Abstract
Unhealthy diet, lack of exercise, psychosocial stress and insufficient sleep are increasingly prevalent modifiable risk factors for cardiovascular disease. Accumulating evidence indicates that these risk factors may fuel chronic inflammatory processes that are active in atherosclerosis and lead to myocardial infarction and stroke. In concert with hyperlipidemia, maladaptive immune system activities can contribute to disease progression and increase the probability of adverse events. In this review, we discuss recent insight into how the above modifiable risk factors influence innate immunity. Specifically, we focus on pathways that raise systemic myeloid cell numbers and modulate immune cell phenotypes, reviewing hematopoiesis, leukocyte trafficking and innate immune cell accumulation in cardiovascular organs. Often, relevant mechanisms that begin with lifestyle choices and lead to cardiovascular events span multiple organ systems, including the central nervous, endocrine, metabolic, hematopoietic, immune and, finally, the cardiovascular system. We argue that deciphering such pathways provides not only support for preventive interventions but also opportunities to develop biomimetic immunomodulatory therapeutics that mitigate cardiovascular inflammation.
Keywords: atherosclerosis, hematopoiesis, macrophages, lifestyle, arteriosclerosis, inflammation, sleep, stress, metabolism, bone marrow
Subject Terms: Atherosclerosis, Lifestyle, Risk Factors
Introduction
Our highly dynamic understanding of atherosclerosis has generated certainty: the involvement of an overactive and maladaptive immune system is no longer debated1–4. Indeed, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), which reduced cardiovascular events by targeting the inflammatory cytokine interleukin-1β5, 6, marks the end of the era during which the role of inflammation was emerging. With the notion that dampening inflammation may reduce cardiovascular events now widely accepted, the field is entering a new phase of discovery, with the goal of translating this insight into immunomodulatory cardiovascular therapeutics with acceptable safety profiles. A key challenge is to find therapeutic targets that reduce harmful inflammation without compromising host defense and immune system function. It may take considerable time and resources to meet this and other challenges, but with a growing understanding of the complexity and heterogeneity of the immune system and rapidly improving research tools, we are now identifying previously unknown mechanisms and pathways linking inflammation and atherosclerosis. The motivation for this work could not be more clear: atherosclerosis and its complications are still the leading cause of mortality world-wide7.
It has long been evident that lifestyle vastly affects cardiovascular health8–15. Five years ago, we argued that it may be worthwhile to probe how lifestyle affects cardiovascular inflammation16. Meanwhile, the strong connections between lifestyle-related risk factors and the immune system have become increasingly clear, as has these connections’ influence on cardiovascular health. We here revisit this argument to review new data, discuss emerging opportunities and highlight promising areas that require further investigation. Specifically, we discuss how diet, sleep, psychosocial stress and physical activity influence systemic numbers and phenotypes of innate immune cells, as well as their production and supply to atherosclerotic lesions.
Risk factors and importance of lifestyle for atherosclerosis
The growing understanding of how specific cardiovascular risk factors lead to the development and progression of atherosclerosis has enhanced our ability to assess each individual patient’s risk more precisely. Epidemiological studies have identified the relative importance of atherosclerotic risk factors, which can be categorized into non-modifiable and modifiable. Non-modifiable factors include age, sex, ethnicity and hereditary factors. Modifiable risk factors include smoking, hypertension, obesity, diabetes, high levels of low-density lipoprotein, low levels of high-density lipoprotein and increased cholesterol and triglycerides17. The prevalence and potency of these risk factors vary. Often, risk factors act in concert to promote atherosclerosis, which is a multifactorial disease. Delving into risk factors’ precise contributions to and mechanistic roles in the pathogenesis of atherosclerosis has fueled important therapeutical advances18, such as HMG-CoA reductase inhibitors that lower low-density lipoprotein cholesterol19, 20 and antihypertensive drugs that reduce blood pressure21–24, both of which potently inhibit atherosclerotic progression and reduce its complications. A lifestyle-related example is smoking, which was estimated to contribute to approximately one third of coronary deaths in the 1980s25.
In addition to these well-established risk factors, we recognize that certain lifestyle choices such as a sedentary behavior26 and exposure to psychosocial stress27, 28, air pollution29 and environmental noise30 associate with increased CVD risk. Several large-scale cohorts, including a prospective observational study in 20,721 Swedish men, demonstrated that adhering to a healthy lifestyle associated with a 79% reduction (95% CI: 34% to 93%) in the incidence of myocardial infarction31–33, and another study with 461,211 participants reported a hazard ratio of 0.61 (95% CI: 0.56 to 0.66) for ischemic stroke12, 34. Such health benefits likely also extend to high-quality sleep35, 36, regular physical activity14 and a healthy diet37–39. Mechanisms by which lifestyle exerts beneficial effects are likely manifold and remain incompletely understood; we argue that protective mechanisms likely involve the immune and hematopoietic systems.
Hematopoiesis in CVD
Hematopoiesis is the process by which blood cells are continuously replenished. This life-long renewal process is well regulated and able to respond to increased demands, as when blood cells are lost due to bleeding or infection. Hematopoietic stem cells (HSCs) form the apex of the differentiation cascade and are unique in their ability to self-renew and diversify into any blood cell. They reside almost exclusively in the bone marrow and are relatively rare and mostly quiescent40–43. HSC give rise to various downstream hematopoietic stem and progenitor cells (HSPC) that proliferate at higher frequencies than HSC and are more restricted to distinct lineage differentiation potentials44. This process of differentiation and self-renewal is tightly regulated by a complex interplay of cell-intrinsic and microenvironmental factors in order to maintain hematopoietic homeostasis45. Within the bone marrow, there are special localized areas with a highly regulated microenvironment referred to as the ‘stem cell niche’. These niches are formed by several stromal cells, including endothelial cells, osteoblasts, macrophages and various mesenchymal stromal cells, which secrete messengers known as ‘niche factors’. In concert with growth factors (e.g., M-CSF, GM-CSF), niche factors such as the cytokines Cxcl12 and Scf1 regulate HSPC proliferation and maintenance. Other molecules, e.g. angiopoietin-1, thrombopoietin and osteopontin, contribute to HSPC maintenance by binding to receptors expressed by these cells46–50. Additionally, adhesion molecules like Vcam-1 and E-selectin retain HSPC and leukocytes in the bone marrow51. Once committed, myeloid progenitor cells differentiate into monocytes and neutrophils. Their release into circulation is rigidly controlled by various signals, including the Ccr2 ligand Ccl2 and the Cxcr4 ligand Cxcl12. Hematopoiesis and leukocyte trafficking between tissues and circulation are influenced by circadian rhythms52–58. For further reading on how the circadian rhythm controls hematopoiesis, we refer to several excellent reviews54, 59–61.
The hematopoietic system responds sensitively to cardiovascular events such as myocardial infarction, after which bone marrow myeloid progenitors and monocytes expand62 and circulating leukocytes are recruited to the ischemic myocardium63, 64. After stroke, myelopoiesis increases while the hypothalamic-pituitary-adrenal axis mediates B lymphopoiesis defects65–70. Myeloid cells recruited to the injured brain may contribute to neural recovery but also to reperfusion injury71, 72. Atherosclerosis leads to low-grade, systems-wide chronic inflammation during which, as described in ApoE−/− mice with atherosclerosis, blood monocyte levels increase progressively73–75.
The increased bone marrow activity in CVD centers on myelopoiesis and is elicited by an expanding number of signals. Hyperlipidemia is prominently among these, acting directly on HSPC and leukocytes via cholesterol efflux pathways76, 77. For example, ABCA1, ABCG1 and HDL, which clear cholesterol from macrophages, inhibit HSPC proliferation, reducing the production of atherogenic leukocytes77. Experimentally, Abca1−/− and Abcg1−/−deficient mice exhibit leukocytosis and increased numbers of stem and progenitor cells, thus exaggerating atherogenesis77. The mechanistic links connecting hypercholesterolemia with hematopoiesis were clarified by studies reporting that apoA-I binding protein (AIBP), which mediates cholesterol efflux, controls HSPC activity76, 77. AIBP activates Srebp2, which is a transcription factor relevant for cholesterol synthesis76. Srebp2 up-regulates Notch signaling, thereby enhancing hematopoietic stem cell expansion. In human blood, LDL cholesterol correlates with HPSC numbers and Srebp2, and HPSC from individuals with high LDL cholesterol had higher Notch gene expression than HSPC from individuals with low LDL cholesterol76. In mice, Srebp2 inhibition can prevent HSPC emergence. These studies76, 77 link hypercholesterolemia with hematopoiesis in atherosclerosis and identify cholesterol efflux pathway genes as key regulators of hematopoiesis. For a more detailed overview of how atherosclerosis influences hematopoiesis and the bone marrow niche, we refer to a related review78.
Immune cells in cardiovascular pathology
Macrophages, white blood cells that are part of the innate immune system, are found in large numbers in all healthy organs, where they closely interact with their surroundings79, 80. During homeostasis, macrophages self-renew through local proliferation, with only a small proportion arising from monocyte recruitment. Macrophages’ primary functions involve removing debris and pathogens. Over the past decade, it has become clear that macrophages pursue many additional functions, which depend on their origins, microenvironment and phenotype. For example, macrophages support cardiac conduction by interacting with cardiomyocytes81. Yolk sac-derived embryonic macrophages promote coronary artery development82 and aortic LYVE-1+ macrophages moderate steady-state arterial tone by interfacing with with smooth muscle cells and collagen83. These tasks may be disrupted during inflammatory conditions such as myocardial infarction and atherosclerosis, when local resident macrophages die84 and inflammatory, monocyte-derived macrophages engage with surrounding stromal and immune cells, secreting proinflammatory molecules that contribute to tissue repair or destruction85. After myocardial infarction, tissue-resident macrophage numbers and phenotypes also shift in remote, uninjured organs such as the lung, liver, brain and kidney86. Other systemic inflammatory conditions, particularly sepsis, likewise evoke systemic macrophage adaptations that may affect cardiovascular health86. It is largely unknown how lifestyle factors shape resident leukocytes in tissue.
The pathogenesis and progression of atherosclerosis are closely linked to inflammatory processes within the arterial wall, where modified lipoproteins are rendered proinflammatory and activate the overlying endothelium87. The consequence is a chronic, low-grade immune response that recruits additional leukocytes, including monocyte-derived phagocytes, into the subendothelial space. During atherosclerosis development, increased levels of circulating LDL cholesterol lead to vascular wall deposits, which are taken up by macrophages that then become foam cells. These cholesterol-laden plaque macrophages do not readily remove the lipid material from the vascular wall. Rather, these cells accumulate locally, enhance inflammatory processes and eventually die, contributing to the formation of the necrotic core often seen in ruptured plaques. In healthy and diseased mice, the arterial macrophage population is heterogeneous and consists of several subtypes that vary in function and gene expression profile2, 87–89. Although some data suggest some lesional macrophage-like cells may arise from smooth muscle cells90, 91, most macrophages stem from hematopoietic progenitors in the bone marrow and spleen73–75. Plaque macrophages’ comparatively short four-week lifespans, measured in mice with atherosclerosis92, necessitate continuous replenishment. Macrophages in early atherosclerotic lesions almost all derive from recruited monocytes, while in later lesions most arise from local macrophage proliferation, though their local ancestry still descends from monocytes92. In the lesion, macrophages accumulate progressively and adopt phenotypes that fuel inflammatory processes, leading to tissue destruction93, 94. These inflammatory macrophages can activate their surroundings, include tissue-resident stromal and other immune cells, and release inflammatory chemokines and cytokines, which recruit more leukocytes to the lesion. In the long run, macrophages contribute to plaque destabilization by amplifying local inflammation and secreting various proteases that destabilize fibrous components of the plaque.
Other immune cells, including neutrophils and lymphocytes, also inhabit the atherosclerotic plaque and contribute to its growth and destabilization. Because lesional neutrophils are relatively rare, their contributions were long underestimated95. Recent studies, however, have demonstrated their presence and role in the pathogenesis of atherosclerosis96–98. For instance, neutrophils can deposit granule proteins (e.g., proteinase-3, azurocidin, cathepsin G, neutrophil elastase) on the endothelium, impairing endothelial function, inducing adhesion and promoting inflammatory monocyte recruitment99–103. The cells promote erosion of the endothelium104 via neutrophil extracellular traps. Lesional neutrophils may activate plaque macrophages and shift their phenotype towards a more inflammatory state105, 106. Additionally, neutrophils can render the plaque more vulnerable by exhibiting myeloperoxidase-dependent oxidative stress107, 108 and by secreting matrix metalloproteinases (e.g., MMP2, MMP8 MMP9) that degrade endothelial layers109–111. The presence and function of lymphocytes in atherosclerotic lesions have been well studied112–114, and we refer the interested reader to reviews on that subject115–117. Despite our detailed understanding of these processes, translational success in the form of clinical immunotherapy targeting plaque leukocyte numbers, phenotypes or functions in atherosclerosis has not yet been realized. We believe that a better understanding how risk and lifestyle factors modulate cellular immunity may provide new therapeutic points of attack, hopefully with a similar impact as the research on hypercholesterolemia which led to the development of statin therapy.
Diet, metabolism and obesity influence innate immunity
In the U.S., obesity is increasingly prevalent in both adults and children and is now a major public health concern118, 119. Obesity contributes to dyslipidemia, insulin resistance, diabetes, hypertension, sleep apnea and metabolic syndrome, all of which increase the risk of ischemic events120, 121. Abdominal obesity, in which excessive fat accumulates in the visceral area, has particularly strong links to cardiovascular disease122. For further reading on the connections between obesity and cardiovascular disease, we refer to several excellent reviews123–125.
Healthy diets, such as the Mediterranean diet, may associate with reduced cardiovascular events38. A recent randomized multi-center trial of 7,447 participants revealed that the hazard ratio for major cardiovascular events was 0.69 (95% confidence interval, 0.53 to 0.91) for participants consuming a Mediterranean diet with extra-virgin olive oil supplementation as compared to a control diet38. In contrast, consuming red meat may promote the progression of atherosclerosis126, 127. Saturated fat, sodium and preservatives in red meat have been attributed to elevated blood pressure and cholesterol levels. Bacterial metabolism of dietary L-carnitine, a trimethylamine abundant in red meat, produces trimethylamine N-oxide (TMAO). This microbiotic metabolite impairs reverse cholesterol transport in macrophages126, 127 and expands inflammatory monocyte numbers128, thus aggravating atherosclerosis.
Intermittent fasting, among other health-promoting diets, are currently receiving attention for their potential beneficial effects on cardiovascular health. Specifically, intermittent fasting lowers blood glucose129, 130, triglycerides131, cholesterol132, 133, blood pressure131, 134, 135 and circulating proinflammatory cytokines136. Short-term fasting reduces circulating monocyte numbers and their metabolic and inflammatory activity137. Healthy humans and mice exposed to 4h and 20h of fasting had fewer circulating monocytes while neutrophils remained unchanged. These effects were mediated by dietary carbohydrate and protein levels but not triglycerides, highlighting the importance of specific dietary components. During fasting, circulating monocytes are retained in the bone marrow, while their cell death and production rates appeared to be unaffected. Blocking glycolysis similarly decreased circulating monocyte numbers, suggesting this metabolic pathway is one driving factor. Indeed, fasting in mice with hepatocyte-specific loss of AMPK or PPARα, both involved in nutrient sensing, did not reduce circulating monocyte numbers. Fasting in humans and mice also lead to reduced Ccl2 plasma levels. The chemokine Ccl2 is a major regulator of monocyte migration from the bone marrow to inflammatory sites138. Intriguingly, fasting in PPARα-deficient mice did not curtail circulating Ccl2, thereby highlighting the mechanistic role of hepatic metabolic pathways. In the liver, fasting affected cytokines and chemokines that regulate Ccl2 production, revealing that hepatic metabolic pathways have a previously unknown function in regulating inflammation. Re-feeding mice after hours of fasting normalized circulating monocyte numbers, indicating that diet-induced shifts in monocytes are only temporary. This study137 directly links dietary glucose intake with innate immune cell trafficking and demonstrates the interconnections among hematopoietic organs, metabolism and systemic inflammation. The authors also clarified that fasting does not compromise monocyte emergency mobilization during acute infection; however, it remains unclear if fasting affects emergency hematopoiesis, i.e. leukocyte production during host defense or tissue repair.
Other work demonstrated that during caloric restriction, defined as 50% reduced food intake, memory T cells depart from secondary lymphoid organs and accumulate within the bone marrow in a process coordinated by glucocorticoids and bone marrow adipocytes139. During caloric restriction, circulating glucocorticoids levels increased, promoting memory T cell death in peripheral lymphoid organs, blood and adipose tissue. T cells accumulated in the bone marrow and assumed a state of energy conservation, resulting in protection against cell death. The authors observed that caloric restriction triggered bone marrow remodeling, as reflected by increased numbers of T cell-recruiting factors, erythrocytes and adipocytes139. These mechanisms could be interpreted as strategies to maintain immune function during times of food restriction by redistributing immune cells to different compartments in which they are protected from diet-induced stress.
Both the studies detailed above reveal that caloric intake controls tissue immune tone and link dietary habits to immunity. This research demonstrates the close interplay between metabolism and immunity140 and should motivate further efforts to explore how the metabolic-immune axis controls immune cell trafficking and production. It is tempting to speculate that chronic intermittent fasting affects the magnitude of immune cell responses. A number of interesting questions arise: What happens to the immune system during fasting in obese individuals? Does fasting positively affect atherosclerotic lesion size or phenotype by reducing monocytosis and lesional macrophage numbers? Does fasting affect the bone marrow niche and hematopoiesis?
There is growing interest in understanding how organ-specific metabolic sensors affect systemic metabolic processes, especially those relevant to obesity’s development and complications. A recent study described that T cells may serve as such sensors141. Integrin β7+ natural gut intraepithelial T lymphocytes (natural IELs) can fine-tune systemic metabolism. Integrin β7−/− mice, which are deficient in natural IELs, are metabolically hyperactive and protected against obesity, hypercholesterolemia, hypertension and diabetes when fed a high-fat diet. Further, Integrin β7−/− mice developed smaller atherosclerotic lesions in the aorta, with reduced plaque macrophage numbers, due to an overall improved metabolic state. Mechanistically, the study showed that these protective properties were driven by IELs’ ability to control the availability of GLP-1, a potent incretin hormone141. This study describes an immuno-metabolic checkpoint that functions as a nutrient sensor in the gut and mediates systemic metabolic activity, thereby affecting cardiovascular health.
Obesity is known to be associated with Ccl2-dependent infiltration of monocytes and macrophages into adipose tissue142–144, a phenomenon that contributes to the development of insulin resistance and diabetes142, 143. Unbiased cell profiling of leukocytes in adipose tissue maps the heterogenous immune cell landscape with increasing resolution, identifying macrophage subsets that regulate adipocyte size and function145. In mice and humans, obesity leads to monocytosis and neutrophilia with increased numbers of bone marrow myeloid progenitors146–148. Transplantation experiments of adipose tissue into lean recipient mice confirmed an adipose tissue-driven elevation in bone marrow myeloid progenitor numbers147. Weight loss in both mice and humans reduced monocytosis and neutrophilia147. Mechanistically, adipose tissue-derived S100A8/A9 promoted tissue macrophages to express Tlr4 and produce Il-1β, which signaled to Il-1 receptor-expressing bone marrow myeloid progenitor cells and increased neutrophil and monocyte production147. Within human bone marrow, adipocytes are among the most abundant stromal cells149, 150 and evidence of their putative roles in regulating hematopoiesis is currently emerging. Recent studies have shown that bone marrow adipocytes are a relevant source of stem cell factor (Scf) and that adipocyte-specific Scf deletion reduces HSC numbers151, 152. Interestingly, in mice, Scf-deletion in adipocytes more potently affected hematopoiesis in the tail vertebrae than in long bones, such as the femur, which contain fewer adipocytes152. This supports the interesting yet not well studied concept of bone marrow heterogeneity and should be investigated further.
Another rapidly growing area of study that connects dietary habits and cardiovascular disease focuses on trained immunity, i.e. the process by which epigenetic reprogramming of innate immune cells induces memory within the innate immune system153. A recent study146 confirmed previous reports77, 154 that hyperlipidemia engenders systemic inflammation via increased hematopoiesis, adding an intriguing mechanistic angle related to trained immunity in HSPC146. Western diet feeding of Ldlr−/− mice created long-lasting epigenetic reprograming in granulocyte-monocyte progenitors (GMP), changes that affected their function even four weeks after terminating high-fat diet. This reprogramming was NLRP3-dependent, as high-fat diet in Nlrp3−/−/Ldlr−/− mice did not induce leukocytosis or GMP activation and resulted in smaller atherosclerotic lesions compared to Ldlr−/− mice146. This study identified the NLRP3 inflammasome as a key mediator of high-fat diet-induced systemic inflammation and expanded epigenetic reprogramming in the context of trained immunity to hematopoietic progenitors.
These studies are specific examples and in no way represent the entire wealth of work on how diet and metabolism affect the immune and hematopoietic systems (please see a recent, more comprehensive position paper140). However, these studies converge on the idea that diet profoundly affects immunity and thus inflammatory diseases such as atherosclerosis.
Sleep
Although sleep is an essential part of life, epidemiological studies show that more than half of adults in the United States sleep fewer than the recommended eight hours a day155. Sleep can be impaired by either insufficient duration, representing quantity, or insufficient depth, representing quality. Fewer than seven hours of sleep are considered insufficient, and the consequences of acute sleep deprivation include reduced general alertness and cognitive performance, impaired short- and long-term memory156 and diminished decision-making abilities. However, in healthy middle-aged men, one night of quality sleep was able to restore cognitive performance deficits resulting from insufficient acute or chronic sleep157. Long-term lack of sleep has detrimental effects on health and associates with increased risk of obesity158, diabetes and impaired glucose tolerance159, cancer160, cardiovascular disease35, 161–163 and anxiety and depression163, 164. A cross-sectional study including 30,397 participants revealed that participants who sleep <5 hours per night had an odds ratio of 2.2 (95% confidence interval, 1.78–2.71) for developing cardiovascular diseases162. Participants sleeping > 9 hours also had increased risk, with an odds ratio of 1.57 (95% confidence interval, 1.31–1.89)162.
Despite mounting evidence for links between lack of sleep and disease, little is known about the mechanisms underlying this connection. Studies investigating the effects of sleep deprivation on cardiovascular health36 have been done in patients with sleep apnea, a chronic condition that affects about 4% of the population in the United States165. Sleep apnea is characterized by frequently interrupted breathing, mostly due to upper airway collapse during sleep166–168. Patients suffer from multiple arousals per night, resulting in fragmented sleep. This is associated with higher coronary169, 170 and cerebrovascular171 morbidity and mortality. Sleep apnea patients have elevated plasma levels of inflammatory cytokines including TNF-α172, IL-6173, IL-8172 and C-reactive protein173 compared with either an obese-only control group or healthy individuals. Individuals with sleep apnea also have higher circulating leukocyte counts174–176; however, whether this is due to increased hematopoiesis remains unclear. Another potentially important mechanistic factor in sleep apnea is that during arrested breathing, blood oxygen drops, resulting in multiple hypoxic events that stimulate the sympathetic nervous system. This complex scenario makes it challenging to distinguish the discrete contributions of sleep fragmentation, recurrent hypoxia and stress to the pro-inflammatory conditions observed in sleep apnea patients.
A recent study on sleep fragmentation’s effect on innate immunity and atherosclerosis provides some clarity (Figure 2)177. Mice subjected to sleep fragmentation had higher bone marrow hematopoietic stem cell numbers and more circulating myeloid cells. Hypocretin, a neuropeptide that regulates arousal, wakefulness and appetite, is produced in the lateral hypothalamus and was reduced in the plasma and bone marrow of mice subjected to sleep fragmentation. Circulating hypocretin suppressed hematopoiesis and atherosclerosis, as Hcrt−/− mice had more hematopoietic stem cells in their bone marrow and larger atherosclerotic lesions. Parabiosis of wildtype and Hcrt−/− mice did not result in increased hematopoiesis in Hcrt−/− parabionts, as circulating wildtype hypocretin sufficiently suppressed hematopoiesis. Mechanistically, circulating hypocretin signaled to hypocretin receptor-expressing pre-neutrophils in the bone marrow, controlling their CSF1 production and, consequently, HSPC proliferation. Bone marrow transplant experiments revealed that hematopoietic knockout of Hcrtr1, including in pre-neutrophils, elevated hematopoiesis by impairing hypocretin signaling suppression. Sleep-fragmented Ldlr–/– mice fed a high-fat diet over 12 weeks had larger atherosclerotic plaques with higher lesion macrophage numbers than Ldlr–/– mice with uninterrupted sleep. Finally, sleep-fragmented mice with hematopoietic Csf1 deletion did not show increased hematopoiesis or atherosclerosis. Intriguingly, hypocretin supplementation in mice was able to counteract sleep fragmentation-induced hematopoiesis and atherosclerosis, possibly offering novel therapy options177. This study described a neuro-immune axis in which sufficient sleep sustains circulating hypocretin levels that constrain hematopoiesis, circulating innate immune cells and thus atherosclerosis.
Fig. 2. Sleep controls hematopoiesis and protects against atherosclerosis.
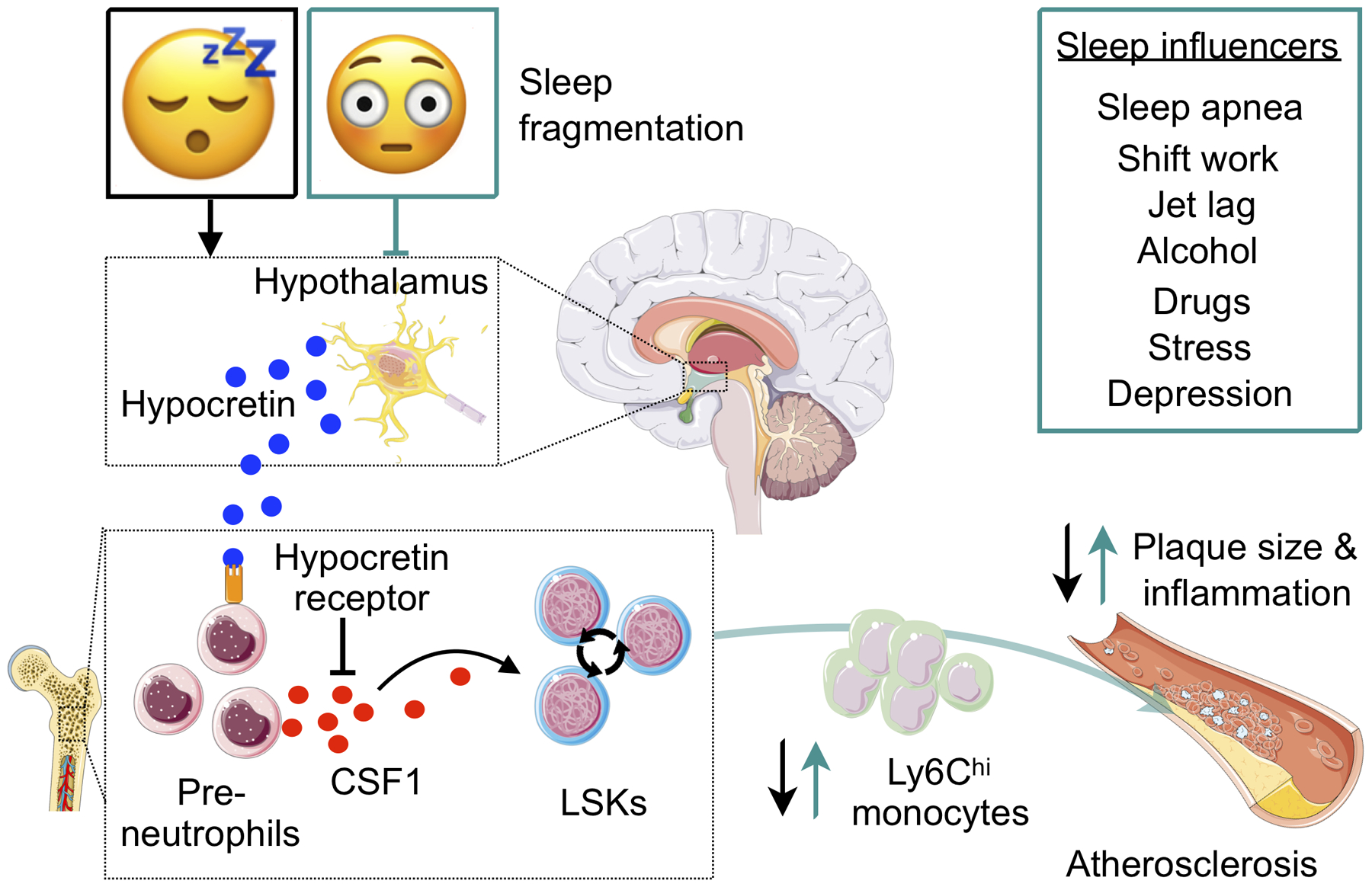
The cartoon illustrates how sleep influences hematopoiesis and moderates leukocyte supply to the atherosclerotic lesions. Sleep induces hypocretin release from the lateral hypothalamus, which circulates to the bone marrow and reduces CSF1 secretion from hypocretinreceptor-expressing pre-neutrophils. Sleep fragmentation leads to decreased release of hypocretin, heightening bone marrow CSF1 levels. CSF1 stimulates hematopoietic stem and progenitor cell proliferation, resulting in increased supply of inflammatory leukocytes. The cartoon was adapted from McAlpine et al. (ref177). CSF1 indicates colony stimulating factor 1.
Circadian rhythms also affect the hematopoietic and immune systems, and therefore cardiovascular heath. Sleep is a major entrainment factor for circadian rhythm, and irregular sleeping habits, e.g. those experienced by shift workers, lead to misalignment with the central clock, rendering individuals at higher risk for cardiovascular events178. It has long been observed that cardiovascular events, such as stroke and myocardial infarction, exhibit circadian incidences and peak in the early morning hours179, 180. Indeed, myocardial infarction during that time of day correlates with worsened outcomes137, 181, 182. Animal studies have demonstrated that an overactive immune response at the beginning of the active phase impairs cardiac wound healing and function after myocardial infarction57, 58, 183. Although the development of atherosclerosis is a chronic process, a recent study suggests that diurnal myeloid cell invasion into the arterial wall could perpetuate atherosclerosis progression184. The authors show in atherosclerotic mice that monocyte and neutrophil recruitment to plaque lesions varies by time of day, peaking during the transition from active to resting phase. This circadian recruitment pattern depends on Ccl2 production by neutrophils, monocytes, B cells and T cells. In support of this mechanistic link, systemically blocking Ccl2 or genetically deleting Ccr2 abrogated the temporal aspect of leukocyte recruitment to atherosclerotic lesions. Intriguingly, daily application of a Ccr2 antagonist specifically at the beginning of the active phase reduced monocyte and neutrophil adhesion to the microcirculatory endothelium, consequently decreasing plaque size and lesional macrophage numbers184. This study indicates that daily leukocyte shifts between circulation and arteries contribute to atherosclerotic growth, and anti-inflammatory strategies applied at a specific time of day may prevent atherosclerosis progression.
During steady state, the circadian rhythm regulates bone marrow hematopoiesis through adrenergic signals transmitted to Nestin+ stromal cells by the β3-adrenergic receptor, leading to rapidly regulated Cxcl12 levels53, 54, 185. In humans and mice, circulating leukocytes, particularly Ly6Chigh monocytes55, 58, oscillate throughout the day and traffic between tissues and blood in a process regulated by chemokines and adhesion molecules expressed by leukocytes and tissue endothelial cells respectively55–58, 186–188. During the resting phase (i.e. daytime as mice are active at night), aged Cxcr4high neutrophils return to the bone marrow, where they are cleared by macrophages that secrete feedback signals to the bone marrow niche, thereby regulating hematopoiesis and leukocyte egress189. These pathways are likely affected by lack of sleep and sleep fragmentation, including experiences such as shift work and jet lag. Conversely, sufficient and uninterrupted sleep will restore these biological rhythms.
Psychosocial stress
Acute and chronic stress are well-recognized risk factors for the development of obesity, diabetes and hypertension, all of which give rise to cardiovascular diseases, particularly atherosclerosis190–193. Acute stress, as is experienced during earthquakes, the World Cup soccer championships or short-term experimental stressors, increases the risk of myocardial infarction and pulmonary embolism27, 194. Chronic stressors, such as post traumatic stress disorder in veterans195, job loss or divorce, lead to increased risk for cardiovascular events. Chronic psychosocial stress also often overlaps with confounding unhealthy lifestyle choices including smoking, alcohol consumption, irregular sleeping habits and a sedentary lifestyle196. Stress has multiple, interdependent impacts on health.
Recent work has shed light on how stress affects immunity and hematopoiesis. There are two major pathways through which stress activates immunity: the limbic-hypothalamic-pituitary-adrenal axis and the sympathetic-adreno medullar axis197. Behavioral and physical stress activate the limbic-hypothalamic-pituitary-adrenal axis, initiating a hormone release cascade that includes glucocorticoid secretion from the adrenal glands197. Fear and other stressors are processed by the amygdala and hypothalamus, triggering the sympathetic nervous system and raising levels of systemic catecholamines (e.g. epinephrine, norepinephrine)198. Circulating catecholamines, especially noradrenaline, signal through α- and β-adrenergic receptors expressed by bone marrow stromal53, 185 and most immune cells199, including lymphocytes, myeloid and hematopoietic progenitors.
In the bone marrow, sympathetic nerve fibers along the arteriolar vasculature innervate Nestin+ stromal cells, which are part of the hematopoietic niche and express β3-adrenergic receptors. In steady state, sympathetic nerve fibers secrete norepinephrine, which decreases Cxcl12 production in Nestin+ stromal cells53, 54, 185, 200. Cxcl12 is a cytokine that retains stem and progenitor cells and Cxcr4-expressing neutrophils within the marrow and promotes HSC quiescence, among many other functions. Stress-related mechanisms of sympathetic bone marrow innervation are not always harmful, as recently described by a study demonstrating that sympathetic bone marrow signaling counteracts the declining hematopoietic differentiation potential that comes with hematopoietic aging201. The authors showed that loss of sympathetic nerves or adrenergic β3-receptor signaling in the bone marrow leads to premature hematopoietic aging in young mice. Intriguingly, treatment with a sympathomimetic drug that selectively activates the adrenergic β3-receptor was able to reverse hematopoietic stem cell aging in old mice201. These findings reveal that the sympathetic nervous systems plays a previously unidentified beneficial role in maintaining bone marrow niche function.
Chronic psychosocial stress over several weeks produces monocytosis and neutrophilia in mice and humans202, 203. Stress and stress-induced hormones raise leukocyte levels across other species including fish, hen, rat and cattle204–208. Administering 5-fluorouracil, which eliminates proliferating progenitors, completely neutralized stress-induced leukocytosis in mice202, thus linking stress-induced leukocytosis with enhanced progenitor proliferation. Indeed, several murine studies have shown that stress increases hemopoietic stem and progenitor cell proliferation in and release from the bone marrow into the circulation, thereby accelerating atherosclerosis202, 209–212. Mechanistically, stress causes bone marrow sympathetic nerve fibers to release noradrenaline, which reduces Cxcl12 production by β3-expressing bone marrow stromal cells202. Treating stressed mice with β3-adrenergic receptor blocker abolished the stress-induced drop in Cxcl12 and curtailed hematopoiesis. Apoe−/− mice subjected to chronic stress over several weeks showed accelerated hematopoiesis leading to higher output of disease-propagating neutrophils and inflammatory monocytes, which promoted fibrotic cap thinning and plaque inflammation202. Taken together, these studies characterize a stress-sensitive neuro-immune axis between the sympathetic nervous system and bone marrow stromal cells that regulates hematopoiesis and affects cardiovascular health.
In support of these mechanistic data, a recent study linked activity in the amygdala, a brain region involved in processing emotions such as stress and fear, with cardiovascular disease risk213. The longitudinal study included 293 patients, 22 of whom had a cardiovascular event within the 3.7-year followup period. PET imaging revealed that enhanced amygdalar 18F-fluorodeoxyglucose activity correlated with increased bone marrow and arterial 18F-fluorodeoxyglucose signal and associated with elevated cardiovascular disease risk, with a hazard ratio of 1.59 (95% confidence interval, 1.27–1.98). Patients with high amygdalar PET signal had significantly decreased event-free survival compared to patients with low amygdalar PET activity. These observations align well with the concept of a neuro-immune axis in which the amygdala is a key structure linking fear and stress to immunity and cardiovascular disease.
The work summarized above indicates the need to enhance our mechanistic understanding of how the central nervous system interacts with the immune and hematopoietic systems. In particular, there is growing interest in resilience mechanisms, i.e. how the pro-inflammatory effects of psychosocial stress, which is often unavoidable, may be mitigated, especially with respect to inflammatory and cardiovascular pathways. To this end, it would be interesting, for instance, to modulate amygdalar activity in an experimental setting and decipher specific stress hormones’ effects on select leukocyte subsets, and their consequences for cardiovascular health. There are numerous additional questions that merit investigation: Which autonomic signals and hormones regulate leukocyte, progenitor and bone marrow niche function when the sympathetic nervous system is not activated by stress? How do lifestyle factors such as sleep, diet and exercise affect this balance, which likely influences hematopoiesis, immunity and cardiovascular health? Do exposure to pollution and environmental noise act on immune cells and their progenitors via stress-related or alternative pathways?
Physical inactivity and exercise
The beneficial effects of physical activity on health, particularly cardiovascular health, have been extensively studied. A sedentary lifestyle distinctly affects the immune system and raises atherosclerotic risk26, 214–216. A large-scale study including 55,137 participants investigated if regular physical activity in the form of leisure-time running was associated with lower cardiovascular mortality215. Indeed, the study found that individuals who ran as little as 51–80min per week had lower hazard ratios for cardiovascular mortality (0.56, 95% CI: 0.43 to 0.73)215. The potentially protective effects of regular exercise involve metabolic sequelae such as improving glucose tolerance217, insulin sensitivity218–221 and decreasing blood lipid concentrations222, thereby guarding against obesity214. Regular exercise can induce anti-inflammatory pathways223; however, the precise mechanisms of this effect remain unclear. Several studies have observed that regular exercise associates with lower circulating monocyte and neutrophil numbers in humans and mice224–226. Mice fed a high-fat diet over several weeks and exposed to regular exercise had fewer skeletal muscle inflammatory cytokines, lower macrophage muscle infiltration227 and less inflammatory macrophage phenotypes228. A human study investigating how 12 weeks of exercise affect inflammatory markers in the skeletal muscle tissue of elderly patients paralleled these animal studies, showing that exercise resulted in 50% less IL-6 and TNF-α mRNA229. In contrast to research documenting regular exercise’s anti-inflammatory effects, several human and animal studies indicate that strenuous bouts of exercise lead to increased inflammatory cytokine levels, leukocytosis and bone marrow cell release into the circulation, immediately after the physical activity230–233. These sudden effects may not be solely mediated by the metabolic shifts described above. Exercise activates both the somatomotor cortex and the autonomic nervous system, enabling skeletal muscle contraction, movement pattern coordination and adjustments in cardiovascular physiology according to system demand234, 235. Strenuous exercise modulates stress-related brain regions, including the amygdala236 and hypothalamic-pituitary adrenal axis, and increases peripheral sympathetic tone triggering leukocytosis237, 238 and progenitor release239–241. Supporting the observation that strenuous exercise leads to stress-like immune responses, injecting epinephrine or noradrenaline into rats resulted in elevated blood leukocyte levels similar to those produced by acute strenuous exercise242, 243. Of note, higher blood pressure and heart rate during exercise may raise the risk of acute ischemic events in the setting of existing CVD244, 245. Caution should thus be used when evaluating research studies on exercise and immunity, as some animal studies do not use voluntary exercise. In some experimental settings, mice are forced to swim or forced to run on a treadmill motivated by mild electric shocks. Both of these forced exercise models likely induce psychological stress and may hence result in a mixed phenotype that reflects more than the positive effects of physical activity. Precise mechanisms by which voluntary exercise may affect short-term immune activation or bone marrow cell release have not yet been described, although stress hormones and sympathetic nervous signaling likely contribute, as they do for humans under stress202, 203. It would be of great interest to experimentally address how the bone marrow niche and hematopoietic factors respond during and shortly after voluntary physical activity, especially beyond stress-induced mechanisms.
A physically active lifestyle associates with better cardiovascular health, perhaps by improving energy consumption and protecting against obesity. Interestingly, long-term regular physical activity in humans has immunosuppressive effects that lower circulating monocyte and lymphocyte numbers246–250. A recent study of individuals with increased cardiovascular risk showed that 16 weeks of daily walking, 1–2 hours per day, did not significantly lower circulating cytokines or oxidative stress markers but did curtail peripheral blood mononuclear cells’ ability to produce cytokines upon stimulation247. Individuals with the longest walking times showed the strongest reduction in cytokine production capacity, suggesting an inverse correlation between physical activity and immunity in this setting. Similar to previous observations on exercise and metabolism, this study revealed that walking decreased peripheral blood mononuclear cells’ oxygen consumption and lactate production, thereby positing enhanced cellular metabolism as one potential mechanism driving these cells’ attenuated immune response after physical activity, but this explanation lacks mechanistic proof.
One possible explanation for the lower circulating leukocyte counts after long-term physical activity is dampened hematopoiesis. Indeed, recent work demonstrated that regular voluntary exercise in mice calms the bone marrow niche and promotes hematopoietic quiescence (Figure 3)224. In this study, mice were given permanent access to a treadmill in their cage for 6 weeks and voluntarily ran, on average, 11.6 km per day. Compared to sedentary mice, running mice had reduced bone marrow HSPC numbers and less proliferation, resulting in lower circulating leukocyte counts for all subsets except erythrocytes. Running decreased leptin production in adipose tissue, consequently lowering the levels in circulation and the bone marrow. Reduced leptin signaling to leptin-expressing stromal cells in the bone marrow niche of running mice elevated HSC quiescence-promoting niche factors, including Cxcl12. Giving mice access to running wheels for 6 weeks followed by 3 weeks of running wheel withdrawal revealed that although leptin levels quickly reversed, circulating leukocytes and their production remained lower even weeks after exercise termination. ATAC sequencing of hematopoietic progenitors sorted from these mice showed that running induces long-term epigenetic changes impacting chromatin accessibility and affecting progenitor proliferation weeks after exercise ends. Specifically deleting the leptin receptor in leptin-expressing stromal bone marrow niche cells, using Prrx1-creERT2; Leprfl/fl mice, mimicked the exercise-induced dampening effect on hematopoiesis and improved outcomes after MI and in atherosclerosis. To test whether the reduced leukocyte levels in physically active mice would impair host defense and bacterial clearing, running mice and sedentary controls were subjected to two different models of sepsis, intraperitoneally injected lipopolysaccharides and cecal ligation and puncture. Interestingly, running mice responded to sepsis with elevated bone marrow HSPC activity and higher circulating leukocyte numbers, resulting in improved survival compared to sedentary mice. These findings indicate that physical activity in mice dampens hematopoiesis via modulating the bone marrow niche, consequently improving cardiovascular disease outcomes without impairing host defense mechanisms that are crucial for surviving infections. These data support previous studies that show physical activity has anti-inflammatory effects in humans and mice225, 226, 247, 251, 252. These studies also raise the concern that nearly all mice in research facilities are kept in cages without access to running wheels. Rather than being classified as neutral controls, these mice should be considered exposed to sedentary lifestyle, and the possible effects on experimental research should be anticipated.
Fig. 3. Interrelated pathways and modifiable risk factors for cardiovascular disease.
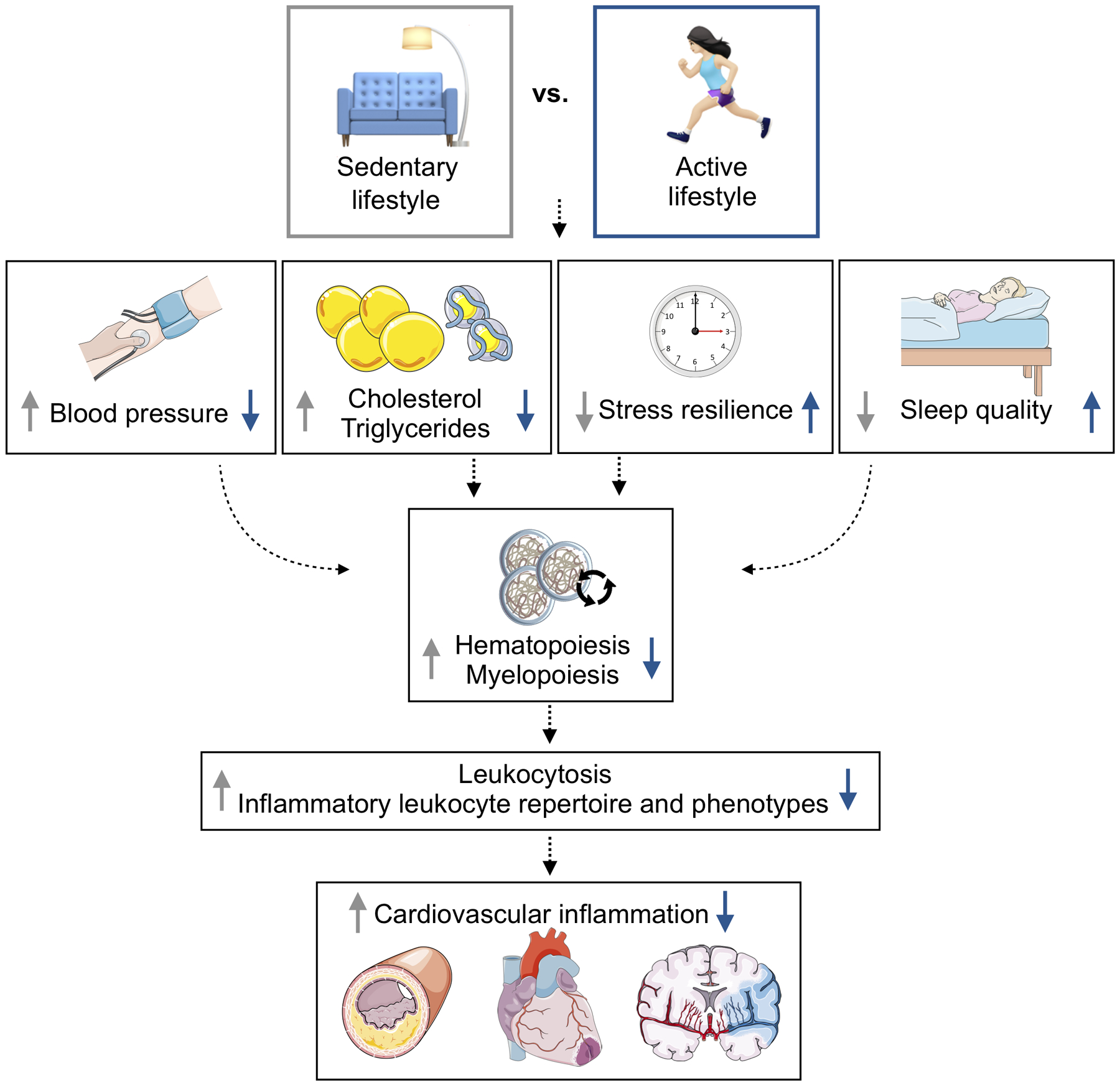
The cartoon depicts how physical activity may moderate innate immunity through multiple organs systems, ultimately affecting cardiovascular disease inception and progression.
Several questions remain unanswered: Does exercise affect extramedullary hematopoiesis in the spleen? Is the type, timing and intensity of exercise important, as some authors232, 253, 254 suggest exercise has a u-shaped effect on immunity? What cell-cell interactions are influenced by sedentary behavior, in particular, are there additional local and long-distance adipocyte-, stromal- and neural-immune cell interactions? Future research efforts aimed at clarifying these questions will help us understand how a physically active lifestyle can benefit health and guide physicians in making specific exercise recommendations to their patients. Given the increasing prevalence of obesity and diabetes, propagating physical activity is a highly cost-effective health intervention that is currently underused. Societal development has reduced the necessity of physical activity in employment and transportation, probably contributing to the obesity epidemic. Despite its low entry costs, exercise is now a leisure activity and is becoming a luxury for many people who work long hours sitting at desks, preceded and followed by sedentary commutes. Solving this problem requires creative prevention strategies built around behavior. For example, why are elevators typically centrally located in the lobby, beautiful and air conditioned while staircases are often hidden and less attractive?
Conclusion
Epidemiology research established a strong correlation between modifiable risk factors and cardiovascular outcomes. Motivated by these clinical data, and by the insight that atherosclerosis is not only a lipid storage but also an inflammatory disease, the field has begun to investigate how lifestyle-related behavior influences pathways that involve immunity. While these studies clarify that leukocyte production and phenotype are shaped by exposure to stress, diet choice and sleep hygiene, to date the uncovered mechanisms have relied on cellular and molecular components well known from work in neuroscience, metabolism, hematology and immunology. Connecting these fields in interdisciplinary teams reveals those cross-cutting pathways. However, the currently known mechanisms are unlikely to be the only important players; rather, they establish a proof-of-principle. The experimental platforms described herein, including sleep disruption, chronic mild stress and voluntary exercise are robust, have been used in their respective fields for multiple years and are straightforward to implement in any laboratory. We posit that these tools can serve as discovery platforms in the search for currently unknown pathways and targets that build resilience against cardiovascular inflammation. One promising avenue for such work is to investigate the microbiome, metabolome, proteome, transcriptome and epigenome, perhaps even in meaningful combinations, with unbiased assays. Such work would give rise to large data sets to help discover currently unknown health-promoting pathways and related drug targets. Importantly, drug targets discovered with these platforms may have desirable toxicity profiles. For example, mice with access to running wheels produced fewer leukocytes but survived peritonitis better, an observation that suggests voluntary running may serve as a discovery platform for anti-inflammatory therapeutics that do not compromise host defense. Thus, the currently available and quickly growing body of data serves as a motivation to focus on unbiased discovery work into how health-promoting behavior reduces modifiable cardiovascular risk.
Fig. 1. Lifestyle factors affect hematopoiesis in cardiovascular disease.
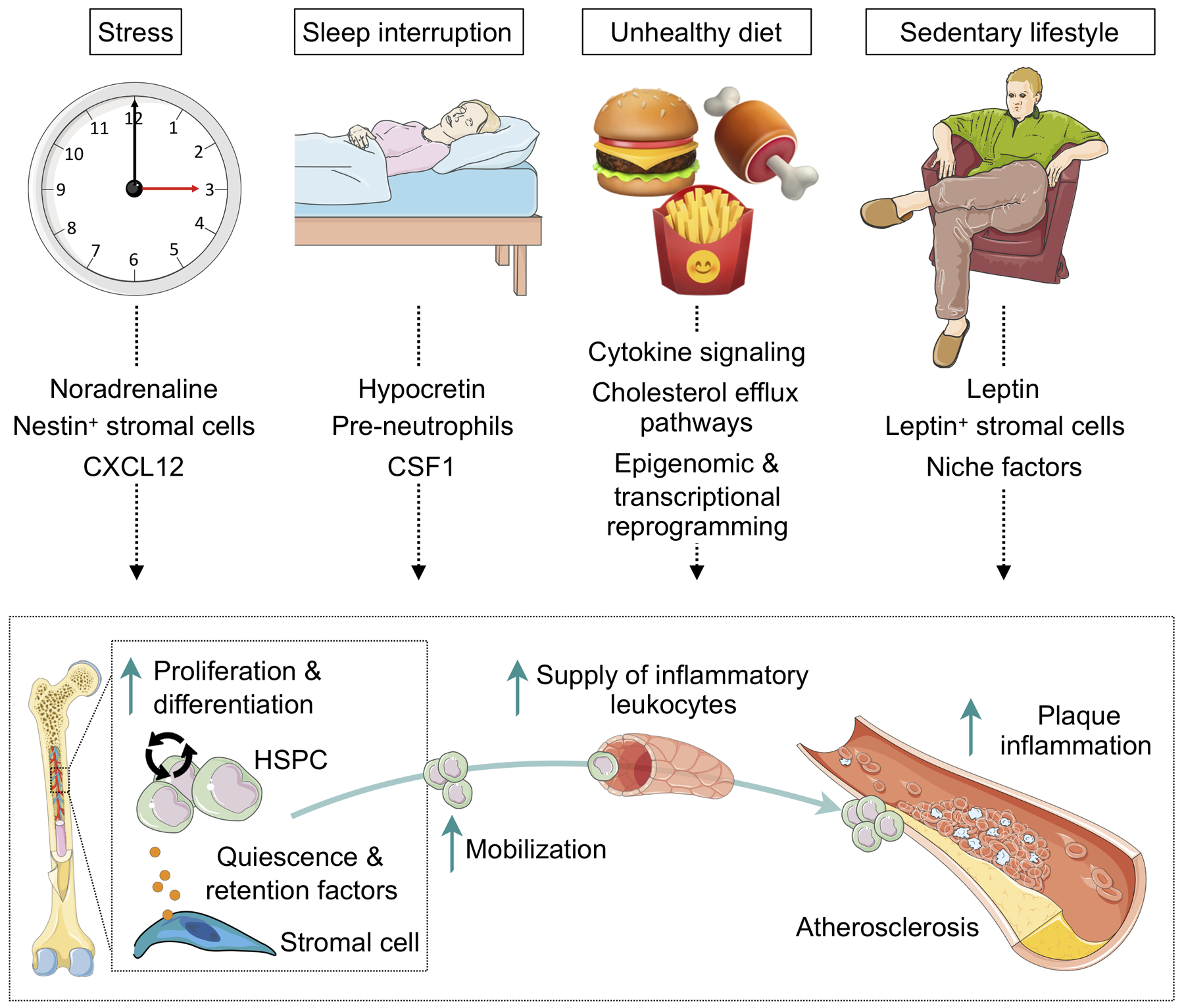
The cartoon illustrates the various pathways how lifestyle factors influence hematopoiesis and increase inflammatory leukocyte supply to the atherosclerotic lesion, accelerating its progression. CXCL indicates C-x-c chemokine ligand; CSF1, colony stimulating factor 1; HSPC, hematopoietic stem and progenitor cell. Cartoon summarizes data in references76, 77, 146, 177, 202, 224, 255.
Acknowledgements
We thank Kaley Joyes, PhD, for editing the article. Figures were designed using Servier Medical Art (http://www.servier.com).
Sources of Funding
This work was supported in part by the National Institutes of Health grants HL135752, HL131478, HL142494, HL139598 and the MGH Research Scholar program. M.J. Schloss was funded by Deutsche Forschungsgemeinschaft (SCHL 2221/1-1).
Non-standard Abbreviations and Acronyms:
- CSF
colony stimulating factor
- CVD
cardiovascular disease
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cells
- MMP
matrix metalloproteinase
- SCF
stem cell factor
- PET
postiron emission tomography
Footnotes
Disclosures
Dr. Nahrendorf received consulting fees from Verseau Therapeutics and IFM Therapeutics. Dr. Swirski received consulting fees from Verseau Therapeutics, Partner Therapeutics and Novartis.
REFERENCES
- 1.Libby P Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- 2.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randolph GJ. The fate of monocytes in atherosclerosis. J Thromb Haemost. 2009;7 Suppl 1:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37(22):1720–1722. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS TG. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 7.Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Hamandi A, Mowafi M. The global economic burden of noncommunicable diseases. 2012 [Google Scholar]
- 8.Arsenault BJ, Després JP. Cardiovascular disease prevention: lifestyle attenuation of genetic risk. Nat Rev Cardiol. 2017;14(4):187–188. [DOI] [PubMed] [Google Scholar]
- 9.Gaziano TA. Lifestyle and Cardiovascular Disease: More Work to Do. J Am Coll Cardiol. 2017;69(9):1126–1128. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ Res. 2019;124(5):799–815. [DOI] [PubMed] [Google Scholar]
- 11.Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, Tielemans MJ, Voortman T, Freak-Poli R, Veloso GGV, Chowdhury R, Kavousi M, Muka T, Franco OH. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(9):831–845. [DOI] [PubMed] [Google Scholar]
- 12.Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Tang X, Zhang W, Qian Y, Huang Y, Wang X, Chen J, Chen Z, Qi L, Li L, China KBCG. Adherence to Healthy Lifestyle and Cardiovascular Diseases in the Chinese Population. J Am Coll Cardiol. 2017;69(9):1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odegaard AO, Koh WP, Gross MD, Yuan JM, Pereira MA. Combined lifestyle factors and cardiovascular disease mortality in Chinese men and women: the Singapore Chinese health study. Circulation. 2011;124(25):2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–752. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART SI. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. [DOI] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116(5):884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 Suppl 1):III15–9. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res. 2016;118(4):531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol TTCTTC. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. [DOI] [PubMed] [Google Scholar]
- 20.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET SG. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. [DOI] [PubMed] [Google Scholar]
- 22.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827–838. [DOI] [PubMed] [Google Scholar]
- 23.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350(9080):757–764. [DOI] [PubMed] [Google Scholar]
- 25.Fielding JE. Smoking: health effects and control (1). N Engl J Med. 1985;313(8):491–498. [DOI] [PubMed] [Google Scholar]
- 26.Kohl HW, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, Kahlmeier S, Lancet PASWG. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380(9838):294–305. [DOI] [PubMed] [Google Scholar]
- 27.Dimsdale JE. Psychological stress and cardiovascular disease. Journal of the American College of Cardiology. 2008;51(13):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, Warner ET, Wasfy J, Fayad ZA, Koenen K, Ridker PM, Pitman RK, Armstrong KA. Stress-Associated Neurobiological Pathway Linking Socioeconomic Disparities to Cardiovascular Disease. J Am Coll Cardiol. 2019;73(25):3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajagopalan S, Al-Kindi SG, Brook RD. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(17):2054–2070. [DOI] [PubMed] [Google Scholar]
- 30.Munzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014;35(13):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64(13):1299–1306. [DOI] [PubMed] [Google Scholar]
- 32.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. [DOI] [PubMed] [Google Scholar]
- 34.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118(9):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 36.Domínguez F, Fuster V, Fernández-Alvira JM, Fernández-Friera L, López-Melgar B, Blanco-Rojo R, Fernández-Ortiz A, García-Pavía P, Sanz J, Mendiguren JM, Ibañez B, Bueno H, Lara-Pezzi E, Ordovás JM. Association of Sleep Duration and Quality With Subclinical Atherosclerosis. J Am Coll Cardiol. 2019;73(2):134–144. [DOI] [PubMed] [Google Scholar]
- 37.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E, PREDIMED SI. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. [DOI] [PubMed] [Google Scholar]
- 38.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA, PREDIMED SI. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 39.Sofi F, Dinu M, Pagliai G, Cesari F, Gori AM, Sereni A, Becatti M, Fiorillo C, Marcucci R, Casini A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation. 2018;137(11):1103–1113. [DOI] [PubMed] [Google Scholar]
- 40.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. [DOI] [PubMed] [Google Scholar]
- 41.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. [DOI] [PubMed] [Google Scholar]
- 42.Passegué E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202(11):1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514(7522):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553(7689):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19(3):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–1657. [DOI] [PubMed] [Google Scholar]
- 52.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. [DOI] [PubMed] [Google Scholar]
- 54.Méndez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16(4):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37(2):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med. 2016;8:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schloss MJ, Hilby M, Nitz K, Guillamat Prats R, Ferraro B, Leoni G, Soehnlein O, Kessler T, He W, Luckow B, Horckmans M, Weber C, Duchene J, Steffens S. Ly6Chigh Monocytes Oscillate in the Heart During Homeostasis and After Myocardial Infarction-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37(9):1640–1645. [DOI] [PubMed] [Google Scholar]
- 59.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheiermann C, Frenette PS, Hidalgo A. Regulation of leucocyte homeostasis in the circulation. Cardiovasc Res. 2015;107(3):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steffens S, Winter C, Schloss MJ, Hidalgo A, Weber C, Soehnlein O. Circadian Control of Inflammatory Processes in Atherosclerosis and Its Complications. Arterioscler Thromb Vasc Biol. 2017;37(6):1022–1028. [DOI] [PubMed] [Google Scholar]
- 62.Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2015;16(5):477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257(17):2318–2324. [PubMed] [Google Scholar]
- 64.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. [DOI] [PubMed] [Google Scholar]
- 65.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097–1103. [DOI] [PubMed] [Google Scholar]
- 66.Chapman KZ, Dale VQ, Dénes A, Bennett G, Rothwell NJ, Allan SM, McColl BW. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1764–1768. [DOI] [PubMed] [Google Scholar]
- 67.Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res. 2015;116(3):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Courties G, Frodermann V, Honold L, Zheng Y, Herisson F, Schloss MJ, Sun Y, Presumey J, Severe N, Engblom C, Hulsmans M, Cremer S, Rohde D, Pittet MJ, Scadden DT, Swirski FK, Kim DE, Moskowitz MA, Nahrendorf M. Glucocorticoids Regulate Bone Marrow B Lymphopoiesis After Stroke. Circ Res. 2019;124(9):1372–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26(5):654–665. [DOI] [PubMed] [Google Scholar]
- 70.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176(11):6523–6531. [DOI] [PubMed] [Google Scholar]
- 71.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol. 1994;144(1):188–199. [PMC free article] [PubMed] [Google Scholar]
- 72.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. [DOI] [PubMed] [Google Scholar]
- 73.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121(5):2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu Q, Yang X, Lv J, Zhang J, Xia B, Kim JD, Wang R, Xiong F, Meng S, Clements TP, Tandon B, Wagner DS, Diaz MF, Wenzel PL, Miller YI, Traver D, Cooke JP, Li W, Zon LI, Chen K, Bai Y, Fang L. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science. 2019;363(6431):1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poller WC, Nahrendorf M, Swirski FK. Hematopoiesis and cardiovascular disease. Circulation Research, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404. [DOI] [PubMed] [Google Scholar]
- 81.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169(3):510–522.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ Res. 2016;118(10):1498–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim HY, Lim SY, Tan CK, Thiam CH, Goh CC, Carbajo D, Chew SHS, See P, Chakarov S, Wang XN, Lim LH, Johnson LA, Lum J, Fong CY, Bongso A, Biswas A, Goh C, Evrard M, Yeo KP, Basu R, Wang JK, Tan Y, Jain R, Tikoo S, Choong C, Weninger W, Poidinger M, Stanley RE, Collin M, Tan NS, Ng LG, Jackson DG, Ginhoux F, Angeli V. Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity. 2018;49(2):326–341.e7. [DOI] [PubMed] [Google Scholar]
- 84.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209(1):123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, Wojtkiewicz G, Rohde D, Frodermann V, Vandoorne K, Courties G, Iwamoto Y, Garris CS, Williams DL, Breton S, Brown D, Whalen M, Libby P, Pittet MJ, King KR, Weissleder R, Swirski FK, Nahrendorf M. Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity. 2019;51(5):899–914.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122(12):1661–1674. [DOI] [PubMed] [Google Scholar]
- 89.Lin JD, Nishi H, Poles J, Niu X, Mccauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA, Loke P. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115(7):662–667. [DOI] [PubMed] [Google Scholar]
- 91.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21(6):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(6):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soehnlein O Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110(6):875–888. [DOI] [PubMed] [Google Scholar]
- 96.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. [DOI] [PubMed] [Google Scholar]
- 97.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177(1):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28(1):84–89. [DOI] [PubMed] [Google Scholar]
- 99.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11(2):257–266. [PubMed] [Google Scholar]
- 100.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Neutrophil antimicrobial peptide alpha-defensin causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2006;43(2):357–363. [DOI] [PubMed] [Google Scholar]
- 101.Lee TD, Gonzalez ML, Kumar P, Grammas P, Pereira HA. CAP37, a neutrophil-derived inflammatory mediator, augments leukocyte adhesion to endothelial monolayers. Microvasc Res. 2003;66(1):38–48. [DOI] [PubMed] [Google Scholar]
- 102.Pereira HA, Moore P, Grammas P. CAP37, a neutrophil granule-derived protein stimulates protein kinase C activity in endothelial cells. J Leukoc Biol. 1996;60(3):415–422. [DOI] [PubMed] [Google Scholar]
- 103.Taekema-Roelvink ME, Kooten C, Kooij SV, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol. 2001;12(5):932–940. [DOI] [PubMed] [Google Scholar]
- 104.Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ Res. 2018;123(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald H, Agerberth B, Lindbom L. Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008;118(10):3491–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soehnlein O, Kenne E, Rotzius P, Eriksson EE, Lindbom L. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin Exp Immunol. 2008;151(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sedgwick JB, Hwang YS, Gerbyshak HA, Kita H, Busse WW. Oxidized low-density lipoprotein activates migration and degranulation of human granulocytes. Am J Respir Cell Mol Biol. 2003;29(6):702–709. [DOI] [PubMed] [Google Scholar]
- 108.Singh U, Devaraj S, Jialal I. C-reactive protein stimulates myeloperoxidase release from polymorphonuclear cells and monocytes: implications for acute coronary syndromes. Clin Chem. 2009;55(2):361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosokawa T, Kumon Y, Kobayashi T, Enzan H, Nishioka Y, Yuri K, Wakiguchi H, Sugiura T. Neutrophil infiltration and oxidant-production in human atherosclerotic carotid plaques. Histol Histopathol. 2011;26(1):1–11. [DOI] [PubMed] [Google Scholar]
- 110.Leclercq A, Houard X, Philippe M, Ollivier V, Sebbag U, Meilhac O, Michel JB. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82(6):1420–1429. [DOI] [PubMed] [Google Scholar]
- 111.Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A. 1986;83(12):4322–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12(2):178–180. [DOI] [PubMed] [Google Scholar]
- 113.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207(8):1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108(10):1232–1237. [DOI] [PubMed] [Google Scholar]
- 115.Hedrick CC. Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(2):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2015;35(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25(11):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. [DOI] [PubMed] [Google Scholar]
- 119.Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and Prevalence of Cardiovascular Diseases and Prognosis-The Obesity Paradox Updated. Prog Cardiovasc Dis. 2016;58(5):537–547. [DOI] [PubMed] [Google Scholar]
- 120.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. [DOI] [PubMed] [Google Scholar]
- 121.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. [DOI] [PubMed] [Google Scholar]
- 122.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–1496. [DOI] [PubMed] [Google Scholar]
- 123.Mathis D Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17(6):851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. [DOI] [PubMed] [Google Scholar]
- 125.Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res. 2016;118(11):1752–1770. [DOI] [PubMed] [Google Scholar]
- 126.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, Krankel N, Widera C, Sonnenschein K, Haghikia A, Weissenborn K, Fraccarollo D, Heimesaat MM, Bauersachs J, Wang Z, Zhu W, Bavendiek U, Hazen SL, Endres M, Landmesser U. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol. 2018;38(9):2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khan NB, Khan MH, Shaikh MZ, Khanani MR. Effects of Ramadan fasting on glucose levels and serum lipid profile among type 2 diabetic patients. Saudi Med J. 2010;31(11):1269–1270. [PubMed] [Google Scholar]
- 130.Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90(5):1138–1143. [DOI] [PubMed] [Google Scholar]
- 132.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring). 2013;21(7):1370–1379. [DOI] [PubMed] [Google Scholar]
- 133.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. Eur J Clin Nutr. 2013;67(7):783–785. [DOI] [PubMed] [Google Scholar]
- 134.Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73(10):661–674. [DOI] [PubMed] [Google Scholar]
- 135.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86(1):7–13. [DOI] [PubMed] [Google Scholar]
- 136.Faris MA, Kacimi S, Al-Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32(12):947–955. [DOI] [PubMed] [Google Scholar]
- 137.Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, Wirtz TH, Naik S, Rose SA, Brocker CN, Gainullina A, Hornburg D, Horng S, Maier BB, Cravedi P, LeRoith D, Gonzalez FJ, Meissner F, Ochando J, Rahman A, Chipuk JE, Artyomov MN, Frenette PS, Piccio L, Berres ML, Gallagher EJ, Merad M. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019;178(5):1102–1114.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7(3):311–317. [DOI] [PubMed] [Google Scholar]
- 139.Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, Kishton RJ, Shannon JP, Dixit D, Schwab SR, Hickman HD, Restifo NP, McGavern DB, Schwartzberg PL, Belkaid Y. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell. 2019;178(5):1088–1101.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ketelhuth DFJ, Lutgens E, Back M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res. 2019;115(9):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, Selgrade D, Fenn AM, Chan CT, Mindur JE, Valet C, Poller WC, Halle L, Rotllan N, Iwamoto Y, Wojtkiewicz GR, Weissleder R, Libby P, Fernández-Hernando C, Drucker DJ, Nahrendorf M, Swirski FK. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 2019;566(7742):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. [DOI] [PubMed] [Google Scholar]
- 143.Kanda H, Tateya S, Tamori Y, Kotani K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of …. 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E, Amit I. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178(3):686–698.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag SJCFM, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172(1–2):162–175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Lumeng CN. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gimble JM. The function of adipocytes in the bone marrow stroma. The New Biologist. 1990;2(4):304–312. [PubMed] [Google Scholar]
- 150.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19(5):421–428. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Z, Huang Z, Ong B, Sahu C, Zeng H, Ruan H-B. Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica. 2019haematol. 2018. 205856.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nature cell biology. 2017;19(8):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125(2):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep. 2015;38(5):829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Krishnan HC, Gandour CE, Ramos JL, Wrinkle MC, Sanchez-Pacheco JJ, Lyons LC. Acute sleep deprivation blocks short-and long-term operant memory in Aplysia. Sleep. 2016;39(12):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Philip P, Sagaspe P, Prague M, Tassi P, Capelli A, Bioulac B, Commenges D, Taillard J. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness. Sleep. 2012;35(7):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. [DOI] [PubMed] [Google Scholar]
- 160.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. [DOI] [PubMed] [Google Scholar]
- 161.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. [DOI] [PubMed] [Google Scholar]
- 162.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33(8):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. [DOI] [PubMed] [Google Scholar]
- 164.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 165.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ali SS, Oni ET, Warraich HJ, Blaha MJ, Blumenthal RS, Karim A, Shaharyar S, Jamal O, Fialkow J, Cury R. Systematic review on noninvasive assessment of subclinical cardiovascular disease in obstructive sleep apnea: new kid on the block. Sleep medicine reviews. 2014;18(5):379–391. [DOI] [PubMed] [Google Scholar]
- 167.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. Journal of the American College of Cardiology. 2013;62(7):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–728. [DOI] [PubMed] [Google Scholar]
- 170.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 171.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. [DOI] [PubMed] [Google Scholar]
- 172.Ming H, Tian A, Liu B, Hu Y, Liu C, Chen R, Cheng L. Inflammatory cytokines tumor necrosis factor‑α, interleukin‑8 and sleep monitoring in patients with obstructive sleep apnea syndrome. Experimental and therapeutic medicine. 2019;17(3):1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. [DOI] [PubMed] [Google Scholar]
- 174.Fan Z, Lu X, Long H, Li T, Zhang Y. The association of hemocyte profile and obstructive sleep apnea. J Clin Lab Anal. 2019;33(2):e22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Geovanini GR, Wang R, Weng J, Tracy R, Jenny NS, Goldberger AL, Costa MD, Liu Y, Libby P, Redline S. Elevations in neutrophils with obstructive sleep apnea: The Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol. 2018;257:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Inonu Koseoglu H, Pazarli AC, Kanbay A, Demir O. Monocyte Count/HDL Cholesterol Ratio and Cardiovascular Disease in Patients With Obstructive Sleep Apnea Syndrome: A Multicenter Study. Clin Appl Thromb Hemost. 2018;24(1):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen L, Yang G. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol. 2015;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75(1):131–138. [DOI] [PubMed] [Google Scholar]
- 181.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res. 2012;110(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suárez-Barrientos A, López-Romero P, Vivas D, Castro-Ferreira F, Núñez-Gil I, Franco E, Ruiz-Mateos B, García-Rubira JC, Fernández-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97(12):970–976. [DOI] [PubMed] [Google Scholar]
- 183.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106(3):546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, Sperandio M, Hristov M, Zeller T, Nicolaes GAF, Weber C, Viola JR, Hidalgo A, Scheiermann C, Soehnlein O. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018;28(1):175–182.e5. [DOI] [PubMed] [Google Scholar]
- 185.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Juan A, Ince LM, Pick R, Chen CS, Molica F, Zuchtriegel G, Wang C, Zhang D, Druzd D, Hessenauer MET, Pelli G, Kolbe I, Oster H, Prophete C, Hergenhan SM, Albrecht U, Ripperger J, Montanez E, Reichel CA, Soehnlein O, Kwak BR, Frenette PS, Scheiermann C. Artery-Associated Sympathetic Innervation Drives Rhythmic Vascular Inflammation of Arteries and Veins. Circulation. 2019;140(13):1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 2017;46(1):120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, Bradfield P, Grenier JMP, Pelletier J, Druzd D, Chen CS, Ince LM, Bierschenk S, Pick R, Sperandio M, Aurrand-Lions M, Scheiermann C. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity. 2018;49(6):1175–1190.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis. 2014;236(2):385–388. [DOI] [PubMed] [Google Scholar]
- 191.Nabi H, Kivimäki M, Batty GD, Shipley MJ, Britton A, Brunner EJ, Vahtera J, Lemogne C, Elbaz A, Singh-Manoux A. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J. 2013;34:2697–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, INTERHEART I. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. [DOI] [PubMed] [Google Scholar]
- 193.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. [DOI] [PubMed] [Google Scholar]
- 194.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C, Güthlin D, Plasse A, Knez A, Küchenhoff H, Steinbeck G. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358(5):475–483. [DOI] [PubMed] [Google Scholar]
- 195.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29–33. [DOI] [PubMed] [Google Scholar]
- 196.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. [DOI] [PubMed] [Google Scholar]
- 197.Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci. 2018;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. [DOI] [PubMed] [Google Scholar]
- 201.Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, Wei Q, Wang X, Ciero P, Xu J, Leftin A, Frenette PS. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110(41):16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, Stefanski V. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol. 2004;156(1–2):153–162. [DOI] [PubMed] [Google Scholar]
- 205.Jaedicke KM, Fuhrmann MD, Stefanski V. Lactation modifies stress-induced immune changes in laboratory rats. Brain Behav Immun. 2009;23(5):700–708. [DOI] [PubMed] [Google Scholar]
- 206.Grzelak AK, Davis DJ, Caraker SM, Crim MJ, Spitsbergen JM, Wiedmeyer CE. Stress Leukogram Induced by Acute and Chronic Stress in Zebrafish (Danio rerio). Comp Med. 2017;67(3):263–269. [PMC free article] [PubMed] [Google Scholar]
- 207.Gray HG, Paradis TJ, Chang PW. Physiological effects of adrenocorticotropic hormone and hydrocortisone in laying hens. Poult Sci. 1989;68(12):1710–1713. [DOI] [PubMed] [Google Scholar]
- 208.Anderson BH, Watson DL, Colditz IG. The effect of dexamethasone on some immunological parameters in cattle. Vet Res Commun. 1999;23(7):399–413. [DOI] [PubMed] [Google Scholar]
- 209.Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis. 2012;221(2):359–365. [DOI] [PubMed] [Google Scholar]
- 210.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb Res. 2010;126(5):386–392. [DOI] [PubMed] [Google Scholar]
- 213.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389(10071):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training--what’s the link. Exp Physiol. 2002;87(4):423–435. [DOI] [PubMed] [Google Scholar]
- 215.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64(5):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith JK. Exercise and atherogenesis. Exerc Sport Sci Rev. 2001;29(2):49–53. [DOI] [PubMed] [Google Scholar]
- 217.Slentz CA, Bateman LA, Willis LH, Granville EO, Piner LW, Samsa GP, Setji TL, Muehlbauer MJ, Huffman KM, Bales CW, Kraus WE. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59(10):2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291(1):E99–E107. [DOI] [PubMed] [Google Scholar]
- 219.Conn VS, Koopman RJ, Ruppar TM, Phillips LJ, Mehr DR, Hafdahl AR. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J Prim Care Community Health. 2014;5(3):211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54(11):1472–1479. [DOI] [PubMed] [Google Scholar]
- 221.Ringseis R, Mooren FC, Keller J, Couturier A, Wen G, Hirche F, Stangl GI, Eder K, Krüger K. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol Nutr Food Res. 2011;55 Suppl 2:S193–202. [DOI] [PubMed] [Google Scholar]
- 222.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity. Nature Reviews Immunology. 20191. [DOI] [PubMed] [Google Scholar]
- 224.Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, van Koeverden ID, Herisson F, Honold L, Masson GS, Zhang S, Grune J, Iwamoto Y, Schmidt SP, Wojtkiewicz GR, Lee IH, Gustafsson K, Pasterkamp G, de Jager SCA, Sadreyev RI, MacFadyen J, Libby P, Ridker P, Scadden DT, Naxerova K, Jeffrey KL, Swirski FK, Nahrendorf M. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25(11):1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Node K, Michishita R, Tsuruta T, Shono N, Inoue T. Effect of exercise therapy on monocyte and neutrophil counts in overweight women. The American journal of the medical sciences. 2010;339(2):152–156. [DOI] [PubMed] [Google Scholar]
- 226.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise. J Leukoc Biol. 2008;84(5):1271–1278. [DOI] [PubMed] [Google Scholar]
- 227.Samaan MC, Marcinko K, Sikkema S, Fullerton MD, Ziafazeli T, Khan MI, Steinberg GR. Endurance interval training in obese mice reduces muscle inflammation and macrophage content independently of weight loss. Physiol Rep. 2014;2(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 229.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. Journal of Applied Physiology. 2008;105(2):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109(2):220–226. [DOI] [PubMed] [Google Scholar]
- 231.Morici G, Zangla D, Santoro A, Pelosi E, Petrucci E, Gioia M, Bonanno A, Profita M, Bellia V, Testa U, Bonsignore MR. Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1496–503. [DOI] [PubMed] [Google Scholar]
- 232.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–1081. [DOI] [PubMed] [Google Scholar]
- 233.Sand KL, Flatebo T, Andersen MB, Maghazachi AA. Effects of exercise on leukocytosis and blood hemostasis in 800 healthy young females and males. World journal of experimental medicine. 2013;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. The Journal of Physiology. 1972;226(1):173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Krogh A, Lindhard J. A comparison between voluntary and electrically induced muscular work in man. The Journal of physiology. 1917;51(3):182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen YC, Chen C, Martínez RM, Etnier JL, Cheng Y. Habitual physical activity mediates the acute exercise-induced modulation of anxiety-related amygdala functional connectivity. Sci Rep. 2019;9(1):19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone MH, Butterworth DE, Davis JM. The acute immune response to exhaustive resistance exercise. Int J Sports Med. 1995;16(5):322–328. [DOI] [PubMed] [Google Scholar]
- 238.Risøy BA, Raastad T, Hallén J, Lappegård KT, Baeverfjord K, Kravdal A, Siebke EM, Benestad HB. Delayed leukocytosis after hard strength and endurance exercise: aspects of regulatory mechanisms. BMC Physiol. 2003;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kroepfl JM, Pekovits K, Stelzer I, Fuchs R, Zelzer S, Hofmann P, Sedlmayr P, Dohr G, Wallner-Liebmann S, Domej W, Mueller W. Exercise increases the frequency of circulating hematopoietic progenitor cells, but reduces hematopoietic colony-forming capacity. Stem Cells Dev. 2012;21(16):2915–2925. [DOI] [PubMed] [Google Scholar]
- 240.Morici G, Zangla D, Santoro A, Pelosi E, Petrucci E, Gioia M, Bonanno A, Profita M, Bellia V, Testa U, Bonsignore MR. Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1496–503. [DOI] [PubMed] [Google Scholar]
- 241.Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol (1985). 2009;107(6):1943–1950. [DOI] [PubMed] [Google Scholar]
- 242.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells—From barracks to boulevards to battlefields: A tale of three hormones–Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Iversen PO, Stokland A, Rolstad B, Benestad HB. Adrenaline-induced leucocytosis: recruitment of blood cells from rat spleen, bone marrow and lymphatics. European journal of applied physiology and occupational physiology. 1994;68(3):219–227. [DOI] [PubMed] [Google Scholar]
- 244.Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest. 1989;83(6):1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F, American Heart Association Council on Nutrition PA, and Metabolism, American HACOCC, American COSM. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. [DOI] [PubMed] [Google Scholar]
- 246.Johannsen NM, Swift DL, Johnson WD, Dixit VD, Earnest CP, Blair SN, Church TS. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in postmenopausal women: results from DREW. PloS one. 2012;7(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Noz MP, Hartman YAW, Hopman MTE, Willems PHGM, Tack CJ, Joosten LAB, Netea MG, Thijssen DHJ, Riksen NP. Sixteen-Week Physical Activity Intervention in Subjects With Increased Cardiometabolic Risk Shifts Innate Immune Function Towards a Less Proinflammatory State. J Am Heart Assoc. 2019;8(21):e013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Palmefors H, DuttaRoy S, Rundqvist B, Börjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis--a systematic review. Atherosclerosis. 2014;235(1):150–161. [DOI] [PubMed] [Google Scholar]
- 249.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017;37:371–393. [DOI] [PubMed] [Google Scholar]
- 250.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training‐induced lowering of inflammatory (CD14+ CD16+) monocytes: a role in the anti‐inflammatory influence of exercise. Journal of leukocyte biology. 2008;84(5):1271–1278. [DOI] [PubMed] [Google Scholar]
- 251.Broderick TL, Sennott JM, Gutkowska J, Jankowski M. Anti-inflammatory and angiogenic effects of exercise training in cardiac muscle of diabetic mice. Diabetes, metabolic syndrome and obesity: targets and therapy. 2019;12:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ziegler AK, Damgaard A, Mackey AL, Schjerling P, Magnusson P, Olesen AT, Kjaer M, Scheele C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Scientific reports. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Frontiers in immunology. 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nieman DC. Exercise, infection, and immunity. International journal of sports medicine. 1994;15(S 3):S131–S141. [DOI] [PubMed] [Google Scholar]
- 255.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. [DOI] [PubMed] [Google Scholar]


