Abstract
Background
The current outbreak of coronavirus disease 2019 (COVID-19) caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, Hubei, China, spreads across national and international borders.
Methods
We prospectively collected medical records of 14 health care workers (HCWs) who were infected with SARS-CoV-2, in neurosurgery department of Wuhan Union Hospital, China.
Results
Among the 14 HCWs, 12 were conformed cases, the other 2 were suspected cases. Most of them were either exposed to the two index patients or infected coworkers, without knowing they were COVID-19 patients. There were 4 male and 10 female infected HCWs in this cohort, whose mean age was 36 years (SD, 6 years). The main symptoms included myalgia or fatigue (100%), fever (86%) and dry cough (71%). On admission, 79% of infected HCWs showed leucopenia and 43% lymphopenia. Reduced complement C3 could be seen in 57% of the infected HCWs and IL-6 was significantly elevated in 86% of them. The proportion of lymphocytes subsets, concentrations of immunoglobulins, complement C4, IL-2, IL-4, IL-10, TNF-α and IFN-γ were within normal range in these 14 infected HCWs. The most frequent findings on pulmonary computed tomographic images were bilateral multifocal ground-glass opacifications (86%).
Conclusions
Human-to-human transmission of COVID-19 pneumonia has occurred among HCWs, and most of these infected HCWs with confirmed COVID-19 are mild cases. Our data suggest that in the epidemic area of COVID-19, stringent and urgent surveillance and infection-control measures should be implemented to protect doctors and nurses from COVID-19 infection.
Keywords: COVID-19, SARS-CoV-2, Health care workers
Introduction
Since December 2019, the emergence and spread of Corona Virus Disease 2019 (COVID-19) caused by the Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from Wuhan city, Hubei province, China,1, 2, 3, 4 has become a global health concern.5, 6, 7, 8 As of March 18, 2020, a total of 191, 127 confirmed cases had been reported globally, with 7807 deaths.9 80928 confirmed cases and 3245 death cases were documented in China, as of March 18, 2020.10 The overwhelming burden of illness stressed the health system capacity and increased the risk of infection among health care workers (HCWs).
As of February 11, 2020, the Chinese Center for Disease Control and Prevention reported that a total of 1716 HCWs were confirmed to be COVID-19 patients in China.11 Among them, 63% (1080) were in Wuhan, at least 5 had been dead.11 During the early outbreak of COVID-19 in Wuhan, inadequate personal protection of health care workers maybe an important reason for the COVID-19 infection in HCWs.
Here, we report a cluster of infected HCWs from department of neurosurgery in Wuhan Union Hospital.
Methods
Patients
One male patient (69 years old) was admitted to the department of neurosurgery of Wuhan Union Hospital for pituitary adenoma resection on December 25, 2019. He demonstrated a fever of 38 °C on January 6 and pulmonary computed tomography (CT) revealed “virus pneumonia” on January 11. Another patient in the same ward also complained of fever, on January 11. Both of them were confirmed to be COVID-19 patients with positive result of SARS-CoV-2 on January 16 and 18, respectively. They were transferred to an isolation ward for patients with infectious diseases, immediately. Meanwhile, all health care workers in the neurosurgery department, not only those exposed to the two index patients, were enquired if they had any uncomfort, took test for SARS-CoV-2 from throat swab and CT scan, to see if they had been infected.
Ethics approval was approved by institutional ethics board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (2020-0120). Oral consent was obtained from all patients and infected HCWs. Written informed consents were exempted according to the approved ethics approval (2020-0120).
Data sources
All the infected 14 health care workers were hospitalized in isolation wards. All the medical records were extracted and analyzed.
The criteria for confirmed and suspected cases of COVID-19 were followed “the diagnosis and treatment protocol for novel coronavirus pneumonia” (trial version 7, released by National Health Commission & State Administration of Traditional Chinese Medicine on March 3, 2020).10
Throat-swab specimens from the upper respiratory tract were obtained from all patients and infected HCWs, and the detection of SARS-CoV-2 was performed in accordance with WHO recommendation.12 SARS-CoV-2 was confirmed by real-time RT-PCR using the same protocol as previously described in the studies from Wuhan Jinyintan Hospital.3 , 4
Serum cytokine levels (IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ) were assayed using the BD Biosciences Th1/Th2 cytokine kit, as per the manufacturer's instructions (BD Ltd., Franklin Lakes, NJ, USA).
For those confirmed infected HCWs, they were permitted to discharge, if (1) their body temperature was back to normal for more than three days, (2) respiratory symptoms improve obviously, (3) pulmonary imaging shows obvious absorption of inflammation (4) and nuclei acid tests negative twice consecutively on nasopharyngeal swabs (sampling interval being at least 24 h).10 For those suspected infected HCWs, they also were permitted to discharge if they met the above criteria (1), (2) and (3). All discharged HCWs were required to live in an isolation hotel for another 14 days, before they could go home.
Outcomes
We describe demographics, clinical presentation, laboratory results, pulmonary CT findings, response to therapy, and clinical outcomes.
Statistical analysis
Continuous measurements are presented as mean (SD) and categorical variables as count (%). Comparisons were determined by paired t test or repeated measures analysis of variance as appropriate. We also assessed whether the laboratory results were outside the normal range. All statistical analyses were performed with SPSS22.0 (SPSS Inc., Chicago, IL).
Results
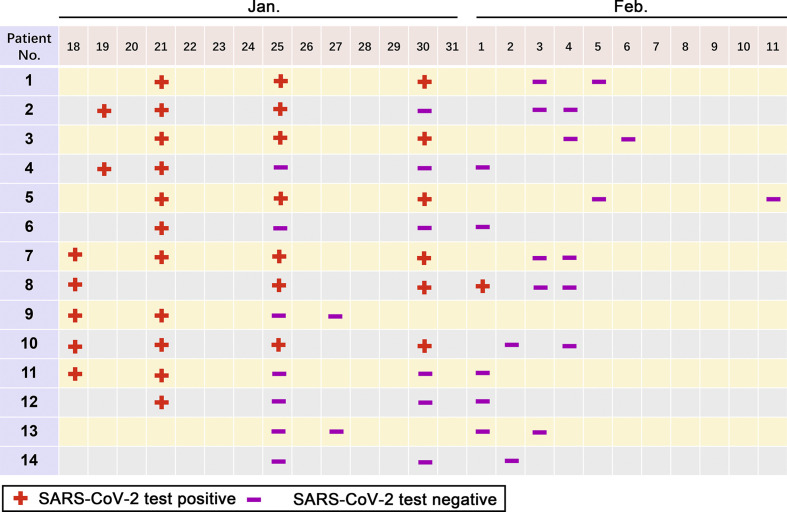
As of January 31, fourteen HCWs in the neurosurgery department were hospitalized to isolation wards. According “the diagnosis and treatment protocol for novel coronavirus pneumonia” (trial version 7, released by National Health Commission & State Administration of Traditional Chinese Medicine on March 3, 2020), 12 infected HCWs were confirmed with a positive result of SARS-CoV-2 test from throat swab. Another two were diagnosed as suspected cases, due to an epidemiological history and typical manifestations (In contact with novel coronavirus infected people [with positive results for the nucleic acid test] within 14 days prior to the onset of the disease; Fever and/or respiratory symptoms; The imaging characteristics of NCP; Normal or decreases WBC count, normal or decreased lymphocyte count in the early stage of onset), as their SARS-CoV-2 test results were negative. After a period of treatment, the SARS-CoV-2 test turned to be negative in the 12 confirmed HCWs (Fig. 1 ). In addition, all infected HCWs were tested for the nucleic acid of influenza viruses A and B as well as local common bacteria and fungi spectra, and influenza virus A test was positive in one patient (patient 2).
Fig. 1.
Results of SARS-CoV-2 detection in health care workers during hospitalization.
The mean age of the infected HCWs was 36 years (SD, 6 years), and 4 of the 14 infected HCWs (29%) were male (Table 1 ). No one was smoker and had been exposed to Huana seafood wholesale market in Wuhan, which was thought to be associated with the early outbreak of COVID-19 pneumonia.3 , 4 , 13 Except patient 13 had cough variant asthma, none of the remaining 13 infected HCWs had any kinds of underlying chronic illness.
Table 1.
Demography and Symptoms in Health Care Workers with COVID-19 at admission.
| Sex | Patient No. |
Summary | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
||
| Male | Male | Female | Female | Female | Female | Female | Female | Female | Female | Female | Male | Male | Female | ||
| Age (yr) | 32 | 34 | 34 | 32 | 41 | 41 | 35 | 36 | 35 | 43 | 51 | 33 | 27 | 36 | 36±6a |
| Occupation | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Nurse | Doctor | Doctor | Nurse | – |
| Smoking history | Never | Never | Never | Never | Never | Never | Never | Never | Never | Never | Never | Never | Never | Never | – |
| Underlying disease | – | – | – | – | – | – | – | – | – | – | – | – | + | – | 1+ |
| Symptoms | |||||||||||||||
| Myalgia or fatigue | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 14+ |
| Fever | + | – | + | + | + | + | + | + | + | + | – | + | + | + | 12+ |
| Headache | – | + | – | + | + | + | + | + | – | + | + | – | – | – | 8+ |
| Pharyngalgia | + | + | + | + | – | – | + | – | – | + | + | – | – | – | 7+ |
| Cough | + | + | + | + | – | + | – | + | + | + | + | + | – | – | 10+ |
| Sputum | – | + | + | + | – | + | – | – | – | – | + | – | – | – | 5+ |
| Dyspnea | – | – | – | – | + | + | – | – | – | – | – | – | – | – | 2+ |
| Diarrhea | + | + | + | + | + | + | – | + | + | – | + | – | – | – | 9+ |
| Vomiting | – | – | – | – | + | + | – | – | – | – | – | – | – | – | 2+ |
Means ±SD.
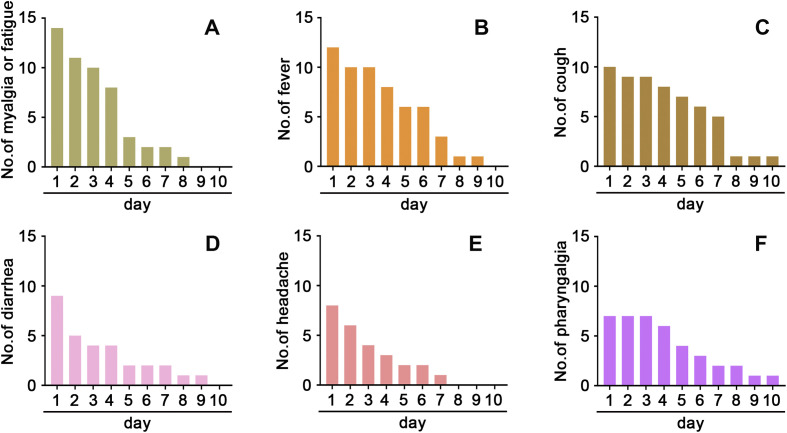
The main symptoms included myalgia or fatigue (100% of the patients), fever (86%), dry cough (71%), diarrhea (64%), headache (57%), and pharyngalgia (50%), on admission to hospital (Table 1). Myalgia or fatigue, fever, dry cough, diarrhea (Fig. 2 ) and the other symptoms resolved within two weeks after the disease onset.
Fig. 2.
Time of Onset of Symptoms in the Clinical Course. The distribution and lasting days of myalgia or fatigue (A), fever (B), dry cough (C), and diarrhea (D), headache (E), and pharyngalgia (F) in the infected HCWs with COVID-19 pneumonia.
On admission, 79% of infected HCWs showed leucopenia and 43% lymphopenia (Table 2 ). The numbers of white blood cells and lymphocytes in most infected HCWs with leucopenia and/or lymphopenia increased to normal range within 10 days after admission. Unlike the laboratory findings previously reported in the first and second cohorts,3 , 4 all blood biochemical and the other parameters determined in our cohort were not outside the normal range, although a couple of parameters, such as aspartate aminotransferase and albumin, demonstrated some an extent of fluctuation (Table 2). It was noted that COVID-19 pneumonia onset did not influence the proportion of lymphocytes subsets (Appendix Table 1) and the concentrations of immunoglobulins (Appendix Table 2). Reduced complement C3 could be seen in 57% of the infected HCWs at Day 1 and 29% at Day 7, while complement C4 was not affected (Appendix Table 2). We determined the concentrations of IL-2, IL-4, IL-6, IL-10, TNF-α, as well as IFN-γ, and found that IL-6 was the only one cytokine whose level was significantly elevated in serum of COVID-19 pneumonia infected HCWs, indicating IL-6 may play an important role in the pathogenesis of COVID-19 pneumonia (Appendix Table 3).
Table 2.
Laboratory findings in Health Care Workers with COVID-19 during hospitalization (N = 14).
| Variable | Day 1 | Day 3 | Day 5 | Day 10 |
|---|---|---|---|---|
| White blood cell counts, × 109/L | 3.4 ± 1.3 | 4.6 ± 2.8 | 5.3 ± 1.6a | 4.3 ± 1.3 |
| <3.5 -- no. (%) | 11 (79) | 6 (43) | 1 (7)a | 3 (21)a |
| 3.5–9.5 -- no. (%) | 3 (21) | 7 (50) | 13 (93) | 11 (79) |
| >9.5 -- no. (%) | 0 | 1 (7) | 0 | 0 |
| Neutrophils, × 109/L | 1.8 ± 0.9 | 2.6 ± 1.8 | 3.8 ± 1.6a | 2.5 ± 0.8a |
| Lymphocytes, × 109/L | 1.1 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.4 | 1.5 ± 0.4a |
| <1.1 -- no. (%) | 6 (43) | 5 (36) | 5 (36) | 3 (21) |
| 1.1–3.2 -- no. (%) | 8 (57) | 9 (64) | 9 (64) | 11 (79) |
| Platelets, × 109/L | 174 ± 63 | 180 ± 51 | 188 ± 60 | 242 ± 50a |
| Haemoglobin, g/L | 126.0 ± 14.2 | 125.1 ± 16.4 | 123.9 ± 16.7 | 119.7 ± 15.9 |
| Alanine aminotransferase, U/L | 17.1 ± 7.0 | 18.3 ± 6.1 | 17.6 ± 6.9 | 17.7 ± 7.2 |
| Aspartate aminotransferase, U/L | 22.4 ± 4.2 | 26.6 ± 5.4a | 27.9 ± 6.2a | 24.5 ± 4.9 |
| Albumin, g/L | 41.3 ± 2.9 | 44.4 ± 3.5a | 49.1 ± 4.7a | 45.2 ± 4.1a |
| Total bilirubin, μmol/L | 7.5 ± 1.6 | 7.7 ± 1.4 | 7.5 ± 1.9 | 8.1 ± 1.8 |
| Direct bilirubin, μmol/L | 2.7 ± 0.9 | 3.1 ± 0.7 | 2.8 ± 1.1 | 2.8 ± 0.9 |
| Creatinine, μmol/L | 65.2 ± 13.1 | 64.8 ± 19.4 | 71.1 ± 15.3 | 70.9 ± 14.1 |
| Blood urea nitrogen, mmol/L | 3.3 ± 1.1 | 3.0 ± 0.9 | 3.9 ± 1.3 | 3.4 ± 1.2 |
| Creatine kinase, U/L | 63.3 ± 22.7 | 79.9 ± 31.8 | 73.8 ± 24.6 | 70.9 ± 29.7 |
| Lactic dehydrogenase, U/L | 203.9 ± 51.0 | 231.9 ± 48.9 | 208.4 ± 42.8 | 187.8 ± 45.9 |
| Prothrombin time, s | 13.9 ± 2.9 | 14.0 ± 2.1 | 13.1 ± 2.2 | 13.5 ± 2.6 |
| Activated partial thromboplastin time, s | 40.2 ± 4.2 | 38.3 ± 4.1 | 36.1 ± 3.2a | 39.2 ± 4.9 |
| D-Dimer, mg/L | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.3 |
| C-reaction protein, mg/L -- no. (%) | ||||
| <8.0 | 8 (57.1) | 6 (42.9) | 7 (50) | 9 (64.3) |
| ≥8.0 | 6 (42.9) | 8 (57.1) | 7 (50) | 5 (35.7) |
| Procalcitonin, μg/L -- no. (%) | ||||
| <0.5 | 14 (100) | 12 (86) | 14 (100) | 14 (100) |
| Erythrocyte sedimentation rate, mm/h | 13.2 ± 9.4 | 21.7 ± 8.5a | 17.2 ± 7.3 | 10.9 ± 5.2 |
P < 0.05 compared with Day 1.
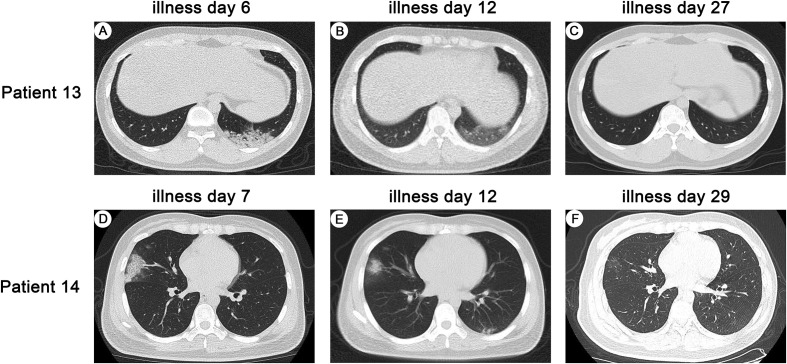
Pulmonary involvement was seen on CT images of all infected HCWs, and most of the CT abnormalities were bilateral pneumonia (Appendix Table 4). The CT scans of the two suspected infected HCWs were shown in Fig. 3 . The most frequent findings on CT images were ground-glass opacifications (86%), which located in peripheral parenchyma (50%) or in both peripheral and central parenchyma (36%). The ground-glass opacifications in all infected HCWs were multifocal. Subsegmental areas of consolidation could be identified in six (43%) infected HCWs and patchy reticular changes subpleural areas in two (14%). Importantly, ground-glass opacification and/or consolidation stably resolved in 10 (71%) infected HCWs on serial CT images during hospitalization; however, such abnormalities demonstrated a deterioration for a couple of days and then an improvement in 4 (29%) infected HCWs. Bilateral pleural effusion appeared in one (7%).
Fig. 3.
Pulmonary CTs of two suspected infected HCWs with COVID-19 pneumonia. Transverse chest CT images from Patient 13 (A) showed patchy consolidation in the left lower lobe of lung, (B) the density of consolidation decreases, showing as ground-glass opacity, (C) lesion completely resolved; Transverse chest CT images from Patient 14 (D) showed patchy shadows and ground glass density in the middle lobe of the right lung, (E) the lesion was reduced in the middle lobe of the right lung, and newly small patchy shadows appeared in the left lower lobe of the lung, (F) the lesions in the middle lobe of the right lung and the lower lobe of the left lung were almost resloved, showing as ground-glass opacity.
During hospitalization, all these 14 HCWs received antibiotic and antiviral treatments. The antiviral treatment they took were arbidol (200 mg tid) and recombinant human interferon α2b (5 million U each time for adults, adding 2 ml of sterilized water, atomization inhalation twice daily). Three infected HCWs needed oxygen inhalation through nasal tube. None of these infected HCWs were given systematic corticosteroids, non-invasive ventilation, or ICU admission. As of February 26, all the 14 HCWs had discharged and none of them died.
Discussion
In the beginning of the outbreak of COVID-19 in China, COVID-19 patients increased substantially and HCWs were a high-risk group to get infected. Of the 138 admitted patients in Zhongnan Hospital from Wuhan during January 1 to January 28, 40 patients were health care workers.14 Here, we describe a cluster of COVID-19 infected HCWs who worked in the same department.
In the present study, when the first index patient was hospitalized for pituitary adenoma resection, he did not have fever or any respiratory symptom, his chest X-ray did not shown any signs of virus pneumonia on admission. Thus he was not suspected to be a COVID-19 patient. Eleven days after hospitalization, he accepted the pituitary adenoma resection and had a fever of 38 °C after the surgery. Postoperative fever was considered to the cause of the patient's fever. However, five days after the fever onset, the patient had a chest CT scan with typical signs of viral pneumonia. Meanwhile, another patient who lived in the same ward had fever, too. Further, several days later, the two patients were confirmed to be COVID-19 patients with positive results for SARS-CoV-2 from throat swab and transferred to isolation wards. However, during their stay in the department of neurosurgery, especially before the day of the first patient's chest CT scan, HCWs did not have enough personal protective equipment. Sometimes, they weared nursing mask but not the surgical mask, when they contact these two patient, as we did not know that was a COVID-19 patient at that time and the infectiousness of the SARS-CoV-2 was also underestimated. Moreover, HCWs also contacted each other in daily work and meetings, went to other departments in this hospital (where there were COVID-19 patients possibly), without wearing any masks. Among all the HCWs in this department, twelve nurses and two doctors had been identified to be COVID-19 infected HCWs. Besides exposure to the infected patients, exposure to the infected colleague maybe another important reason for the infection of SARS-CoV-2 in HCWs.
During the outbreak of COVID-19 in China, the Chinese government had taken containment measures to reduce new cases. From 00:00 to 24:00 March 18, 2020, there were none any new confirmed cases in Hubei Province, China.10 As of March 1, 2020, China had dispatched 42322 heath care workers from 30 provinces and municipalities to reinforce the medical treatment in Hubei province.10 Encouragely, none of these 42322 HCWs were infected with SARS-CoV-2, as of March 18, 2020.10 Proper personal protective equipment including a gown, gloves, N95 respirator, a face shield or goggles, protected HCWs from infection.
None of these 14 infected HCWs were admitted to ICU or dead. One explanation might be that all these HCWs with COVID-19 pneumonia were young people and mild cases. Another explanation might be that our infected HCWs did not had significant underlying chronic diseases. In the largest case series to date of COVID-19 in mainland China (72,314 cases, updated February 11, 2020), no deaths were reported in mild and severe cases.11 But in critical cases, the overall case-fatality rate (CFR) was 49.0%.11 CFR was increased in those with underlying diseases, such as cardiovascular disease, diabetes, chronic respiratory diseas, hypertension and cancer.11
We noticed that IL-6 was significantly elevated in 86% of the infected HCWs and complement C3 was decreased in 57% of them. It indicated that a dysregulated and excessive host inflammatory response may exist in COVID-19 pneumonia.15 As a multieffective cytokine with anti-inflammatory and pro-inflammatory effects, IL-6 plays an important role in cytokine release syndrome of COVID-19 patients.15 Blocking the signal transduction pathway of IL-6 maybe a promising method for the treatment of COVID-19 patients.16 A latest small single-centre study has revealed the effectiveness of Tocilizumab (IL-6R blocker) in treating COVID-19 patients.16 However, multicenter randomized controlled trial is still needed to document the effectiveness of Tocilizumab in COVID-19.
The proportion of lymphocytes subsets were within normal range in these 14 infected HCWs, including CD4+T cells and CD8+T cells. Published studies indicated that CD4+T cells and CD8+T cells decreased significantly in severe cases, as compared with mild cases.17 , 18 All these 14 infected HCWs were mild cases, it might be the reason that the proportion of lymphocytes subsets did not exceed the normal range, as well as the concentration of immunoglobulins, complement C4, IL-2, IL-4, IL-10, TNF-α and IFN-γ in this small case series.
It was quite reasonable to consider that some of the HCWs were infected by the two index patients, as they did not contact any other COVID-19 patients. However, the laboratory-confirmed evidence of SARS-CoV-2 transmission chain among them was not available yet. Another limitation of this study was the small number of cases. Observation with a large number of infected HCWs is needed to document the route of nosocomial transmission.
Conclusions
Our current study demonstrated a cluster of HCWs in the same department due to unsufficient personal protective equipment. Future studies are necessary to determine what is the best protocol to protect HCWs from infected with SAR-CoV-2 while they contact the COVID-19 patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81973990) and the Fundamental Research Funds for the Central Universities, HUST COVID-19 Rapid Response Call (No. 2020kfyXGYJ030). All these funding bodies had no impact in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Authors' contribution
QZ and HZS conceived the idea, designed and supervised the study, had full access to all data and took responsibility for the integrity of the data. XSW, XRW, JCZ, and BHY. collected and analyzed the clinical and laboratory data. NCJ evaluated pulmonary computed tomographic images. WBY, WLM, and ZCG analyzed data and performed statistical analysis. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We are grateful to all who participated in this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2020.04.013.
List of Abbreviations
- COVID-19
Corona Virus Disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- HCWs
health care workers
- CT
computed tomography
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke R.M., Midgley C.M., Dratch A., Fenstersheib M., Haupt T., Holshue M. Active monitoring of persons exposed to patients with confirmed COVID-19 - United States, january-february 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO main website. 2020. https://www.who.int [Google Scholar]
- 10.National Health Commission of the People's Republic of China. 2020. http://www.nhc.gov.cn/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance [Google Scholar]
- 13.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.