vaccination, provide evidence that may support strategic vaccination at the time of cattle arrival, reducing quarantine periods and intervals between vaccination and commingling with other cattle, which might facilitate farm management practices. Further, this study tested the strategy of using ITM concurrent with vaccination in an attempt to improve the protective immune response to BVDV in newly received beef calves challenged few days after vaccination.
Severe BVDV-induced leukopenia and thrombocytopenia were observed in the unvaccinated calves, while vaccinated groups experienced a slight decrease in white blood cells after BVDV2 challenge. In addition, platelet counts were significantly greater in the VAC + ITM group compared with the other groups. A known hallmark of BVDV2 infection is the reduction in leukocyte and platelet counts. The degree of leukopenia and thrombocytopenia is correlated to virulence of some specific strains of BVDV (Walz et al., 2001). Leukopenia induced by BVDV has been associated with apoptosis and necrosis of leukocytes, as well as leukocyte migration from circulation into tissues where viral replication occurs (Walz et al., 2010). BVDV-induced leukopenia results in immunosuppression and plays a major role in the pathogenesis of BRD, as it potentiates secondary infection by commensal flora in the upper respiratory tract responsible for causing mild to fatal bronchitis and pneumonia (Brogden and Guthmiller, 2002). Reduction in platelet number is a common hematological finding in infections with some BVDV2 strains and has been associated with a hemorrhagic syndrome (Rebhun et al., 1989). Administration of ITM at the time of MLV vaccination induced a significant increase in platelet counts in calves challenged with BVDV2. The benefits of injectable trace mineral (Se, Cu, Mn and Zn) supplementation for mitigating the negative effects of BVDV2 infection on platelets number is a remarkable finding of this study. Comparable effects of ITM administration on platelet counts were observed in our previous trial evaluating the long-term protection elicited by MLV vaccination and ITM supplementation against BVDV2 infection in dairy calves (Bittar et al., 2018b).
The commercial vaccine used in this study was able to reduce viremia and viral shedding after BVDV infection, as represented by the lower number of BVDV2 positive buffy coat and nasal swab samples in both vaccinated groups on days 5 and 7 after experimental BVDV2 challenge compared with the unvaccinated group, which is in agreement with previous studies using different BVDV strains (Brock et al., 2007; Palomares et al., 2012). However, supplementation with ITM showed no additional beneficial effects reducing BVDV detection in peripheral leukocytes and nasal swab samples.
The humoral immune response post BVDV challenge was greater in both vaccinated groups compared to the unvaccinated calves. Calves treated with ITM had greater SNA titers to BVDV1 and BVDV2 compared to the other groups. Previous field studies have shown association between higher SNA titers and health performance in calves at feedlot receiving (Fulton et al., 2002; Campbell, 2004). Production of neutralizing antibodies is one of the major features of the immune system to prevent viral infections such as BVDV. Neutralizing antibodies hamper BVDV replication and transmission in the host cells, diminishing the chances of a successful viral infection and shedding within cattle populations. Similarly, positive effects of using ITM concurrent with MLV vaccination on improvement of SNA production against BRD viruses and animal health and performance have been shown in previous studies in dairy and beef cattle (Richeson and Kegley, 2011; Arthington and Havenga, 2012; Palomares et al., 2016). In contrast, our previous study evaluating the effects of ITM given at the time of vaccination on the long-term protection against BVDV infection in dairy calves showed no improvement in BVDV SNA titers or protection from clinical disease in calves treated with ITM (Bittar et al., 2018b). The absence of clinically remarkable differences between groups was attributed to the moderate virulence BVDV strain used for challenge, low level of stress and limited infectious pressure, which favored protection elicited by two doses of MLV vaccination. On the other hand, the greater BRD risk scenario of the current trial (one dose of MLV vaccine given to naïve cattle, short interval between vaccination and BVDV challenge, 8-h transportation before vaccination and diet change) might explain discordance between these studies. Thus, the experimental conditions of the current study appear to optimize the effects of ITM concurrently with MLV BRD vaccine, especially when a rapid onset of an immune response and early protection are needed.
Infection with BVDV caused a significant reduction in number of CD4+ and CD8+ T lymphocytes in the unvaccinated calves, which agrees with previous reports (Howard et al., 1992; Chase et al., 2004). Calves treated with ITM had a less pronounced reduction in CD4+ T-cells (with higher percentage on days 3 and 7) and a lower number of activated CD4+ CD25+ and CD8+ CD25+ T-cells compared to the other groups. Lymphocytes expressing the surface protein CD4 (T helper cells), play an important role in the humoral and cell mediated immune responses after vaccination and/or infection. They contribute to the activity of other immune cells by releasing cytokines that help stimulating or regulating the immune response (Abbas et al., 2012). They are essential in B cell antibody isotype switching, activation and proliferation of cytotoxic T-cells (CD8+), and in maximizing phagocytic and bactericidal activity by neutrophils and macrophages, among other functions. Cytotoxic T lymphocytes or CD8+ T-cells have important effector functions killing virus-infected cells, through the release of the cytotoxins perforins, granzymes, and granulysin (Abbas et al., 2012). It is known that CD4+ T-cell depletion during BVDV acute infection may increase the period of virus shedding, as demonstrated by the higher proportion of unvaccinated calves carrying BVDV in nasal secretions and peripheral blood leukocytes in this trial and previous studies (Howard et al., 1992). Total and activated WC1+ T-cells were consistently increased in the unvaccinated group after BVDV2 challenge. Treatment with ITM appeared to prevent a significant increase in WC1+ T-cells. In cattle, gamma delta T-cells expressing the WC1 phenotype accounts for up to 60 % of the total circulating lymphocytes and have been described to have regulatory functions with spontaneous IL-10 secretion, (Hoek et al., 2009; Guzman et al., 2014). Therefore, increasing activation and trafficking of gamma delta T lymphocytes may be one of the mechanisms used by BVDV to down-regulate the immune system causing immune suppression, as it has been previously reported (Palomares et al., 2015).
It is possible to infer that ITM supplementation positively influenced both humoral and cell mediated immune responses elicited by MLV primary vaccination, as previously shown (Palomares et al., 2016; Bittar et al., 2018a), resulting in an enhanced protection upon BVDV exposure. In addition, ITM may have contributed to a more efficacious innate immunity (e.g. type I interferon antiviral state, antigen processing and presentation) after vaccination, which may have limited BVDV replication and viral load at the site of infection, reduced virus-induced T-cell depletion (resulting in higher number of CD4+ T-cells) and lowered the proportion of T-cells trafficking to secondary lymphoid tissues after virus infection, reducing T-cell activation, proliferation and differentiation. This inference might explain the lower percentage of activated CD4+CD25+ and CD8+CD25+ in the peripheral blood circulation in the VAC + ITM group.
This study had the limitations that T-cell populations were not evaluated at the tissue level (respiratory tract mucosa and lymphoid tissue), which would have provided a better understanding of the T-lymphocyte phenotypic dynamics after the BVDV challenge. Nonetheless, to the authors’ knowledge, this is the first study evaluating the ex-vivo dynamics of circulating T-cells upon BVDV challenge occurring shortly after MLV-BRD vaccination.
It would have been interesting to include an ‘ITM alone group’ in the experimental design to test the effects of ITM administration on the immune response after challenge with BVDV2, as ITM administration was suggested to improve the general health status of young calves (Teixeira et al., 2014). However, administration of ITM is not intended to induce specific protective immunity against respiratory pathogens by itself or replace vaccination.
In summary, MLV-BRD vaccination concurrent with ITM or not, induced a rapid protection against BVDV infection. In addition, administration of ITM at the time of vaccination was associated with increased humoral immune response to BVDV1 and 2, enhanced health status, appeared to mitigate the decrease in the percentage of peripheral blood circulating CD4+ and CD8+ T cells, and reduced their activation in beef calves challenged with BVDV2 five days after vaccination. Moreover, administration of ITM at the time of vaccination was associated with significantly increased platelet counts after BVDV challenge. These results support the strategic use of ITM concurrently with vaccination protocols, especially when a rapid immune response and protection is needed in newly received beef calves.
Declaration of Competing Interest
The authors of this article, declare that there are no conflicts of interest.
Acknowledgements
The authors thank The University of Georgia and Multimin® USA, INC for the financial support. Thanks to Drs. Brenton Credille and Amelia Woolums for their support at University of Georgia. The authors extend their gratitude to Mark Chastain, Troupe Tabb and Dr. Seth Stowers for providing collaboration on animal husbandry during this research. The authors also thank Boehringer Ingelheim Vetmedica Inc., for the donation of vaccines Express 5.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.vetimm.2020.110055.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abbas A.K., Lichtman A.H., Pillai S. 7th edition. Elsevier Saunders; Philadelphia, PA: 2012. Cellular and Molecular Immunology. [Google Scholar]
- Arthington J.D., Havenga L.J. Effect of injectable trace minerals on the humoral immune response to multivalent vaccine administration in beef calves. J. Anim. Sci. 2012;90:1966–1971. doi: 10.2527/jas.2011-4024. [DOI] [PubMed] [Google Scholar]
- Bittar J.H.J., Hurley D.J., Woolums A.R., Norton N.A., Barber C.E., Moliere F., Havenga L.J., Palomares R.A. Effects of injectable trace minerals on the immune response to Mannheimia haemolytica and Pasteurella multocida following vaccination of dairy calves with a commercial attenuated-live bacterin vaccine. Prof. Anim. Sci. 2018;34:59–66. [Google Scholar]
- Bittar J.H.J., Hoyos-Jaramillo A., Hurley D.J., Woolums A.R., Havenga L.J., Lourenço J.M., Barnett G., Gomes V., Saliki J.T., Harmon D.D., Palomares R.A. Effects of injectable trace minerals administered concurrently with a modified live virus vaccine on long-term protection against bovine viral diarrhea virus acute infection in dairy calves. Res. Vet. Sci. 2018;119:250–258. doi: 10.1016/j.rvsc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarède F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Brock K. Strategies for the control and prevention of bovine viral diarrhea virus. Vet. Clin. North Am: Food Anim. Pract. 2004;20:171–180. doi: 10.1016/j.cvfa.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Brock K.V., Widel P., Walz P., Walz H.L. Onset of protection form experimental infection with type 2 bovine viral diarrhea virus following vaccination with a modified-live vaccine. Vet. Ther. 2007;8:88–96. [PubMed] [Google Scholar]
- Brogden K.A., Guthmiller J.M. ASM Press; Washington (DC): 2002. Polymicrobial Diseases; p. 2002. [PubMed] [Google Scholar]
- Campbell J.R. Effect of bovine viral diarrhea virus in the feedlot. Vet. Clin. North. Amer. Food Anim. Pract. 2004;20:39–50. doi: 10.1016/j.cvfa.2003.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase C.C., Elmowalid G., Yousif A.A. The immune response to bovine viral diarrhea virus: a constantly changing picture. Vet. Clin. North. Am. Food. Anim. Pract. 2004;20:95–114. doi: 10.1016/j.cvfa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Chirase N.K., Hutcheson D.P., Thompson G.B., Spears J.W. Recovery rate and plasma zinc and copper concentrations of steer calves fed organic and inorganic zinc and manganese sources with or without injectable copper and challenged with infectious bovine rhinotracheitis virus. J. Anim. Sci. 1994;72:212–219. doi: 10.2527/1994.721212x. [DOI] [PubMed] [Google Scholar]
- Dean H.J., Leyh R. Cross-protective efficacy of a bovine viral diarrhea virus (BVDV) type 1 vaccine against BVDV type 2 challenge. Vaccine. 1999;17:1117–1124. doi: 10.1016/s0264-410x(98)00329-6. [DOI] [PubMed] [Google Scholar]
- Duff G.C., Galyean M.L. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 2007;85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Purdy C.W., Confer A.W., Saliki J.T., Loan R.W., Briggs R.E., Burge L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000;64:151–159. [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Cook B.J., Step D.L., Confer A.W., Saliki J.T., Payton M.E., Burge L.J., Welsh R.D., Blood K.S. Evaluation of health status of calves and the impact on feedlot performance: assessment of a retained ownership program for postweaning calves. Can. J. Vet. Res. 2002;66:173–180. [PMC free article] [PubMed] [Google Scholar]
- Griffin D. Economic impact associated to respiratory disease in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 1997;13:367–378. doi: 10.1016/s0749-0720(15)30302-9. [DOI] [PubMed] [Google Scholar]
- Guzman E., Hope J., Taylor G., Smith A.L., Cubillos-Zapata C., Charleston B. Bovine γδ T cells are a major regulatory T cell subset. J. Immunol. 2014;193:208–222. doi: 10.4049/jimmunol.1303398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H., Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- Harpin S., Hurley D.J., Mbikay M., Talbot B., Elazhary Y. Vaccination of cattle with a DNA plasmid encoding the bovine viral diarrhoea virus major glycoprotein E2. J. Gen. Virol. 1999;80:3137–3144. doi: 10.1099/0022-1317-80-12-3137. [DOI] [PubMed] [Google Scholar]
- Herdt T.H., Hoff B. The use of blood analysis to evaluate trace mineral Status in ruminant livestock. Vet. Clin. Food Anim. 2011;27:255–283. doi: 10.1016/j.cvfa.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Hoek A., Rutten V.P., Kool J., Arkesteijn G.J., Bouwstra R.J., Van Rhijn I., Koets A.P. Subpopulations of bovine WC1+ γδ T cells rather than CD4+CD25high Foxp3+ T cells act as immune regulatory cells ex vivo. Vet. Res. 2009;40:6–20. doi: 10.1051/vetres:2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C.J., Clarke M.C., Sopp P., Brownlie J. Immunity to bovine virus diarrhea virus in calves: the role of different T-cell subpopulations analysed by specific depletion in vivo with monoclonal antibodies. Vet. Immunol. Immunopathol. 1992;32:303–314. doi: 10.1016/0165-2427(92)90052-r. [DOI] [PubMed] [Google Scholar]
- Letellier C., Kerkhofs P. Real-time PCR for simultaneous detection and genotyping of bovine viral diarrhea virus. J. Virol. Meth. 2003;114:21–27. doi: 10.1016/j.jviromet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Newcomer B.W., Chamorro M.F., Walz P.H. Vaccination of cattle against bovine viral diarrhea virus. Vet. Microbiol. 2017;206:78–83. doi: 10.1016/j.vetmic.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Nockels C.F., DeBonis J., Torrent J. Stress induction affects copper and zinc balance in calves fed organic and inorganic copper and zinc sources. J. Anim. Sci. 1993;71:2539–2545. doi: 10.2527/1993.7192539x. [DOI] [PubMed] [Google Scholar]
- Palomares R.A., Givens M.D., Wright J.C., Walz P.H., Brock K.Y. Evaluation of the onset of protection induced by a modified-live virus vaccine in calves challenge inoculated with type 1b bovine viral diarrhea virus. Am. J. Vet. Res. 2012;73:567–574. doi: 10.2460/ajvr.73.4.567. [DOI] [PubMed] [Google Scholar]
- Palomares R.A., Sakamoto K., Walz H.L., Brock K.V., Hurley D.J. Acute infection with bovine viral diarrhea virus of low or high virulence leads to depletion and redistribution of WC1(+) γδ T cells in lymphoid tissues of beef calves. Vet. Immunol. Immunopathol. 2015;167:190–195. doi: 10.1016/j.vetimm.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Palomares R.A., Hurley D.J., Bittar J.H.J., Saliki J.T., Woolums A.R., Moliere F., Havenga L.J., Norton N.A., Sigmund A.J., Barber C.E., Berger M.L., Clark M.J., Fratto M.A. Effects of injectable trace minerals on humoral and cell-mediated immune responses to bovine viral diarrhea virus, bovine herpes virus 1 and bovine respiratory syncytial virus following administration of a modified-live virus vaccine in dairy calves. Vet. Immunol. Immunopat. 2016;178:88–98. doi: 10.1016/j.vetimm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Percival S.S. Copper and immunity. Am. J. Clin. Nutr. 1998;67:1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- Pinna K., Kelley D.S., Taylor P.C., King J.C. Immune functions are maintained in healthy men with low zinc intake. J. Nutr. 2002;132:2033–2036. doi: 10.1093/jn/132.7.2033. [DOI] [PubMed] [Google Scholar]
- Reber A.J., Tanner M., Okinaga T., Woolums A.R., Williams S., Ensley D.T., Hurley D.J. Evaluation of multiple immune parameters after vaccination with modified live or killed bovine viral diarrhea virus vaccine. Comp. Immun. Microbiol. Infect. Dis. 2006;29:61–77. doi: 10.1016/j.cimid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Rebhun W.C., French T.W., Perdrizet J.A., Dubovi E.J., Dill S.G., Karcher L.F. Thrombocytopenia associated with acute bovine virus diarrhea infection in cattle. J. Vet. Intern. Med. 1989;3:42–46. doi: 10.1111/j.1939-1676.1989.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Rhodes S.G., Cocksedge J.M., Collins R.A., Morrison W.I. Differential cytokine responses of CD4+ and CD8+ t cells in response to bovine viral diarrhoea virus in cattle. J. Gen. Vir. 1999;80:1673–1679. doi: 10.1099/0022-1317-80-7-1673. [DOI] [PubMed] [Google Scholar]
- Richeson J.T., Kegley E.B. Effect of supplemental trace minerals from injection on health and performance of highly stressed, newly received beef calves. Prof. Anim. Sci. 2011;27:461–466. [Google Scholar]
- Roberts S.L., May N.D., Brauer C.L., Gentry W.W., Weiss C.P., Jennings J.S., Richeson J.T. Effect of injectable trace mineral administration on health, performance and vaccine response of newly received beef cattle. Prof. Anim. Sci. 2016;32:842–848. [Google Scholar]
- Rosenquist B.D., English J.E., Johnson D.W., Loan R.W. Mixed viral etiology of a shipping fever epizootic in cattle. Am. J. Vet. Res. 1970;31:989–994. [PubMed] [Google Scholar]
- Spears J.W., Kegley E.B. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 2002;80:2747–2752. doi: 10.2527/2002.80102747x. [DOI] [PubMed] [Google Scholar]
- Taylor J.D., Fulton R.W., Lehenbauer T.W., Step D.L., Confer A.W. The epidemiology of bovine respiratory disease: what is the evidence for predisposing factors? Can. Vet. J. 2010;51:1095–1102. [PMC free article] [PubMed] [Google Scholar]
- Teixeira A.G., Lima F.S., Bicalho M.L., Kussler A., Lima S.F., Felippe M.J., Bicalho R.C. Effect of an injectable trace mineral supplement containing selenium, copper, zinc, and manganese on immunity, health, and growth of dairy calves. J. Dairy Sci. 2014;97:4216–4226. doi: 10.3168/jds.2013-7625. [DOI] [PubMed] [Google Scholar]
- Tomlinson D.J., Socha M.T., DeFrain J.M. Role of trace minerals in the immune system. 2008 Penn State Dairy Cattle Nutrition Workshop; November 12–13. Grantville, PA; 2008. pp. 39–52. [Google Scholar]
- Underwood E.J., Suttle N.F. Third edition. CABI Publishing, CAB International; Wallingford, Oxon, UK: 1999. The Mineral Nutrition of Livestock. [Google Scholar]
- USDA . 2010. National Animal Health Monitoring System. Beef 2007-08. Part IV: Reference of Beef Bow-Calf Management Practices in the United States. Available at: https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef0708/Beef0708_dr_PartIV.pdf. (Accessed May 3, 2019) [Google Scholar]
- Walz P.H., Bell T.G., Grooms D.L., Kaiser L., Maes R.K., Baker J.C. Platelet aggregation responses and virus isolation from platelets in calves experimentally infected with type I or type II bovine viral diarrhea virus. Can. J. Vet. Res. 2001;65:214–247. [PMC free article] [PubMed] [Google Scholar]
- Walz P.H., Grooms D.L., Passler T., Ridpath J.F., Tremblay R., Step D.L., Callan R.J., Givens M.D. Control of bovine viral diarrhea virus in ruminants. J. Vet. Intern. Med. 2010;24:476–486. doi: 10.1111/j.1939-1676.2010.0502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.